Utilization of damaged and spoiled wheat grains for
bioethanol production
Nadia Razdan* and G.S. Kocher
Department of Microbiology, Punjab Agricultural University, Ludhiana-141004, India
ABSTRACT
First generation ethanol from starchy crops particularly maize is an established technology that being a renewable
and bio-based resource has advantages over gasoline. The second generation ethanol from lignocellulosics owing to
its economic considerations is still at pilot scale and is yet to see commercialization. This has increased the demand
of starchy feed-stocks for energy. The recently released National Policy on Biofuels in May, 2018 categorically also
emphasizes on the potential of different raw materials for ethanol production by consenting the utilization of dam-
aged food grains like wheat, broken rice etc. that area otherwise un t for human consumption. As far as wheat is
concerned, The Comptroller and Auditor General (CAG), India (2017) reported that Food Corporation of India’s (FCI)
wheat stock worth 700 crores was damaged solely in Punjab from 2011 to 2016 as the grain was kept in open areas
attributed to the lack in storage facility. Development of ef cient technology to pretreat and convert damaged starch
into fermentable sugars and optimization of enzymatic hydrolysis using commercial as well as indigenous enzyme
preparation are the key points for the ef cient bioethanol production from damaged wheat. Further, the synergistic
action of alpha and glucoamylase in the hydrolysis of wheat mash have been tried that has revealed 96.25% con-
version ef ciency with an ethanol yield 5.60 % (v/v). The present review discusses research progress in bioethanol
production from damaged wheat grains containing higher starch content. Thus, utilization of especially damaged
and spoiled wheat grains pave better way for commercialization of bioethanol production from an economical
perspective.
KEY WORDS: BIOTHANOL, INDIGENOUS, LIGNOCELLULOSICS, DAMAGED WHEAT
658
Agricultural
Communication
Biosci. Biotech. Res. Comm. 11(4): 658-673 (2018)
ARTICLE INFORMATION:
Corresponding Authors: nadiarazdan2012@gmail.com
Received 25
th
Oct, 2018
Accepted after revision 24
th
Dec, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
Mono of Clarivate Analytics and Crossref Indexed
Journal Mono of CR
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at:
http//www.bbrc.in/
DOI: 10.21786/bbrc/11.4/17

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION 659
Nadia Razdan and G.S. Kocher
INTRODUCTION
Global demand for energy sources and their utilization
determine the economic status and growth of develop-
ing countries all over the world (Xu and Liu 2009). The
major energy demand is still supplied from conventional
fossil fuels such as oil, coal and natural gas which don’t
regenerate at sustainable manners (Twidell and Weir
2003). Fossil Fuels have played an indispensible role in
development of industry but its indiscriminate use and
the resulting environmental pollution led to advent of
alternate fuels. The Energy Information Administration
(EIA) reported that 70% of oil consumed in the United
States was used for transportation (EIA 2015 a). Accord-
ing to EIA’s 2014 report, 27% of petroleum consumed
in the United State was imported from foreign countries
(EIA 2015b). Hence, use of renewable resources to pro-
duce liquid biofuels offer attractive solutions to reducing
greenhouse gas emissions, decreasing reliance on foreign
oils, addressing energy security concerns, strengthening
rural and agricultural economies and increasing the sus-
tainability of the world transportation system. Today,
Bioethanol has long been regarded as a suitable sub-
stitute to fossil fuels. It has immense properties such as
higher compression ratio, shorter burn time and leaner
burn engine, which lead to theoretical ef ciency advan-
tages over gasoline (Hansen et al 2005). Brazil has the
world’s rst sustainable bioethanol economy with 6.19
billion gallons produced in 2014, which represents over
25% of the world’s ethanol fuel and is ranked second in
the world next to the United States (Biofuels 2016).
According to the Renewable Fuels Association (RFA
2018), the global production of bioethanol stood at 27
billion gallons in the year 2017, with the USA (15,800
million gallons) and Brazil (7,600 million gallons) as
the largest producers in the world. India ranks eighth
in ethanol production next to EU, China, US and Brazil
with a total production of 280 million gallons in 2017
(RFA, 2018).
The rst and second generation bioethanol are
commonly referred as rst generation bioethanol
resources and third type as second generation bioetha-
nol resources. The shift from rst to second generation
bioresources is obligatory due to main reasons; one is
that rst generation bio resources have alternate uses
such as food (sugarcane, corn), animal feed (molas-
ses) etc, secondly that these are still unable to meet
the global demand of bioethanol. On the other hand,
Lignocellulosics need costly steps of pretreatment and
range of enzyme requirement for sacchari cation which
is making bioethanol production from lignocellulosics
a costly affair. In this scenario, there is need for a suit-
able economical substitute as an alternative. Therefore
the National Policy on Biofuels –2018 categorically
emphasis on the potential of different raw materials for
ethanol production by consenting the utilization of sug-
arcane juice, sugar containing materials like sugar beet,
sweet sorghum, starch containing materials like corn,
cassava, damaged food grains like wheat, broken rice,
rotten potatoes, un t for human consumption. India is
an agricultural country where wheat and rice are staple
food for its burgeoning population. In terms of produc-
tion, India stands second after china in wheat and rice
production accounting for about 200MT/year out of this
about 12MT (6%) of grains are damaged by post-harvest
storage due to poor storage facilities and hence damaged
by insects, rodents, birds and microbial spoilage (Sharon
et al 2014). As far as wheat is concerned, Comptroller and
Auditor General (CAG) (2017) reported that Food Corpo-
ration of India’s (FCI) wheat stock worth 700 crore was
damaged solely in Punjab from 2011 to 2016 as the grain
was kept in open areas attributed to the lack in stor-
age facility (Anonymous 2017). All this damaged grains
were disposed off as they were not t for consumption
by human and animals. However, the damaged grains
can be put to application by producing ethanol from its
starch content which constitutes 70-80% in wheat and
rice. Theoretically, 30gal/MT of ethanol from damaged
wheat and rice may be obtained (Gawande and Patil
2015). Wheat starch is comprised of one quarter amylose
and around three quarters amylopectin with little pro-
tein and lipid debasements (0.8% and 0.2% respectively)
(Bowler et al 1985).
The process of grain bioethanol production involves
milling (grinding and pretreatments), mashing (enzy-
matic or acid hydrolysis, steaming, adding supplements
etc) and Fermentation (SHF or SSF) which is followed
by distillation and dehydration to produce anhydrous
ethanol. The conversion of starch to ethanol can be
accomplished by acid hydrolysis, but the generation of
by products such as levulinic and formic acid may cause
hampered yeast growth hence, lower yields of alcohol
(Kerr 1944). The acid hydrolysis has now been largely
switched with amylolytic enzymes (-amylase and glu-
coamylase) which deliver 95% more yield of glucose
(Hua and Yang 2016).
Though using damaged grains will incur lower sub-
strate cost, mashing involves costly commercial sac-
chari cation and enzymes which may also be taken
care of by using indigenous culture of Bacillus subti-
lis, Bacillus circulans, Bacillus cereus etc for -amylase
and Aspergillus such as Aspergillus niger, Aspergillus
oryzae etc for glucoamylase. Fermentation is the nal
stage performed after starch pretreatment (digestion) for
bioethanol production The process cost may be further
be reduced by using Simultaneous Sacchari cation and
Fermentation as it reduces the time as well as energy
by using two different vessels for Sacchari cation and

660 UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Nadia Razdan and G.S. Kocher
Fermentation besides lowering the chances of contami-
nation. This review designed to provide an insight into
the process details as well as the update of the damaged
cereals particularly damaged wheat for bioethanol pro-
duction.
FEEDSTOCKS FOR BIOETHANOL PRODUCTION
The raw materials for bioethanol production can broadly
be classi ed as (i) sucrose-containing feedstock (sugar-
cane, sugar beet and sweet sorghum) (ii) starch-con-
taining feedstock (wheat, corn and cassava) and (iii)
cellulosic feedstock (straw, grasses, wood, stovers, agri-
cultural wastes and paper etc) (Table 1) while the bioeth-
anol produced from sucrose-and starch-containing feed-
stock is classi ed as 1st generation bioethanol (ethanol
from corn and sugarcane) that produced utilizing cel-
lulosic feedstock is referred as 2
nd
generation bioethanol.
The source of third generation biofuel is lipolytic com-
pounds obtained predominantly from algae. Most cur-
rent bioethanol production processes utilize more read-
ily degradable biomass feedstock such as cereals (corn
and grain) and molasses. However, the utilization of
edible agricultural crops exclusively for biofuel produc-
tion con ict with food and feed production (Wheals et al
1999). One of the major problems with bioethanol pro-
duction is the variability in available raw materials as
their geographic locations differ from season to season,
place to place and price of substrates which affects and
hence the production cost of bioethanol (Kumar 2006;
Yoosin et al 2007).
UTILIZATION OF DAMAGED CEREAL GRAINS
FOR BIOETHANOL PRODUCTION
Cereal grains are used mostly for food and feed. How-
ever, post heat losses from farm to fork take a signi -
cant portion of damaged/infested cereal grains which
are not t for consumption. But there are rich sources
of sugar (in the form of starch) just like simple sugar
substances such as molasses, sugarcane juice can be fer-
mented. These starch based materials like corn, rice and
wheat have infact proved to be promising raw mate-
rials for their ef cient fermentation into industrial as
well as potable ethanol with the help of appropriate fer-
menting micro-organisms (Awasthi et al 2015). As per
estimates provided by Food Corporation of India (FCI)
huge quantities of cereal grains are getting spoiled every
year due to unfavourable climate conditions and become
un t for human and animal consumption and one mil-
lion tonnes of damaged grains is lying unutilised in FCI
stores (Kumar et al 1999). The damage includes discol-
oration, breakage, cracking, attack by fungi, insect dam-
age, chalky grain, partial softening due to dampness, off
smell etc (Gawande and Patil 2015).
The damaged grains used for ethanol production are
ten times cheaper than ne quality. These damaged or
waste cereal grains can be utilized for the effective pro-
duction of ethanol using fermentation process which
will not only meet (partially) our needs but may pro-
vide some incentive to the farmers who suffers due to
crop damage. The chemical composition of cereal grains
is characterized by the high content of carbohydrates
mainly starch (56–74%) deposited in the endosperm and
bre in the bran (2–13%). The second important group
of constituents is the proteins which fall within an aver-
age range of about 8–11% and high content of B-vita-
mins is, in particular, of nutritional relevance. Hence,
spoiled and damaged starchy grains can also be used for
bioethanol production.
DAMAGED WHEAT: A PROMISING RAW
MATERIAL FOR BIOETHANOL PRODUCTION
Global Scenario of wheat
Wheat is produced in 120 countries and accounts for
about 19 per cent of the world’s calorie supplies. It is
used primarily as our for making bread, pastry, pasta
and noodles etc. It is also used to feed livestock, with the
feed accounting for about 17 per cent of global wheat
consumption. In addition, the by-products from milling
of wheat into our are also used as feed. The annual
global production of dry wheat is about 529 Tg whereby
Asia (43%) and Europe (32%) are the primary producers.
Like rice, China is the largest producer of wheat with
about 18% of global production at an average yield of
3:4 dry mg ha
-1
.The second largest producer is India,
where dry wheat production is 71 Tg (12%), and the
yield is 2:4 dry mg ha
-1
(Seungdo et al 2004).
India produces wheat in appreciable amount that can
be a very good raw material for bioethanol production.
Secondly, a huge quantity of wheat is wasted every year
due to mismanagement; lack of proper storing facilities
in the warehouses and spoiled wheat can also be utilized
for bioethanol production. In the present Indian scenario
as per estimates provided by Food Corporation of India
(FCI) huge quantities of cereal grains are getting spoiled
every year due to unfavorable climatic conditions and
become un t for human and animal consumption. There
are about one million tons of damaged and spoiled
grains lying unutilized in FCI stores (Kumar et al 1999).
As far as wheat is concerned, Comptroller and Audi-
tor General (CAG) (2017) reported that Food Corporation
of India’s (FCI) wheat stock worth 700 crores was dam-
aged solely in Punjab from 2011 to 2016 as the grain
was kept in open areas attributed to the lack in stor-
age facility (Anonymous 2017). All this damaged grains
were disposed off as they were not t for consumption
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION 661
Nadia Razdan and G.S. Kocher
by human and animals. Belboom et al (2015) reported
that the consumption of 1 MJ bioethanol produced from
wheat instead of 1 MJ gasoline can reduce greenhouse
gas emissions by 42.5 - 61.2%.
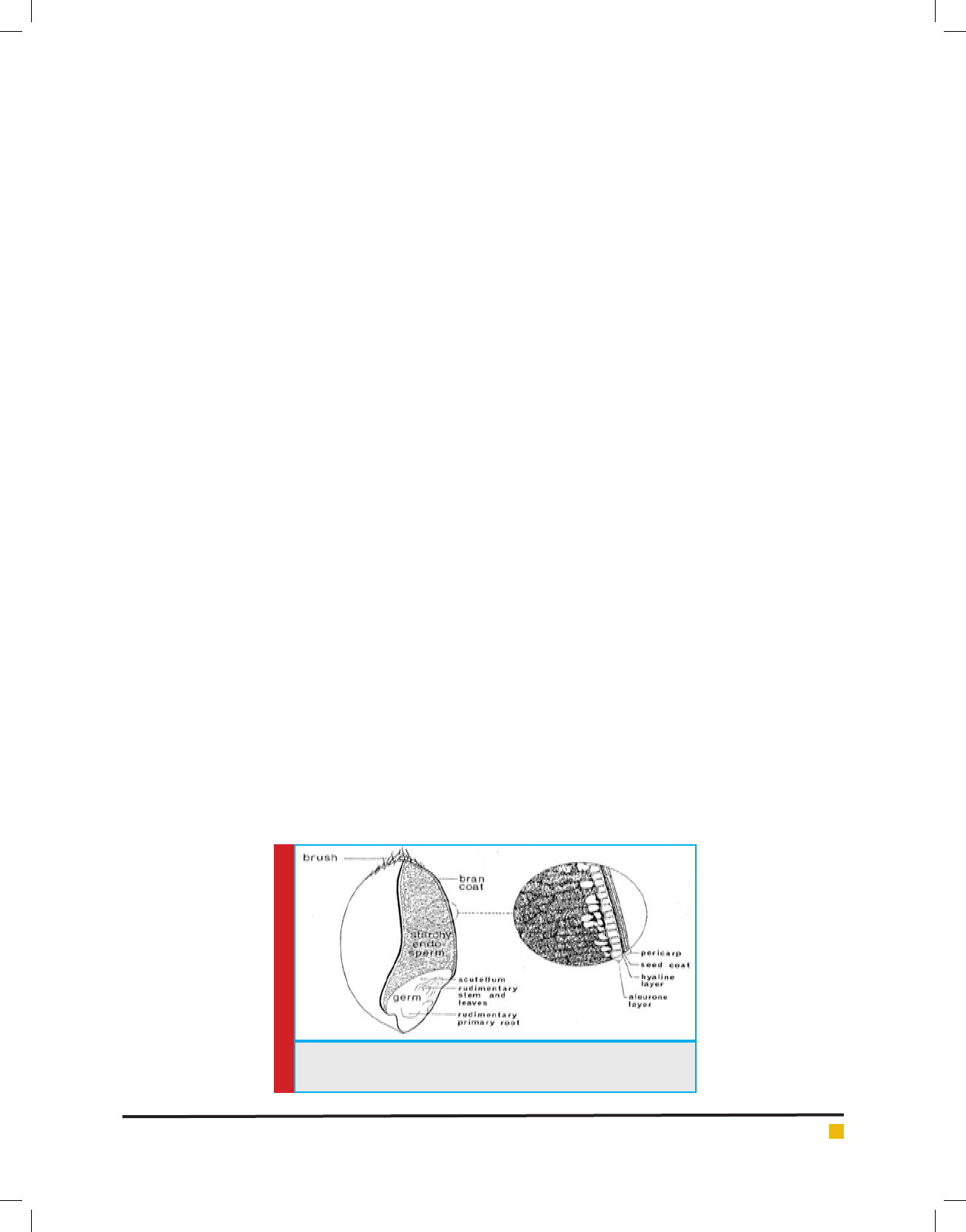
Structure and composition of wheat grain
Wheat, derived from the wild (Triticum aestivum L.) is
today the fth major cereal plant cultivated in the world
(Fig 2.1). Besides growing wheat for food purposes, there
is also an interest in wheat cultivars for non-food and
technical uses starch and bioethanol production (Kust
and Potmesilova 2014). Damaged wheat grains could be
an economical carbon source for ethanol fermentation
in the industry, because of high starch content and low
commercial value. Yan et al (2010) tested eld-sprouted-
sorghum and concluded that the use of these kernels sig-
ni cantly reduced fermentation time and yielded higher
ethanol. Starch or amylum is a polymeric carbohydrate
comprising of a large number of glucose units joined by
glycosidic bonds and contains two main structural com-
ponents, amylose and amylopectin. Amylose is essen-
tially a linear polymer in which the glucose residues are
connected by the -1, 4 linkages. The other main com-
ponent of starch is amylopectin, which is a larger and
branched molecule with both -1, 4 and -1, 6 link-
ages. Most native wheat starch is a mixture of amylose
and amylopectin, in the ratio of 1:3 by weight. The con-
tent of amylose in wheat starch generally ranges from
20–26% (Buresova et al 2010).
STARCH HYDROLYSIS
In industry, starch is converted into sugars or fermented
to produce ethanol. Starch cannot be metabolized
directly by yeast, but must rst be broken down into
simple six carbon sugars (glucose) prior to fermentation.
The conversion of starch-containing feedstock to obtain
fermentable sugars is mainly comprised of three opera-
tions which are: (i) milling, (ii) liquefaction and (iii) sac-
chari cation using enzymes.
The rst stage of starch hydrolysis is gelatinization
which is to break down the intermolecular bonds of
starch with heat in the presence of water. Starch gran-
ules are quite resistant to penetration by both water and
hydrolytic enzymes due to the formation of hydrogen
bonds within the molecule and other molecules. How-
ever, these intra and inter- hydrogen bonds are weak-
ened during gelatinization. During this stage, the tem-
perature of aqueous suspension of starch is elevated,
the water absorption and expanded granules dissolving
starch granules to form a viscous suspension or slurry.
This allows disruption or burst of the starch granules
and exposes it to enzyme attack. This process is known
as gelatinization and the temperature at which starch
properties are changed is named as gelatinization tem-
peratures (Albani 2008). The susceptibility of starch to
amylase attack depends on the properties of the speci c
starch, such as e.g. degree of gelatinization and the char-
acteristics of the speci c amylase (Bijttebier et al 2008).
Different starches have different gelatinization tempera-
tures, implying different ease of cooking. Cassava starch
has a lower temperature, relatively to cereal starches; the
pasting temperatures for cassava, corn, wheat and rice
are 60-65ºC, 75-80ºC, 80-85ºC and 73-75ºC, respectively
(Swinkels 1998; Thirathumthavorn and Charoenrein
2005). The physicochemical properties of starch impose
limitations in the use of higher starch concentrations
as a result of gelatinization of the starch which causes
undesirable viscosity development.
Liquefaction is a step that starch is degraded by an
endo-acting enzyme namely alpha –amylase (EC 3.2.1.1)
which hydrolyzes only -1, 4 and causes dramatically
drop in viscosity of cooked starch. Typically, liquefying
enzymes can have an activity at a high temperature (>
85°C) so that the enzyme can help reduce paste viscos-
ity of starch during cooking. The dextrins, i.e. products
FIGURE 1. Wheat grain cut lengthwise through crease (Pomeranz
1988)

662 UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Nadia Razdan and G.S. Kocher
obtained after liquefaction, is further hydrolyzed ulti-
mately to glucose by glucoamylase enzyme which can
hydrolyze both -1,4 and -1,6 glycosidic linkage in
amylase and amylopectin branches of starch. Glucoam-
ylase (GA), also known as amyloglucosidase (EC 3.2.1.3),
is an inverting type and exo-acting enzyme, capable of
hydrolyzing -1,4 glycosidic linkages in raw or soluble
starches and related oligosaccharides with the inversion
of the anomeric con guration to produce glucose.
The starch-based bioethanol industry has been com-
mercially viable for about 30 years; in that time, tre-
mendous improvements have been made in enzyme
ef ciency, reducing process costs, time, increasing
hydrolysis and bioethanol productivity. Hydrolysis of
starch may be considered as a key step in the process-
ing of starch-based feedstock for the bioethanol produc-
tion. Starch can be hydrolyzed by acid, acid-enzyme and
enzyme-enzyme techniques.
Acid hydrolysis (lintnerization)
Acid hydrolysis is an important chemical modi cation
that can signi cantly change the structural and func-
tional properties of starch without disrupting its granular
morphology. During acid hydrolysis, amorphous regions
are hydrolysed preferentially, enhancing the crystallin-
ity and double helical content of acid hydrolyzed starch
(Wang and Copeland 2013). According to Dziedzic and
Kearsley (2012) acid hydrolysis was discovered at the
beginning of the 19
th
century by boiling wheat starch
with dilute sulphuric acid results in a sweet syrup. Later,
potato starch was used as the starch source and sulphu-
ric acid was replaced by hydrochloric acid and indirect
heating of the reaction vessel was common practice.
Since then, acid has been used to a great extent for the
breakdown of starch into glucose particularly in indus-
try. Bej et al (2008) had investigated on concentrated
acid hydrolysis (H
2
SO
4
) of wheat our in a batch reac-
tor at different temperatures and acid concentrations. A
maximum conversion (42%) of starch to the reducing
sugars was obtained at 95°C and pH 3.
Similarly, Hoseinpour et al (2010) showed that
hydrolysis of starch using dilute sulphuric acid leads to
complete conversion to glucose under optimum condi-
tions of 130°C, 1% acid and 7.5% solids loading for 30
minutes. The mineral acid or acid-base involved in the
hydrolysis can be of diluted or concentrated form and
dilute acid process at 1-5% concentration is conducted
under high temperature, pressure and has fast reaction.
The concentrated acid process on the other hand uses
relatively mild temperatures and reaction times are typi-
cally much longer as compared to dilute acid hydrolysis.
The biggest advantage of dilute acid processes is their
fast reaction rate, which facilitates continuous process-
ing for hydrolysis of both starch and cellulosic materi-
als. Their prime disadvantage however is the low sugar
yield and this has opened up a new challenge to increase
glucose yields higher than 70% (especially in cellulosic
material) in an economically viable industrial process
while maintaining high hydrolysis rate and minimizing
glucose decomposition (Xiang et al 2004; McConnell
2008). The concentrated acid hydrolysis offers high
sugar recovery ef ciency, up to 90% of both hemicellu-
loses and cellulose sugars. However, this technique does
have a number of drawbacks such as relatively low yield
and formation of undesirable by-products (Ramprakash
and Muthukumar 2014).
Enzymatic hydrolysis
In the last decade, the starch industry has transformed
from using acid in the hydrolysis process to enzyme. The
acid was largely replaced by enzyme which gives 95%
more yield of glucose (Hua and Yang 2016). Enzymatic
hydrolysis of starch requires two types of enzymes due
to the fact that starch or amylum comprises of two major
components, namely amylose, a mainly linear polysac-
charide consisting of -1,4-linked -glucopyranose
units and the highly branched amylopectin fraction that
consists of -1,4 and -1,6-linked -glucopyranose
units (Knox et al 2004). These two types of linkages,
-1, 4 and -1,6-linked required an ef cient starch
hydrolysis agent or enzyme that can fraction -1,4 and
promote -1,6 debranching activity which leads to a
reduction in viscosity of gelatinized starch in the lique-
faction process. There are certain type of carbohydrate-
degrading enzymes include a -amylases, b -amylases,
debranching enzymes, cellulases, b -glucanases and glu-
cosidases etc. The process of enzyme hydrolysis involves
hydration of starch by heating the starch in aqueous
suspension to give -amylase an access to hydrolyze
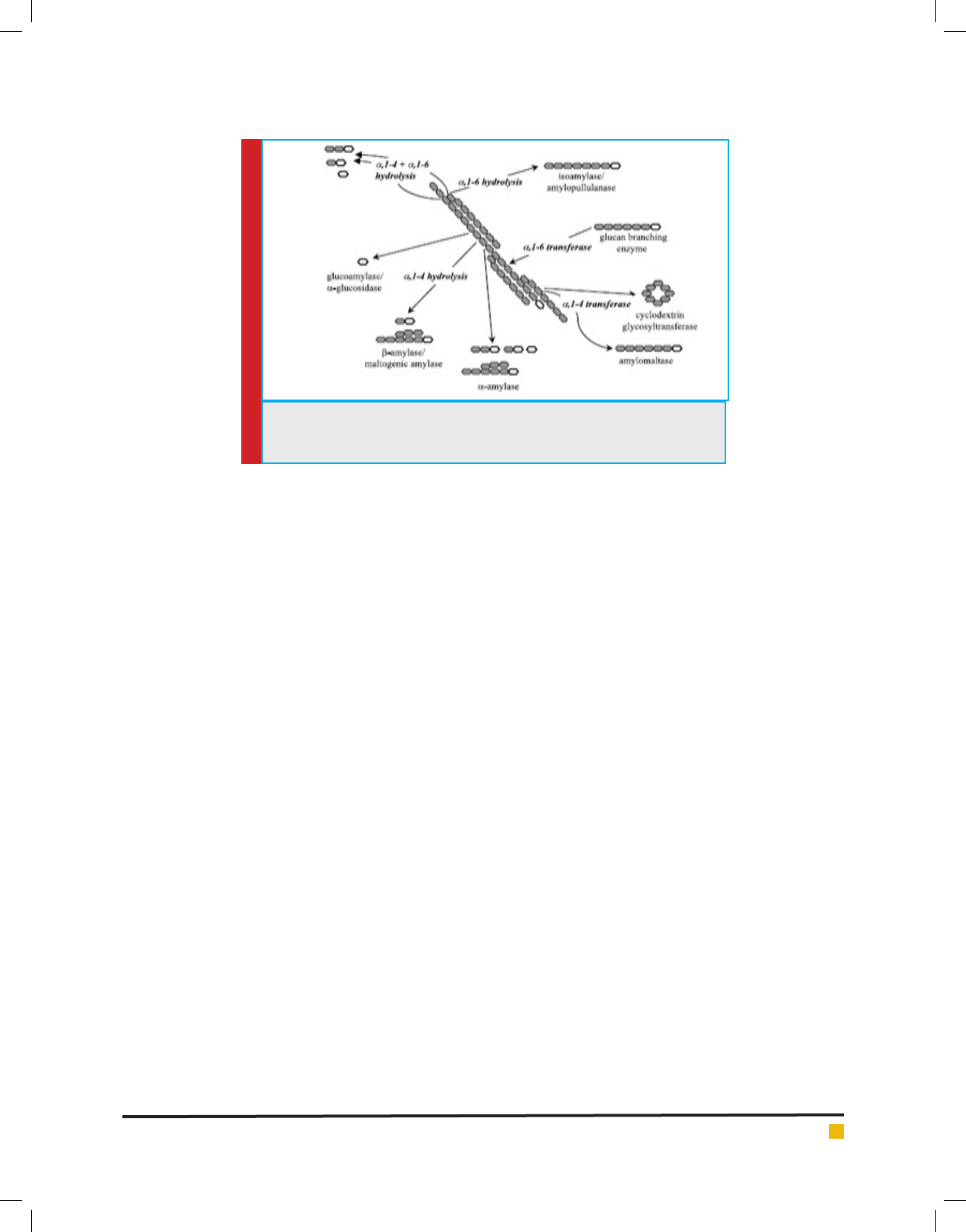
the starch (Fig 2). Exoamylases such as glucoamylase
is added during sacchara cation which hydrolyses 1,4
and 1,6-alpha linkages in lique ed starch(Maarel et al
2002).The important advantages of the sacchari cation
of starch by the amylase mode include higher yield and
purity, easy crystallization, better process control, lower
cost of production, ion exchange capacity, signi cant
reduction in energy requirement, elimination of heavy
depreciations on expensive corrosion resistant equip-
ment, production of new products and formation of
lower by-products (Barfoed 1967; Madsen and Norman
1973; Fullbrook 1984).
MICROBIAL DIVERSITY INVOLVED IN AMYLASE
PRODUCTION
The amylolytic microorganisms have immense applica-
tions in industries as well as in scienti c research as
they are more stable when compared with plant and ani-
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION 663
Nadia Razdan and G.S. Kocher
mal amylase. The major advantage of using microorgan-
isms for the production of amylases is the economical
bulk production capacity and the fact that microbes are
easy to manipulate to obtain enzymes of desired char-
acteristics. Though amylases are produced by several
fungi, yeast, bacteria and actinomycetes, but only a few
selected strains of fungi and bacteria meet the criteria
for commercial amylase production (Table 1).
Microbial production of alpha amylase
Pro ling microorganisms with high potential for amyl-
ase production in submerged fermentation (SmF) using
synthetic media has been widely recognized due to their
myriad applicability in bioethanol production. Raplong
et al (2014) identi ed Bacillus using mannitol egg yolk
polymyxin B (MYP) agar a highly selective media.They
reported that Bacillus cereus strain SB2 had largest zone
of hydrolysis of 12mm on nutrient agar supplemented
with starch. Amylase activity of 2.56U/ml was obtained
at pH (6.5), temperature (35ºC), incubation time (24 hr)
and inoculum concentration (4%) in submerged fermen-
tation. Singh and Kumari (2016) isolated starch degrad-
ing bacteria from soil samples collected from different
environment sources (Banana, Potato and Sugarcane
eld samples). Out of 10 isolated bacterial strains, Bacil-
lus sp. B3 gave positive starch hydrolysis and thus was
suggested for industrial application like starch modi -
cation with better ef ciency with the increase in tem-
perature.
Similarly, Rehman and Saeed (2015) investigated
39 amylase producing Bacillus sp. from soil of which
Bacillus sp. stain AS-2 was reported to have highest
enzyme activity (3179.62 IU/ml/min). Vaseekaran et al
(2015) isolated, identi ed and characterised thermo-
stable amylolytic bacteria from contaminated soil with
decaying materials i.e. kitchen waste and bakery waste
soil etc. Their investigation revealed one strain identi ed
as Bacillus licheniformis with highest -amylase activ-
ity (7.0±0.21 Um/L) at 24 h and enzyme showed neutral
optimum pH and temperature (90
o
C) without additives.
Dash et al (2015) also identi ed and optimized new
B. subtilis strain BI19 that produced appreciable amount
of amylase. Singh et al (2012) produced extracellu-
lar amylase by Bacillus sp. which was optimized in a
submerged fermentation as maximum enzyme activity
was obtained at 35°C and pH 7 and after 10 h inocula-
tion. In submerged fermentation, contents of a synthetic
medium are very expensive and uneconomical, so there
is urgent need of these to be replaced with more eco-
nomically available agricultural, industrial and domestic
by products which are used as substrates for SSF to pro-
duce enzymes in economical way.
SSF holds tremendous potential for the production
of enzymes in view of its economic and engineering
advantages. It can be of particular relevance in those pro-
cesses where a crude fermented product may be used as
an enzyme source (Pandey et al 1999). The major criti-
cal factors affecting microbial synthesis of enzymes in a
SSF system include selection of a suitable substrate and
strains, particle size of the substrate, inoculum concen-
tration, moisture level of the substrate, temperature and
pH. Selection of an appropriate solid substrate plays an
important role in the development of ef cient SSF pro-
cesses (Lonsane et al 1985). Sexena and Singh (2011)
carried out solid state fermentation using various agro-
industrial wastes with best amylase producing strain
isolated from soil. Different physicochemical conditions
were varied for maximum enzyme production. The iso-
FIGURE 2. Different enzymes involved in the degradation of starch. The open
ring structure symbolizes the reducing end of a polyglucose molecule (Maarel
et al 2002).

664 UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Nadia Razdan and G.S. Kocher
Table 1. Over view of amylase producing bacterial and fungal strains (Sundarram et al 2014)
Amylolytic Microorganism type Fermentation type Reference
B.amyloliquefaciens SSF Oboh (2005)
Bacillus licheniformis SSF Babu et al (1995)
Bacillus coagulans SSF Prakash et al (2009)
B. polymyxa SSF Prakash et al (2009)
B. mesentericus SSF Prakash et al (2009)
B. vulgarus SSF Prakash et al (2009)
B. megaterium SSF Prakash et al (2009)
Bacillus licheniformis GCB-U8 SmF Sodhi et al (2005)
Bacillus sp. PS-7 SSF Ramesh and Lonsane (1990)
Bacillus licheniformis M27 SSF Amoozegar et al (2003)
Halobacillus sp MA-2 SmF Gomes and Gomes (2003)
Halomonas meridiana SmF Kathiresan and Manivannan (2006)
Rhodothermus marinus SmF Anto et al (2006)
Bacillus cereus MTCC 1305 SSF Sivaramakrishnan et al (2007)
Fungi
Aspergillus oryzae SSF Leveque et al (2000)
Penicillium fellutanum SmF Erdal et al (2010)
Thermomyces lanuginosus SSF Upgade et al (2011)
Aspergillus niger SSF, Smf Yang and Wang (1999)
Penicillium roquefortii SSF Sivaramakrishnan et al (2006)
Streptomyces rimosus SSF, Smf Sudo et al (1994)
Aspergillus kawachii SSF, Smf Balkan and Ertan (2007)
Penicillium chrysogenumm SSF Sindhu et al (2009)
Penicillium janthinellum (NCIM 4960) SSF Prakasham et al (2007)
Aspergillus awamori SmF Siqueira et al (1997)
Pycnoporus sanguineus SSF Saito et al (1975)
*SSF-Solid state Fermentation ; * SmF- Submerged Fermentation
late produced about 5400 units/g of amylase at 1:3 mois-
ture content, 20% inoculum concentration, temperature
(50ºC), pH 6.0 and after 72 h of incubation with Mustard
Oil seed cake as the substrate. Similarly, Maity et al (2015)
utilized Bacillus subtilis (ATCC 6633) for production of
alpha amylase by optimization of the fermentation media.
They also reported that 80% retention of alpha amylase
activity comparable to puri ed porcine pancreatic amyl-
ase in the presence of drastic conditions of temperature
(60°C), pH (6-11), detergents and utilized various indus-
tries like detergent, food and paper industries.
RSM is a statistical and mathematical tool for design-
ing experiments, building models, evaluating the com-
bined effect of many variables to investigate the optimum
conditions for desirable response with reduced number
of required experiments. Tanyildizi et al (2005) com-
bined effects of macronutrients of media on -amylase
production by Bacillus sp. using response surface meth-
odology. The results showed that yeast extract had no
effect on -amylase production. The optimal combina-
tions of media constituents for maximum -amylase
production were determined as 17.58 g/l starch, 12.37%

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION 665
Nadia Razdan and G.S. Kocher
(v/v) glycerin and 8.77 g/l peptone. Similarly, Sun et
al (2011) optimized the process parameters through the
statistical approach for the production of alpha amyl-
ase by Bacillus subtilis ZJF-1A5 in submerged fermen-
tation. Among the variables screened, the temperature
and time were most signi cant and also showed a posi-
tive interaction. The optimum levels were: temperature
(35.8ºC), pH (5.03) and time (54hrs). Under these condi-
tions -amylase yield was 191.15 U/ml.
Puri cation is a key step in the enzymes produc-
tion where residual cell proteins and contaminants are
removed. The methods used to purify amylases can vary
considerably, but most puri cation protocols involve a
series of steps (Sun et al 2010). Aassar et al (1992) per-
formed the acetone fractionation of Bacillus lentus cul-
ture ltrate yielded the highest -amylase activity and
66.6% fraction reached 13-fold that of the crude enzyme
preparation. -amylase from Bacillus licheniformis was
puri ed 6-fold with a yield of 38% using by two gel
ltration chromatography steps on Sephadex G-100 and
Superose 12 column (Bozic et al 2011). In addition to
the classical chromatographic techniques, immunoaf n-
ity chromatography has been applied for the preparation
of highly puri ed amylases (Jang et al 1994). Abdu et
al (2011) identi ed a novel Bacillus cereus MS6 strain,
which could produce extra cellular amylase that was
puri ed by DEAE-Cellulose anion exchange and sepha-
rose gel ltration chromatography, resulting in high
yield of enzyme. The native protein showed a molecular
mass of 149 kDa being composed of a homo dimer of 78
kDa polypeptide by SDS–PAGE.
Biochemical characterization of alpha amylase
When de ning the proposed unit of activity for any
enzyme, the International Unit of Biochemistry stated
that reaction conditions should be speci ed as optimal.
This implies that enzyme activities are only valid within
a range of physical properties. Therefore, optimum con-
ditions for producing maximum enzyme activities need
to be determined.
Amenaghawon et al (2016) conducted a study of
enzymatic hydrolysis towards cocoyam starch and
found that the rate of hydrolysis was faster at a higher
temperature. Therefore, there has been a need and con-
tinual search for more thermophilic and thermostable
-amylase (Burhan et al 2003). Aassar et al (1992)
observed that the pure enzyme from Bacillus lentus
was stable at higher temperatures in the presence of its
substrate. It exhibited an optimum reaction temperature
of 70°C and retained about 42°-70°C of its activity at
85°C and even at higher temperatures the enzyme still
showed some activity. Weemaes et al (1996) studied sta-
bility of -amylases produced by B. amyloliquefaciens,
B. licheniformis and B. stearothermophilus under com-
bined high temperature and pressure and the results
indicated that -amylase produced by B. licheniformis
was the most stable enzyme.
The pH of a solution affects the structure and activ-
ity of enzymes. Khanna (2010) explained that pH has an
effect on the state of ionization of acidic or basic amino
acids. If the state of ionization of amino acids in a pro-
tein is altered then the ionic bonds that help to deter-
mine the 3D shape of the protein got changed. Sodhi et
al (2005) reported that -amylase of Bacillus sp. PS-7
strain showed pH optima at pH 6.5 and displayed 87
and 52% of peak activity at pH 6.0 and 5.0, respectively.
Elkhalil and Gaffar (2011) analysed the pH activity pro-
le of Bacillus sterothermophilus which showed an opti-
mum activity at pH 7 compared to the B. acidocaldarius,
with an activity optimum at pH 6. The relative activities
of Bacillus sterothermophilus at pH 9 and 10 were about
1.5 and 4.5 times higher than those of the B. acido-
caldarius. Similary, Qader et al (2006), who stated that
the optimum pH of Bacillus sp. AS-1 was around 7.5.
Most of amylases are known to be metal ion-dependent
enzymes, namely divalent ions like Ca
2+
, Mg
2+
, Mn
2+
,
Zn
2+
and Fe
2+
etc (Pandey et al 2000). Naja and Kemb-
havi (2005) studied the effects of chemical modi ers on
-amylase enzyme activity from marine Vibrio sp. The
results suggested the involvement of amino acids such
as Lys, Trp, Asp/Glu and His in enzyme activity. It also
has been reported that heavy metal ions such as Hg
2+
,
Ag
2+
and Cu
2+
inhibited amylase activity (Dey et al 2002).
Asoodeh et al (2013) studied the effect of metal ions
(K
+
, Na
+
, Zn
2+
, Ba
2+,
Mg
2+
, Ca
2+
, Fe
2+
and Hg
2+
), on the
enzyme activity. Among the testi ed metal ions, Mg
2+
,
Fe
2+
and Ba
2+
increased the amylase activity, while Hg
2+
and Zn
2+
were established to inhibit enzyme activity.
Asoodeh et al (2013) determined the kinetic parameters
by incubating 0.1 ml of enzyme (0.1 mg/ml) in the pres-
ence of 0.9 ml starch at different concentrations (0.1–1.2
% w/v). As estimated from Michaelis–Menten equation
the values of Km and Vmax for starch as substrate were
4.5 ± 0.13 mg/ml and 307 ± 12 lM/min/mg, respectively.
SIGNIFICANCE OF FUNGAL GLUCOAMYLASE
(GA) IN STARCH HYDROLYSIS
Glucoamylase (GA), also known as amyloglucosidase (EC
3.2.1.3), is an inverting and exo-acting enzyme, capa-
ble of hydrolyzing -1,4 glycosidic linkages in soluble
starches and related oligosaccharides with the inversion
of the anomeric con guration to produce glucose. In
addition to acting on -1,4 linkages, the enzyme slowly
hydrolyzes -1,6 glycosidic linkages of starch (Weil
et al 1954; Fierobe et al 1998). The widely accepted
mechanism of hydrolysis involves proton transfer from
the catalyst to the glycosidic oxygen of the scissile bond.

666 UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Nadia Razdan and G.S. Kocher
A general acid–base catalyst (McCarter and Withers,
1994; Sinnot, 1990; Tanaka et al 1994) donates hydro-
gen to the glucosidic oxygen and a catalytic base guid-
ing the nucleophilic attack by a water molecule on the
C-1 carbon of the glucose moiety.
Microbial production of glucoamylase
Traditionally, glucoamylase have been produced by
SmF. The development of microbial strains, media com-
position and process control has contributed to the
achievement of high levels of extracellular glucoamyl-
ase. Banakar et al (2012) investigated the amylase pro-
duction from fungal species by submerged fermentation
(SmF). The Production medium was supplemented with
2% (w/v) soluble starch incubated under shake culture at
a temperature of 28±1ºC, pH-7.0 for 7 days. Maximum
amylolytic activity was recorded with crude enzyme at
3rd day of incubation by Penicillium sp. (0.87±0.05 U/
mL) followed by Penicillium chrysogenum (0.69±0.05 U/
mL), Aspergillus candidus (0.67±0.03 U/mL), Aspergillus
fumigatus (0.066±0.06 U/mL) and at 7th day of incuba-
tion was by Penicillium sp. (1.13±0.03 U/mL) followed
by Penicillium chrysogenum (1.12±0.004 U/mL). Wang
et al (2008) investigated food waste (FW) as potential
substrate for the glucoamylase production by Aspergil-
lus niger UV-60 under submerged fermentation. They
reported that optimum concentration of 2.50% (dry
basis), smashed food waste (smashed-FW) produced glu-
coamylase of 126 U/ml after 96 h of incubation, whereas
137 U/ml of glucoamylase could be achieved within the
same time from raw food waste (raw-FW) of 3.75%.
Recently, Okwuenu et al (2017) optimized the produc-
tion of glucoamylase from Aspergillus niger in a sub-
merged fermentation process using amylopectin from
guinea corn starch as the sole carbon source. Speci c
activities for crude enzymes were found to be 729.45 U/
mg and 1046.82 U/mg at ve and twelve days harvested
enzymes, respectively. Benassi et al (2014) investigated
the production of glucoamylase from Aspergillus phoe-
nicis in Machado Benassi (MB) medium using 1% malt-
ose as carbon source. The maximum amylase activity
was recorded with temperature (60–65 °C) and pH (4.5)
after 4 days of incubation in static conditions. However,
the glucoamylase costs are still too high for the estab-
lishment of a cost effective production of energy syrup.
The SSF process has potential to signi cantly reduce
the enzyme production costs because of lower energy
requirements, increased productivity, smaller ef uent
volumes and simpler fermentation equipment (Ellaiah et
al 2002). Cereal bran ours, potato residue and other
starchy waste materials have been utilized as fermen-
tation substrate for glucoamylase production by la-
mentous fungi (Joshi et al 1999; Biesebeke et al 2005).
Glucoamylase production by A. niger was extensively
studied using wheat bran in SmF and SSF by Kaur et al
(2003). Wheat bran, paddy husk, rice processing wastes
or other starch containing wastes have gained impor-
tance as supports for fungal growth during glucoamyl-
ase production (Arasartnam et al 2001).
Sethi and Gupta (2015) isolated amylolytic fungi
from soil and identi ed them as Aspergillus niger, Pen-
cillium chrysogenum, Microsporium sp. and Fusarium
sp on the basis of morphological, biochemical character-
ization and starch hydrolysis assay, of these Pencillium
chrysogenum was most potent alkaline amylase produc-
ing fungi with highest enzyme activity under optimised
conditions i.e pH (8.0), temperature (45°C), wheat bran
(1%) and peptone incubated for 7 days. Indriati et al
(2018) reported that 3, out of 16 thermophile bacteria
produced high amylase activity in media supplemented
with wheat our @ 2% at 40-50ºC.
Zambare (2010) employed response surface method-
ology to optimize SSF medium and various parameters
for production of glucoamylase by Aspergillus oryzae
on the solid surface of rice husk, wheat bran, rice bran,
cotton seed powder, corn steep solids, bagasse powder,
coconut oil cake, and groundnut oil cake as substrates
which resulted in a 24% increase in the glucoamyl-
ase activity. Optimum glucoamylase production (1986
μmoles of glucose/min/g of fermented substrate) was
observed on wheat bran supplemented with 1%, (w/w)
starch, 0.25%, (w/w) urea at pH 6, 100%, (v/w) initial
moisture and 300ºC after incubation of 120 hrs.
Kiran et al (2014) utilized food wastes such as waste
bread, waste cakes, cafeteria waste, fruits, vegetables
and potatoes for glucoamylase production by solid state
fermentation. Response surface methodology was used
to optimize the fermentation conditions for improving
enzyme production and waste cake was the best sub-
strate for glucoamylase production. The highest glucoa-
mylase activity (108.47 U/gds) was achieved at initial pH
(7.9), moisture content (69.6% wt) and inoculum load-
ing 5.2×105 cells/g of substrate and incubation time of
6 days. Kumar and Satyanarayana (2004) improved the
glucoamylase production by a thermophilic mold Ther-
momucor indicae seudaticae in solid-state fermentation
(SSF) by applying response surface methodology (RSM).
The glucoamylase production containing wheat bran as
substrate, under the conditions optimized by RSM, was
455 ± 23 U/g of dry moldy bran (DMB) is higher than
those reported in the literature.
Similarly, Banerjee and Ghosh (2017) applied response
surface methodology, a statistical tool for the optimiza-
tion of glucoamylase production by Aspergillus niger
in solid state fermentation using garden pea peel as a
substrate. The optimized fermentation composition was
incubation time: 5 days; incubation temperature: 30°C;
and substrate amount: 3g, which resulted in GA produc-

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION 667
Nadia Razdan and G.S. Kocher
tion of 90.1728 Ugds-1.
In literature also, Alam et al
(2014) examined the effect of process parameters (pH,
inoculum concentration and agitation speed) on glucoa-
mylase production from bitter cassava by Aspergillus
niger using response surface methodology (RSM).Utmost
glucoamylase production of 38.30 U/ml was attained
under optimized conditions of pH, inoculum concentra-
tion and agitation speed of 4.8, 3.7 % (v/v) and 260 rpm,
respectively. Both the experimental and predicted results
were in agreement with each other as values of 38.30
U/ml and 38.07 U/ml were obtained respectively thus,
con rmed the validity of the developed model as well as
attainment of the optimal points.
Glucoamylase from various sources have been puri-
ed extensively by the procedures using several types of
column fractionations including ion-exchange, hydro-
phobic and gel ltration chromatographic steps. Bagheri
et al (2014) investigated a glucoamylase enzyme from
Aspergillus niger and puri ed it using fractionation, fol-
lowed by anion-exchange chromatography. The results
revealed that molecular mass of glucoamylase enzyme
was estimated to be 62,000 Da, using SDS–PAGE and
57151 Da, based on mass spectrometry. Slivinski et al
(2011) produced glucoamylase by Aspergillus niger in
solid-state fermentation. The enzyme was partially
puri ed by ammonium sulphate precipitation and ion
exchange and gel ltration chromatographies. Its molec-
ular mass was estimated as 118.17 kDa by electrophore-
sis. Okwuenu et al (2017) investigated the production
of glucoamylase from Aspergillus niger in a submerged
fermentation process using amylopectin fractionated
from guinea corn starch as the carbon source. The crude
enzyme with speci c activity 729.45 U/mg was puri ed
to the level of gel ltration (using sephadex G-100) via
ammonium sulphate (70%) precipitation and speci c
activities were found to be 65.98 U/mg and 180.52 U/
mg respectively.
Biochemical characterization of glucoamylase
Many glucoamylases derived from fungi were function-
ally active at thermophilic temperatures, usually 50 to
60 °C. The enzymes from Aspergillus niger NRRL 330
and Aspergillus awamori var. kawachi were optimally
active at 50 °C and 60°C, respectively whereas GAs of
Arthrobotrys amerospora were optimally active at 55.8ºC
(Spinelli et al 1996; Norouzian et al 2000). Ali and Hos-
sain (1991) reported that the optimum temperature for
the action of the glucoamylase was 60°C. The enzyme
was stable at temperatures between 40 and 60°C with
essentially no loss of activity in 30 min.
The rate of an enzyme catalysed reaction varies with
pH of the system. Slivinski et al (2011) produced glucoa-
mylase enzymes by Aspergillus niger in SSF and par-
tially puri ed and characterized them biochemically. The
partially puri ed enzyme had an optimum pH (4.5-5.0)
and temperature (60 °C), with an average activity 152.85
U ml
-1
. Jebor et al (2014) puri ed and characterized glu-
coamylase enzyme from Aspergillus niger The puri ed
glucoamylase (A&B) had a maximum activity at pH (8
and6.5) and temperature (40°C and 30°C) respectively.
It was also found that the K
m
and V
max
value of glucoa-
mylase (B) were (2.8 mM and 9.8 mM/min) respectively
using different concentration of starch. Banerjee and
Ghosh (2017) used garden pea peel as a substrate in SSF
by Aspergillus niger for the production of glucoamyl-
ase. The K
m
and V
max
for glucoamylase were 0.387 mg
of soluble starch ml
-1
and 35.03 U
-1
μl
-1
min
-1
respectively.
Okwuenu et al (2017) obtained Lineweaver-Burk plot
of initial velocity at different substrate concentrations
and K
m
and V
max
of the enzyme were found to be 770.75
mg/ml and 2500 μmol/min respectively. Vivian et al
(2014) reported the activation of glucoamylase from
Aspergillus phoenicis by manganese (Mn
2+
) and calcium
(Ca
2+
) ions. The rise in glucoamylase activity caused by
these metal ions (Ca
2+
, Zn
2+
, Co
2+
, Fe
2+
and Mn
2+
ions)
could be attributed to the ability of these metals ions
to serve as an electron donor or Lewis acid as they
participate directly in the catalytic mechanism of the
enzyme.
SYNERGISTIC USE OF ALPHA AMYLASE AND
GLUCOAMYLASE IN STARCH HYDROLYSIS
Kunamneni and Singh (2005) prepared crude amylases
from Bacillus subtilis ATCC 23350 and Thermomyces
lanuginosus ATCC 58160 under SSF. The effect of vari-
ous process variables was studied for maximum conver-
sion ef ciency of maize starch to glucose using crude
amylase preparations. Doses of pre-cooking and post-
cooking amylase, glucoamylase and sacchari cation
temperature were found to produce maximum conver-
sion ef ciency and were optimization of fermentation
process. Maximum conversion ef ciency (96.25%) were
recorded at pre-cooking and post-cooking -amylase
(2.243 and 3.383 U/mg solids) respectively and glucoa-
mylase (0.073 U/mg solids) at sacchari cation temper-
ature (55.1 ºC). Soni et al (2003) isolated Bacillus sp.
AS-1 and Aspergillus sp. AS-2, producing very high
titres of thermostable -amylase and glucoamylase (198
950 and 3426 U/g fermented dry matter, respectively),
during SSF of wheat bran. Both enzymes were active
and stable over a wide range of temperature and pH.
-Amylase exhibited a high liquefying ef ciency (96%)
while glucoamylase revealed high sacchari cation ef -
ciency (87%), in a 15% starch solution, at 50.8ºC. When
used in combination, these enzymes could effectively
hydrolyzed wheat mash revealing a maximum conver-
sion ef ciency (96%).

668 UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Nadia Razdan and G.S. Kocher
Sodhi et al (2005) used alpha amylase from Bacil-
lus sp. PS-7 in combination with a standard commer-
cial amyloglucosidase (AMG), BioglucanaseTM in the
hydrolysis of malt starch for alcohol production. It was
found that the laboratory alpha amylase preparation
worked very well in the synergistic use with AMG, with
over all mashing ef ciency (89.0 %), overall ef ciency
(79.5 %) and alcohol yields (25.43 %) and also competed
well with the commercial alpha -amylase preparation,
PromaltTM, commonly used in combination with Bio-
glucanaseTM, a commercial amyloglucosidase, for malt
starch hydrolysis in Indian breweries and distilleries.
ETHANOLIC FERMENTATION OF WHEAT
HYDROLYSATE
Direct fermentation of starch using amylolytic micro-
organism offers a better alternative to the conventional
multistage employing commercial amylases for lique-
faction and sacchari cation followed by yeast fermen-
tation (Verma et al 2000; Knox et al 2004). By using this
amylolytic microorganism in direct fermentation, the
ethanol production cost can be reduced via recycling of
microorganism back to fermenters, thereby maintaining
a high cell density, which facilitates rapid conversion
of substrate into ethanol. However, there are very few
types of amylolytic yeasts that are capable of ef ciently
hydrolyzing starch. Recombinant microbes and mix
of amylolytic microorganism with glucose fermenting
yeast in co-culture fermentation can be used to enhance
starch hydrolysis and fermenting ef ciency.
Review of literature has revealed that ability of yeast
strains to achieve high level of ethanol strongly depends
on the nutritional conditions and protective functions.
The immobilization of fermenting organism for the
bioethanol production has been greatly explored as a
strategy to overcome substrate and product inhibition
and to improve the ethanol tolerance (Ljiljanamojovic et
al 2009 In Separate Hydrolysis and Fermentation (SHF)
con guration, the enzyme production, hydrolysis of
biomass, hexose and pentose fermentation are carried
out in separate reactors and at their optimum fermenting
conditions (Lynd et al 2002).
The disadvantages of SHF led to the development of
Simultaneous Sacchari cation and Fermentation (SSF)
process (Wright et al 1988). It is generally accepted that
integration of the enzymatic sacchari cation and fer-
mentation step which are carried out in one vessel so
called simultaneous sacchari cation and fermentation
(SSF) process could reduce the production cost and pro-
cess time compared to conventional separate hydrolysis
and fermentation (SHF) process (Mojovic et al 2006). The
presence of yeast or bacteria along with enzymes mini-
mizes the sugar accumulation in the vessel because the
fermenting organism immediately consumes the released
sugars. Since sugar produced during starch breakdow n
slows down -amylase action, higher rates, yields and
concentrations of ethanol are possible using SSF rather
than SHF, at lower enzyme loading. Additionally, the
presence of ethanol makes the mixture less vulnerable
to contamination by unwanted microorganisms, which
is a frequent burden in case of industrial processes (Bai
et al 2008).
In literature, Kumar et al (1999) used simultaneous
sacchari cation and fermentation to produce ethanol
from starch of damaged quality wheat and sorghum
grains by employing crude amylase preparation from B.
subtilis VB2 and an amylolytic yeast strain S. cerevisiae
VSJ4. They reported that 25% concentration of damaged
wheat and sorghum starch was found to be optimum for
damaged wheat and sorghum starch yielding 4.40%V/V
and 3.50%V/V ethanol respectively. Whereas 25% raw
starch of ne quality wheat and sorghum grains gave
an yield of 5.60%V/V and 5.00%V/V respectively. Simi-
larly, simultaneous sacchari cation and fermentation
(SSF) of damaged grains of sorghum and rice was con-
ducted using Aspergillus niger (NCIM 1248) and Sac-
charomyces cerevisiae VSJl. More yield of ethanol was
produced from the damaged sorghum (2.90% v/v) than
damaged rice (2.09% v/v) under optimal fermentation
conditions (Kumar et al 1998). Recent research studies
on Simultaneous Sacchari cation and Fermentation
(SSF) of damaged corn grains using symbiotic strains
of starch digesting Aspergillus niger (NCIM 1248 and
sugar fermenting Saccharomyces cerevisiae (MTCC 170)
revealed that SSF of damaged corn grains yielded maxi-
mum ethanol concentration of 4.24 (g/100ml) whereas
ne corn grains yielded (6.3 g/100ml) ethanol (Gawande
and Patil 2018).
Waste potato mash was chosen as a renewable carbon
source for ethanol fermentation because it is relatively
inexpensive compared with other feedstock considered
as food sources. Izmirlioglu et al (2012) optimized the
parameters for ethanol fermentation using response sur-
face methodology to achieve maximum ethanol produc-
tion. The study revealed that pH (5.5) and 3% inoculum
size were optimum for maximum ethanol concentration.
The maximum bio-ethanol production rate was attained
at the optimum conditions of 30.99 g/L ethanol. Hence,
waste potato mash was found as a promising carbon.
Current and future perspectives
This review paper investigated the potential for utiliza-
tion of spoiled wheat grains for bioethanol. The main
source for ethanol production in India is still molas-
ses which single handedly cannot sustain the demand.
Hence, there is need to look for alternate substrates for
meeting the increasing ethanol production. Secondly,

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION 669
Nadia Razdan and G.S. Kocher
thermostable alpha amylases are a more recent research
which may reduce energy on cooling the mash prior to
sacchari cation. It is also imperative to standardize the
mash composition by optimizing solid-liquid ratio, addi-
tion of protease etc and mash environment (optimum
temperature and pH). Further, to lower down the cost
of fermentation recombinant glucoamylase-expressing
yeasts were utilized to improve the ef ciency of starch
fermentation. The process cost may be further reduced
by using this process as it reduces the time as well as
energy by using two different vessels for sacchari ca-
tion and fermentation besides lowering the chances of
contamination.
Genetic engineering approaches should be more
focused on developing new improved strains with higher
substrate tolerance and improved production kinetics.
Though using damaged grains will incur lower substrate
cost, mashing involves costly commercial sacchari ca-
tion and enzymes which may also be taken care of by
using indigenous culture of Bacillus subtilis, Bacillus
circulans, Bacillus cereus etc for -amylase and Asper-
gillus sp. such as Aspergillus niger, Aspergillus oryzae
etc for glucoamylase. Thus, utilization of especially
damaged and spoiled wheat grains pave better way for
commercialization of bioethanol production from an
economical perspective.
REFERENCES
Aassar S A E, Omar S H, Gouda M K, Ismail A M, Abdel-Fattah
A F (1992) Puri cation of
-amylase from Bacillus lentus cul-
tures. Appl Microbiol Biotechnol 38: 312-314.
Abu T F A, Enujiugha V N, Sanni D M and Bamidele O S (2014)
Puri cation and characterisation of
-amylase from Bacillus
subtilis isolated from fermented African locust bean (Parkia
biglobosa) seeds. Int J LifeSc Bt Pharm Res 3(4): 1-18.
Alam M Z, Khalaf A, Salleh H M and Salihu A(2014) Process
Optimization of Glucoamylase Production by Aspergillusniger
Using Bitter Cassava (Manihotes culenta). J Environ Biol 8(17):
42-47.
Albani J R (2008) Principles and Applications of Fluorescence
Spectroscopy. Oxford, UK: Blackwell.
Ali S and Hossain H (1991) Characteristics of glucoamylase
from Aspergillus terreus. J Appl Microbiol 71: 144-6.
Amenaghawon N, Osagie E and Ogbeide S (2016) Optimisa-
tion of Combined Acid and Enzymatic Hydrolysis of Cocoyam
Starch to Produce Fermentable Hydrolysate. Pertanika J Sci
Technol 24(1): 123-36.
Amoozegar M A, Malekzadeh F and Malik K A (2003) Produc-
tion of amylase by newly isolated moderate halophile Haloba-
cillus sp. Strain MA-2. J Microbiol 52: 353-59.
Anonymous (2017) FCI wheat worth Rs 700 crores got dam-
aged till Mar 2016 in Punjab: CAG (https://www.tribuneindia.
com/.../punjab/fci-wheat...damaged...punjab.../447086.html)
Anto H, Trivedi U and Patel K (2006)
-Amylase production
by Bacillus cereus MTCC 1305 using solid-state fermentation.
Food Sci Biotechnol 44 (2): 241-45.
Arasaratnam V, Mylvaganam K and Balasubramaniam K (2001)
Improvement of glucoamylase production by Aspergillus niger
in solid-state fermentation with paddy husk as support. J Food
Sci Technol 38: 334-38.
Asoodeh A, Alemi A, Heydari A and Akbari J (2013) Puri-
cation and biochemical characterization of an acidophilic
amylase from a newly isolated Bacillus sp. Extremophiles 17:
339-48.
Awasthi P, Shrivastava S, Kharkwal A C and Verma A (2015)
Biofuel from agricultural waste. Int J Curr Microbiol App Sci
4: 470-77.
Babu K R and Satyanarayana T (1995)
-Amylase production
by thermophilic Bacillus coagulans in solid state fermentation.
Process Biochem 30 (4): 305-09.
Bagheri A, Khodarahmi R,Mostafaie A(2014)Puri cation and
biochemical characterization of glucoamylase from a newly
isolated Aspergillus niger: Relation to starch processing. Food
Chem 161: 270–278.
Bai F W, Anderson W A and Moo-Young M (2008) Biotechnol
Adv 26: 89–105.
Balkan B and Ertan F (2007) Production of a-amylase from
Penicillium chrysogenum under solid-state fermentation by
using some agricultural by-products. Food Technol Biotechnol
45: 439-42.
Ballesteros I, Negro M J, Oliva J M., Cabanas A, Manzanares
P and Ballesteros M (2006) Ethanol production from steam-
explosion pretreated wheat straw. Appl Biochem Biotechnol
130: 496 -508.
Banakar S P, Thippeswamy, Thirumalesh and Naveenkumar K
(2012) Isolation, Production and Partial Puri cation of Fungal
Amylase from Forest Soils of Bhadra Wildlife Sanctuary, West-
ern Ghats. J Pharm Biotech and Microbiol 20(3): 1-8.
Banerjee S and Ghosh U (2017) Production and Characteriza-
tion of Glucoamylase by Aspergillus niger. Appl Food Biotech-
nol 4(1): 19-26.
Banerjee S and Ghosh U (2017) Production and Characteriza-
tion of Glucoamylase by Aspergillus niger. Appl Food Biotech-
nol 4(1): 19-26.
Barfoed H (1967) Die Verwendung von Enzymenbei der Her-
stellung von Dextrose und Starkes irup. Starke 19: 2-8.
Bej B, Basu R K and Ash S N (2008) Kinetic study on acid cata-
lyzed hydrolysis of starch. J Sci Ind Res 67: 295-98.
Belboom S, Bodson B and Leonard A (2015) Does the Produc-
tion of Belgian Bioethanol Fit with European Requirements
on GHG Emissions Case of Wheat. Biomass Bioenergy 74: 58-
65.
Benassi V M, Pasin T M, Facchini1, Joao J A and Lourdes M
D (2014) Novel glucoamylase activated by manganese and
calcium produced in submerged fermentation by Aspergillus
phoenicis. J Basic Microbiol 54: 333-39.

670 UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Nadia Razdan and G.S. Kocher
Biesebeke R, Record E, van Biezen N, Heerikhuisen M, Franken
A, Punt P J, and Hondel C A (2005) Branching mutants of
Aspergillus oryzae with improved amylase and protease produc-
tion on solid substrates. Appl Microbiol Biotechnol 69: 44-50.
Bijttebier A, Goesaert H and Delcour J (2008) Amylase action
pattern on starch polymers. Biologia 63: 989-99.
Bowler P, Towersey P J, Waight S G and Galliard T (1985)
Minor Components of Wheat Starch and Their Technological
Signi cance. In: Hill R D and Munck L (ed) New Approaches to
Research on Cereal Carbohydrates. Pp. 71-9 Elsevier Science,
Amsterdam, Holland.
Bozic N, Ruizb J, Lopez-Santinb J and Vujci Z (2011) Produc-
tion and properties of the highly ef cient raw starch digesting
-amylase from a Bacillus licheniformis. Biochem Eng J 53:
203-09.
Buresova I, Sedlackova I, Famera O and Lipavsky J (2010)
Effect of growing conditions on starch and protein content
in triticale grain and amylose content in starch. Plant Soil
Environ 56: 99-104.
Dash B K, Rahman M M and Sarker P K (2015) Molecular iden-
ti cation of a newly isolated Bacillus subtilis BI19 and opti-
mization of production conditions for enhanced production of
extracellular amylase. Biomed Res Int 2015 859: 1-9.
De Almeida Siqueira E M, Mizuta K and Giglio J R (1997) Pyc-
noporus sanguineus: a novel source of -amylase. Mycol Res
101(2): 188-90.
Dey G, Palit S, Banerjee R and Maiti B R J (2002) Puri cation
and characterization of malto oligosaccharide-forming amyl-
ase from Bacillus circulans GRS 313. Ind Microbiol Biotechnol
28: 193-200.
Dziedzic S Z and Kearsley M W (1995) Handbook of Starch
Hydrolysis Products and their Derivatives DOI 10.1007/978-1-
4615-2159-4 ISBN 978-1-4615-2159-4
EIA. 2015a. Annual energy outlook, Energy Information
Administration. Washington, D.C. Available at http: //www.
eia.gov/forecasts/aeo/MT_ liquid fuels. cfm (accessed on 10
August 2015).
EIA. 2015b. Net petroleum imports data- 2014, U.S Energy
Information Administration. Washington, D.C. Available at
http: //www.eia.gov/tools/faqs/faq.cfm?id=32&t=6 (accessed
on 10 August 2015).
Elkhalil L and Gaffar F Y (2011) Biochemical characterization
of thermophilic amylase enzyme isolated from bacillus strains.
Int J Sci Nat 2(3): 616-20.
Ellaiah P, Adinarayana K, Bhavani Y, Padmaja P and Sriniva-
sula B (2002) Optimization of process parameters for glucoa-
mylase production under solid state fermentation by a newly
isolated Aspergillus species. Process Biochem 38: 615-20.
Erdal S and Taskin M (2010) Production of alpha-amylase by
Penicillium expansum MT-1 in solid-state fermentation using
waste Loquat (Eriobotrya japonica Lindley) kernels as sub-
strate. Rom Biotechnol Lett 15(3): 5342-50.
Fierobe H P, Clarke A J, Tull D and Svensson B (1998) Enzy-
matic properties of ceystein sulfonic acid derivative of the cat-
alytic base mutant Glu400YCys of glucoamylase from Asper-
gillus awamori. Biochem 37: 3753-9.
Fullbrook P D (1984) The enzymatic production of glucose syr-
ups. In: Dziedsic S Z and Kearsley M W (Eds.) Glucose Syrups:
Science and Technology, pp. 65-115. Elsevier, London.
G Indriati* ; R R P Megahati, E Rosba Potency of Amylase-
producing Bacteria and Optimization Amylase Activities. IOP
Conf. Series: Materials Science and Engineering 335 (2018)
012023 doi:10.1088/1757-899X/335/1/012023
Gawande S B and Patil I D (2015) A review on causes for dam-
aged sorghum and corn grains. IJSSBT 3: 5-9.
Gawandea S and Patil I D (2018) Experimental investigation
and optimization for production of bioethanol from damaged
corn grains. Materials Today: Proceedings 5: 1509-17.
Gomes I, Gomes J and Steiner W (2003) Highly thermostable
amylase and pullulanase of the extreme thermophilic eubac-
terium Rhodothermus marinus: production and partial charac-
terization. Bioresource Technol 90(2): 207-14.
Gulten Izmirlioglu 1 and Ali Demirci Ethanol Production from
Waste Potato Mash by Using Saccharomyces cerevisiae Appl.
Sci. 2012, 2, 738-753; doi:10.3390/app2040738.
Hansen A C, Zhang Q and Lyne P W L (2005) Ethanol diesel
fuel blends a review. Bioresource Technol 96: 277-85.
Hoseinpour H, Karimi K, Zilouei H and Taherzadeh M J (2010)
Simultaneous pretreatment of lignocellulose and hydrolysis of
starch in mixtures to sugars. Bioresources 5(4): 2457-69.
Hua X and Yang R (2016) Enzymes in starch processing. In:
Chandrasekaran M (Ed.) Enzymes in Food and Beverage Pro-
cessing, pp. 139-70. Boca Raton: CRC Press.
Hua X and Yang R (2016) Enzymes in starch processing. In:
Chandrasekaran M (Ed.) Enzymes in Food and Beverage Pro-
cessing, pp. 139-70. Boca Raton: CRC Press.
Indian biofuels 2016 Gain report number IN608 (https://gain.
fas.usda.gov/.../Biofuels%20Annual_New%20Delhi_India_6-
24-2016).
Jang S, Cheong T, Him W, Kim J and Park K (1994) Puri cation
of Bacillus licheniformis thermostable -amylase by immune
af nity chromatography. Korean Biochem J 27: 38-41.
Jebor M A, Ali Z A and Hassan B (2014) Puri cation and char-
acterization of the glucoamylase from Aspergillus niger. Int J
Curr Microbiol App Sci 3(1): 63-75.
Joshi V K, Pandey A and Sandhu D K (1999) Waste treat-
ments in fermentation technology. In: Joshi V K and Pandey A
(Eds.) Biotechnology: Food Fermentation. Vol. 2, Trivandrum,
India: Educational Publication and Distribution, pp. 1291-
1338.
Kathiresan K and Manivannan S (2006) Amylase production
by Penicillium fellutanum isolated from mangrove rhizosphere
soil. Afr J Biotechnol 5: 1-10.
Kaur P, Grewal H S and Kocher G S (2003). Production of
-
amylase by Aspergillus niger using wheat bran in submerged
and solid state fermentations. Indian Journal of Microbiology.
43. 143-145.

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION 671
Nadia Razdan and G.S. Kocher
Kerr R W (1944) Chemistry and industry of starch: starch,
sugar and related compounds. Academic press, New York.
Khanna P (2010) Cell and Molecular Biology. I.K. International
Publishing House, New Delhi.
Kiran E U, Trzcinski A P and Liu Y (2014) Glucoamylase pro-
duction from food waste by solid state fermentation and its
evaluation in the hydrolysis of domestic food waste. Biofuel
Res J 3: 98-105.
Knox A M, Preez J C and Kilian S (2004) Starch fermentation
characteristics of Saccharomyces cerevisiae strains transformed
with amylase genes from Lipomyces kononenkoae and Saccha-
mycopsis buligera. Enzyme Microb Technol 34: 453-60.
Knox A M, Preez J C and Kilian S (2004) Starch fermenta-
tion characteristics of Saccharomyces cerevisiae strains trans-
formed with amylase genes from Lipomyces kononenkoae and
Sacchamycopsis buligera. Enzyme Microb Technol 34: 453-
60.
Kumar N V, Dhavala P, Goswami A and Maithel S (2006). Liq-
uid biofuels in South Asia: resources and technologies. Asian
Biotechnol Develop Rev 8: 31-49.
Kumar S and Satyanarayana T (2004) Statistical optimization
of a thermostable and neutral glucoamylase production by a
thermophilic mold Thermomucorindicae-seudaticae in solid-
state fermentation. World J Microbiol Biotechnol 20: 895-
902.
Kumar S, Kiransree N and Rao L V (1999) Production of etha-
nol by raw starch hydrolysis and fermentation of damaged
grains of wheat and sorghum. Bioprocess Eng 21: 165-68.
Kumar S, Kiransree N and Rao L V (1999) Production of etha-
nol by raw starch hydrolysis and fermentation of damaged
grains of wheat and sorghum. Bioprocess Eng 21: 165-68.
Kunamneni A and Singh S (2005) Response surface optimiza-
tion of enzymatic hydrolysis of maize starch for higher glucose
production. Biochem Eng J 27: 179-90.
Kust F and Potmesilova J (2014) Situation and Outlook Report:
Grain. Prague, Ministry of Agriculture of the Czech Republic.
(In Czech).
Leveque E, Janecek S, Haye B and Belarbi A (2000) Thermo-
philic archaeal amylolytic enzymes. Enzyme Microb Technol
26(1): 3-14.
Ljiljanamojovic, Dusankapejin, Olgicagrujic, Sinisamarkov,
Jelenapejin, Maricarakin, Majavukasinovic, Nikolic S, Dra-
gisasavic (2009) Progress in the Production of Bio-ethanol on
Starch Base Feed stocks. Chem Ind Chem Eng 15: 211-26.
Lonsane B K and Ramesh M V (1990) Production of bacterial
thermostable -amylase by solid-state fermentation: a poten-
tial tool for achieving economy in enzyme production and
starch hydrolysis. Adv Appl Microbiol 35: 1-56.
Lynd L R, Weimer P J, van Zyl W H, Pretorius IS microbial cel-
lulose utilization: Fundamentals and biotechnology. Microbiol
Mol Biol Rev. 2002 66(3):506-77.
Maarel M J E C, vander Veen B, Uitdehaag J C M, Leemhuis H
and Dijkhuizen L (2002) Properties and applications of starch-
converting enzymes of the -amylase family. J Biotechnol 94:
137-55.
Madsen G B, and Norman B E (1973) New speciality glucose
syrups. In: Birch G G and Green L G (Eds.) Molecular Structure
and Function of Food Carbohydrates, pp. 50-64. Appl. Sci.,
London
Maity S, Mallik S, Basuthakur M and Gupta S (2015) Optimiza-
tion of Solid State Fermentation Conditions and Characteri-
zation of Thermostable Alpha Amylase from Bacillus subtilis
(ATCC 6633). J Bioprocess Biotech 5: 1-7.
McCarter J D and Withers S G (1994) Mechanism of enzymatic
glycoside hydrolysis. Curr Opin Struct Bio 4: 885-92.
McConnell, C. (2008). Acid Hydrolysis. Qittle. 1st April 2011,
Available at http: //doyouqittle. com/2008/03/08/acid-hydrol-
ysis/
Mojovic L, Nikolic S, Rakin M. and Vukasinovic M (2006) Pro-
duction of Bioethanol from Corn Meal Hydrolyzates. Fuel 85:
1750-55.
Naja M F and Kembhavi A (2005) One-step puri cation and
characterization of an extracellular a-amylase from marine
Vibrio sp. Enzyme Microb Technol 36: 535-39.
Norouzian D, Rostami K, Nouri I D and Saleh M (2000) Sub-
site mapping of puri ed glucoamylases I, II, III produced by
Arthrobotry samerospora ATCC 34468. World J Microbiol Bio-
technol 16: 155-61.
Oboh G (2005) Isolation and characterization of amylase from
fermented cassava (Manihot esculenta Crantz) wastewater. Afr
J Biotechnol 4: 1-10.
Okwuenu P C, Agbo K U, Ezugwu A L, Eze S O and Chilaka F
C (2017) Effect of Divalent Metal Ions on Glucoamylase Activ-
ity of Glucoamylase isolated from Aspergillus niger. Ferment
Technol 6: 141-45.
Okwuenu P C, Ezugwu A L and Chilaka F C (2017) Produc-
tion and optimization of Aspergillus niger glucoamylase using
amylopectin from guinea corn starch as the sole carbon source.
J Sci Ind Res 52: 263-72.
Okwuenu P C, Ezugwu A L and Chilaka F C (2017) Produc-
tion and optimization of Aspergillus niger glucoamylase using
amylopectin from guinea corn starch as the sole carbon source.
J Sci Ind Res 52: 263-72.
Pandey A, Selvakumar P, Soccol C R and Nigam P (1999) Solid
state fermentation for the production of industrial enzymes.
Curr Sci 77: 149-62.
Pandey A, Soccol C R and Mitchell D (2000) New developments
in solid state fermentation: I-bioprocesses and products. Pro-
cess Biochem 35: 1153-69.
Pomeranz Y (1988) Chemical composition of kernel struc-
ture. In: Pomeranz Y (Ed) Wheat: Chemistry and Technology,
Vol I, 3
rd
Edn, St Paul, MN: Am Assoc Cereal Chem, pp. 97-
58.
Prakash B, Vidyasagar M, Madhukumar M S, Muralikrishna G
and Sreeramulu K (2009) Production, puri cation, and char-
acterization of two extremely halotolerant, thermostable and

672 UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Nadia Razdan and G.S. Kocher
alkali-stable amylases from Chromo halobacter sp. TVSP 101.
Process Biochem 44: 210-15.
Prakasham R S, Subba Rao C, Rao R and Sarma P N (2007)
Enhancement of acid amylase production by an isolated
Aspergillus awamori. J Appl Microbiol 102 (1): 204-11.
Qader S A U, Bano S, Aman A, Syed N and Azhar A (2006)
Enhanced production and extracellular activity of commer-
cially important amylolytic enzyme by a newly isolated strain
of Bacillus. sp. AS-1. Turk J Biochem 31(3): 135-40.
Ramprakash B and Muthukumar K (2014) Comparative study
on the production of biohydrogen from rice mill wastewater.
Int J Hydrogen Energy 39: 14613-21.
Raplong H H, Odeleye P O, Hammuel C, Idoko M O, Asanato J
I and Odeke E H (2014) Production of alpha amylase by Bacil-
lus cereus in submerged fermentation. Aceh Int J Sci Technol
3(3): 124-30.
Rehman A and Saeed A (2015) Isolation and screening of
amylase producing Bacillus species from soil. Int J Adv Res
3: 151-64.
Renewable Fuels Association US, Global Ethanol Produc-
tion, 2018. https://ethanolrfa.org/, (Accessed 9 November
2018).
Saito N A and Yamamoto K A (1975) Regulatory factors affect-
ing alpha-amylase production in Bacillus licheniformis. Int J
Bacteriol 121(3): 848-56.
Saxena R and Singh R (2011) Amylase production by solid-
state fermentation of agro-industrial wastes using Bacillus sp.
Brazilian J Microbiol 42: 1334-42.
Sethi S and Gupta S (2015) Isolation, characterization and
optimization of cultural conditions for amylase production
from fungi. J Biosci 9: 3356-63.
Seungdo K and Dale B E (2004) Global potential bioethanol
production from wasted crops and crop residues Biomass and
Bioenergy 26: 361-75
Sharon M E M, Abirami C V K and Alagusundaram K (2014)
Grain Storage Management in India. Postharvest Biol Technol
2: 12-24.
Sindhu R, Suprabha G N and Shashidhar S (2009) Optimiza-
tion of process parameters for the production of a-amylase
from Penicillium janthinellum (NCIM 4960) under solid state
fermentation. Afr J Microbiol Res 3(9): 498-503.
Singh P and Kumari P (2016) Isolation and characterization
of amylase producing Bacillus spp. from selected soil sample.
IJRBS 5: 24-29.
Singh P, Gupta P, Singh R and Sharma R (2012) Factors affect-
ing alpha amylase production on Submerged Fermentation by
Bacillus sp. Int J Pharm and Life Sci 3: 2243-46.
Sinnot M L (1990) Catalytic mechanisms of enzymatic glycosyl
transfer. Chem Rev 90: 1171-202.
Sivaramakrishnan S, Gangadharan D, Nampoothiri K M, Soc-
col C R and Pandey A (2007) Alpha amylase production by
Aspergillus oryzae employing solid-state fermentation. J Sci
Ind Res 66(8): 621-8.
Sivaramakrishnan S, Gangadharan D, Nampoothiri K M, Soc-
col C R and Pandey A (2006) -Amylases from Microbial
Sources-An Overview on Recent Developments. Food Technol
Biotech 44(2): 45-52.
Slivinski C T, Machado A V L, Iulek J, Ayub R A and Braz M M
A (2011) Biochemical characterization of a Glucoamylase from
Aspergillus niger produced by Solid-State fermentation. Arch
Biol Technol 54(3): 559-68.
Sodhi H K, Sharma H, Gupta J K and Soni S K (2005) Produc-
tion of a thermostable -amylase from Bacillus sp. PS-7 by solid
state fermentation and its synergistic use in the hydrolysis of
malt starch for alcohol production Process Biochem 40: 525-34.
Sodhi H K, Sharma K, Gupta J K and Soni S K (2005) Produc-
tion of a thermostable -amylase from Bacillus sp. PS-7 by
solid state fermentation and its synergistic use in the hydroly-
sis of malt starch for alcohol production. Process Biochem 40:
525-34.
Soni S K, Kaur A and Gupta J K (2003) A solid state fermen-
tation based bacterial a-amylase and fungal glucoamylase
system and its suitability for the hydrolysis of wheat starch.
Process Biochem 39: 185-92.
Spinelli B B L, Lourdes M, Polizeli T M, Terenzi H F and Jorge
J A (1996) Biochemical characterization of glucoamylase from
the hyper producer exo-1 mutant strain of Neurospora crassa.
FEMS Microbiol Lett 138: 173-77.
Sudo S, Ishikawa T, Sato K and Oba T (1994) Comparison of
acid-stable -amylase production by Aspergillus kawachii in
solid-state and submerged cultures. J Biosci Bioeng 77(5): 483-
89.
Sun H, Zhao P, Ge X, Xia Y, Hao Z, Liu J and Peng M (2010)
Recent Advances in Microbial Raw Starch Degrading Enzymes.
Appl Biochem Biotechnol 160: 988-1003.
Swinkels, J J M (1985). Composition and properties of com-
mercial native starches. Starch - Stärke, 37, 1-5.
Tanaka Y, Tao W, Blanchard J S and Hehre E J (1994) Transi-
tion state structure for the hydrolysis of a-D-glucopyranosyl
uoride by retaining and inverting reaction of glycosylases. J
Biol Chem 269: 32306-12.
Tanyildizi M S, Ozer D and Elibol M (2005) Optimization of
-amylase production by Bacillus sp. using response surface
methodology. Process Biochem 40: 2291-96.
Thirathumthavorn D and Charoenrein S (2005) Thermal and
Pasting Properties of Acid- Treated Rice Starch. Starch/ Starke
57: 217-22.
Twidell J and Weir T (2003) Renewable Energy Resources. Tay-
lor and Francis Group, New York, pp. 601
Upgade A, Nandeshwar A and Samant L (2011) Assessment
of fungal protease enzyme from French bean using A. niger
by Solid State Fermentation. J Microbiol Biotechnol 1: 45-51.
Vaseekaran S, Balakumar S and Arasaratnam V (2015) Isola-
tion and identi cation of a bacterial strain producing thermo-
stable -amylase. Tropical Agric Res 22(1): 1-11.
Verma G, Nigam P, Singh D and Chaudry K (2000) Bioconver-
sion of starch to ethanol in a single-step process by coculture

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS UTILIZATION OF DAMAGED AND SPOILED WHEAT GRAINS FOR BIOETHANOL PRODUCTION 673
Nadia Razdan and G.S. Kocher
of amylolytic yeasts and Saccharomyces cerevisiae. Bioresour
Technol 72: 261-66.
Vidal B J R (2010) Protease use in ethanol production from
dry fractionated corn. Graduate College University of Illinois,
Urbana-Champaign.
Vivian M B, Thiago M P, Fernanda D A, Joao A J and Maria
de Lourdes T (2014) A novel glucoamylase activated by man-
ganese and calcium produced in submerged fermentation by
Aspergillus phoenicis. J Basic Microbiol 54: 333-39.
Wang Q, Wang X, Wang X H and Ma H (2008) Glucoamylase
production from food waste by Aspergillus niger under sub-
merged fermentation. Process Biochem 43: 280-86.
Wang S and Copeland L (2013) Effect of Acid Hydrolysis on
Starch Structure and Functionality: A Review. Crit Rev Food
Sci Nutr 55(8): 1081-97.
Weemaes C, Cordt S D, Goossens K, Ludikhuyze L, Hen-
drickx M, Heremans K and Tobback P (1996) High pressure,
thermal, and combined pressure-temperature stabilities of
a-amylases from Bacillus species. Biotechnol Bioeng 50: 49-
56.
Weil C E, Burch R J and Van Dyk J W (1954) An a-amylo-
glucosidase that produces h-glucose. Cereal Chem 31: 150-
58.
Wheals A E, Basso L C, Alves D M G and Amorim H V (1999)
Fuel Ethanol after 25 Years. Trends Biotechnol 17: 482-87.
Wright J D, CharlesE Wyman KarelGrohmann Simultaneous
sacchari cation and fermentation of lignocellulose 1988,Vol-
ume 18,Issue1,pp 75–90
Xiang Q, Lee Y Y and Torget R T (2004). Kinetics of Glucose
Decomposition during Dilute-Acid Hydrolysis of Lignocellu-
losic Biomass. Appl Biochem Biotechnol 116: 1127- 39.
Xu Y and Liu H Y (2009) Development and expectation of the
energy plant. Chinese Agric Sci Bull 25: 297-300.
Yan S, Wu X, Dahlberg J, Bean S R, MacRitchie F, Wilson J D
and Wang D (2010) Properties of eld-sprouted sorghum and
its performance in ethanol production. J Cereal Sci 51: 374-80
Yang S S and Wang J Y (1999) Protease and amylase pro-
duction of Streptomyces rimosus in submerged and solid state
cultivations. Bot Bull Acad Sin 40: 259-65.
Yoosin S and Sorapipatana C (2007) A Study of ethanol pro-
duction cost for gasoline substitution in Thailand and its com-
petitiveness. Int J Sci Technol 12: 69-80.
Zambare V (2010) Solid State Fermentation of Aspergillus ory-
zae for Glucoamylase Production on Agro residues. Int J Life
Sci 4: 16-25