
Bioassay guided isolation of -glucosidase inhibitory
compound,
in vivo
postprandial anti hyperglycemia
and docking study of the isolated compound from the
leaves of the methanolic extract of
Quercus serrata
Maibam B. Chanu
1
, Rajendra K. Labala
2
, Yunus Sheikh
1
, Jagat C. Borah
3
, Surajit K. Ghosh
4
,
Dinabandhu Sahoo
5
, Oinam J. Singh
6
, Anshul Shakya
4
and Biseshwori Thongam
7
1
Natural Products Chemistry and Pharmacology Programme, Institute of Bioresources and Sustainable
Development, Imphal-795001, Manipur, India
2
Distributed Information Sub-Centre, Institute of Bioresources & Sustainable Development, Imphal -795001,
Manipur, India
3
Natural Product Chemistry, Institute of Advanced Study in Science & Technology, Guwahati -781035,
Assam, India.
4
Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh, Assam 786004, India
5
Microbial Resources Programme, Institute of Bioresources and Sustainable Development, Imphal-795001,
Manipur, India
6
Department of Pharmacology, Jawaharlal Nehru Institute of Medical Sciences, Imphal 795001, Manipur, India
7
Plant Systematic Conservation Laboratory, Institute of Bioresources and Sustainable Development,
Imphal-795001, Manipur, India
ABSTRACT
Diabetes mellitus is rapidly emerging creating major health problem worldwide. Though synthetic drugs are available, due to their
association with side effect, there is always interest for search of herbal formulation. Quercus serrata is a plant used by traditional
healers of Manipur, India as a folk remedy to treat diabetes mellitus. The objective of this study is bioassay guided isolation of
-glucosidase inhibitory compound from the leaves of Quercus serrata and to check the postprandial antihyperglycaemic effect
of the isolated compound in STZ-induced diabetic albino mice. And to perform molecular docking studies to predict the bind-
647
Pharmaceutical
Communication
Biosci. Biotech. Res. Comm. 11(4): 647-657 (2018)
ARTICLE INFORMATION:
Corresponding Authors: b_thongam07@yahoo.com
Received 30
th
Sep, 2018
Accepted after revision 21
st
Dec, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
Mono of Clarivate Analytics and Crossref Indexed
Journal Mono of CR
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at:
http//www.bbrc.in/
DOI: 10.21786/bbrc/11.4/16

Maibam B. Chanu et al.
648 BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
ing interaction of the isolated compounds with -glucosidase.The methanolic extract of Quercus serrata leaves was screened for
-glucosidase inhibitory activity and fractionated into n-butanol, ethyl acetate and water fraction to isolate the active compound.
Quanti cation of isolated compound was done by HPLC-PDA. Postprandial antihyperglycaemia effect was check in normal and
STZ (100mg/kg) + nicotinamide (120mg/kg) induced diabetic mice after sucrose load (2g/kg). Molecular docking study was done
using AutoDock 4.2.6. Rosmarinic acid was identi ed as the active compound present in Quercus serrata leaves responsible for
the inhibition of -glucosidase with IC50 0.23 ± 0.02 gmL-1 (0.636µmolmL-1). Postprandial blood sugar and Area Under Curve
were signi cantly (p<0.05) reduced by treatment with rosmarinic acid in normal and diabetic mice. Additionally, in silico dock-
ing study elaborated the possible binding between rosmarinic acid and -glucosidase. Above nding con rmed the anti-diabetic
potential of traditionally used Quercus serrata leaves and the isolated compound, rosmarinic acid.
KEY WORDS: -GLUCOSIDASE, DIABETES MELLITUS, MOLECULAR DOCKING, POSTPRANDIAL HYPERGLYCEMIA, QUERCUS SERRATA,
ROSMARINIC ACID
INTRODUCTION
Diabetes mellitus and the spectrum of complications
associated with it impose uncertain healthcare chal-
lenges and economic burdens to the global population.
The catastrophic prevalence of diabetes mellitus, pre-
dominantly type 2 diabetes, has become a global health-
care problem affecting 425 million people worldwide,
(Zimmet et al., 2014, IDF Diabetes Atlas, 2017). In India,
it is reaching extreme epidemic level. A recent study
revealed that prolongation of diabetes leads to neuropa-
thy (24.6%), the most common complication, followed
by cardiovascular complications (23.6%), renal prob-
lems (21.1%), and retinopathy (16.6%) (Kaveeshwar and
Cornwall, 2014).
Diabetes is a life style disease and food habit plays
a major role in the management of diabetes. Con-
trol of postprandial hyperglycemia is also one of the
measures to control severity of the disease. Acarbose,
miglitol and voglibose are available clinically used
drug to control postprandial absorption of glucose by
inhibiting the enzyme -glucosidase and -amylase.
Although substantial quantum of efforts has been made
towards conquering the disease, it is still imperative to
put rigorous effort in research to mitigate the disease.
There is always search for new herbal formulation to
avoid the side effect associated with the synthetic drug,
(Polonsky, 2012, Nakatsu et al., 2017, Brito-Arias et al.,
2018).
Quercus serrata Murray is widely used by traditional
healers of Manipur, India as a folk remedy to treat dia-
betes mellitus (Sheikh et al 2015). No elaborate study
has been reported on the medicinal use of Quercus ser-
rata leaves and the chemical constituent present. So,
the present study is aim at bioassay guided isolation of
the active constituent responsible for the inhibition of
the enzyme -glucosidase, checking its antihypergly-
caemic activity and to do molecular docking study of
the isolated compound to predict the possible binding
interaction of the isolated compound with the enzyme,
-glucosidase.
MATERIALS AND METHODS
Leaves of Quercus serrata, Murray were collected from
Kangla Siphai, Manipur, India and authenticated by
Dr. Biseshwori Thongam, Scientist D, Pant Taxonomist,
IBSD, Manipur. Voucher specimen (No. IBSD/M-202)
was deposited in the IBSD herbarium. Analytical or
HPLC grade organic solvents (Merck Millipore, India)
were used for experiments. For column chromatog-
raphy, 100-200 mesh size silica gel (Merck) was used.
-Glucosidase (Maltase, EC 3.2.1.20), p-nitrophenyl--
D-glucopyranoside and streptozotocin were purchased
from Sisco Research Laboratory. HPLC (Shimadzu LC-
20AD) was performed using Photo diode array (PDA)
detector. NMR spectra were recorded on a Bruker Avance
500 MHz instrument with TMS as an internal standard.
Chemical shifts were expressed in values. Agilent
6520 Accurate mass Q-TOF/LC-MS was used to deter-
mine molecular weight. Absorbance was measured by
Thermo Scienti c Multiskan spectrometer.
Extraction and isolation of active compound: Air-dried
leaves of Quercus serrata (3.5 kg) were extracted three
times with methanol (MeOH) (15 L). Then 150 mL water
was added to the concentrated methanolic extract (3.0
L) to make hydro-alcoholic solution. This solution was
washed with 3.0 L of petroleum ether to remove fatty
matter present in the leaves and then concentrated to
yield 400 g of dried extract. A suspension was prepared
from this using 500 mL of water and fractionated into
ethylacetate , n-butanol and water to yield 152 g of ethyl
acetate fraction, 103 g of butanol fractions and 135g
of water fraction respectively. The butanol fraction was
found to be most active and was subjected to silica gel
column chromatography with increasing polarity: petro-
leum ether-chloroform 1:1 (Fr 1, 1 L), chloroform (Fr 2,
1 L), and chloroform – methanol 9:1 (Fr 3, 2 L), chloro-
form – methanol 4:1 (Fr 4, 2 L), chloroform – methanol
1:1 (Fr 5, 2.5 L) and chloroform – methanol 3:7 (Fr 6,
2.5 L). The sub-fraction 6 (SFr 6) was found to be most
active in inhibition of -glucosidase. Further, SFr 6 was

Maibam B. Chanu et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND 649
subjected to semi- preparative HPLC to yield compound
1 (rosmarinic acid).
Analytical and semi-preparative HPLC: Shimadzu LC-
20AD with PDA detector (SPD-M20A) tted with either
an analytical (CAPCELL PAK C18 MGII S5, 5µ C18 250
× 4.6 mm) or semi-preparative (CAPCELL PAK C18 MGII
S5, 5µ C18 250 × 10.00 mm) column was used for HPLC
analysis. A solution of 10 mg/mL of enriched fraction
(SFr 6) was prepared in HPLC grade water and metha-
nol (7:3) and ltered through 0.45µm Millex-HN syringe
lter. A 20µL or 200µL aliquot of ltered sample solu-
tion was injected for analysis for analytical and semi
preparative separation respectively. The mobile phase
used consisted of 1% acetic acid in water (solvent A)
and methanol (solvent B) following linear gradient over
a total run time of 23min: initially a linear increase of
B up to 100% in 21 min and back to 100% A in 23
min. Flow rate was 1 mL/min and 3 mL/min for analyti-
cal and semi-preparative respectively. Individual peaks
eluting from the column were carefully collected and
the solvents were evaporated in a rotary vacuum evapo-
rator. Instrument control and data handling were per-
formed with the LC solution software on a PC.
Quantitative analysis by HPLC-PDA: Quantitative
analysis was done by analytical HPLC in the protocol
described above. Standard stock solution of 1mg/mL of
rosmarinic acid was prepared and then diluted to yield
the desired test concentrations. Quantitative estimation
of the major active compound present in the methanolic
extract, n-butanol fraction and enriched fraction (SFr6)
was done using calibration curve of the standard solu-
tion and plotted concentration versus area using excel
2007 (Sheikh et al., 2016).
-Glucosidase inhibitory assay: A 0.5U/mL stock solu-
tion of readily available -glucosidase (from Saccharo-
myces cerevisiae) was prepared in 0.1M phosphate buffer
(pH 6.8) and diluted with the same buffer to desired test
concentration. Stock solution of plant extract, fractions
and compounds were prepared in dimethyl sulfoxide
(DMSO) and diluted for assay to the required concen-
tration with same buffer maintaining DMSO concentra-
tion below 1% v/v. -glucosidase inhibitory activities
were determined spectrophotometrically based on an
earlier reported method by using p-nitrophenyl--D-
glucopyranoside as substrate (Laishram et al., 2014)
Acarbose was used as positive control and the unin-
hibited enzyme was taken as negative control (DMSO
control). The assay was performed in three independent
experiments.
Experimental animals: Swiss albino mice (25-30 g)
were used in all the experiment and were procured from
Regional Institute of Medical Sciences (RIMS), Manipur,
India. Ethical clearance was obtained from the Insti-
tutional Animals Ethical Committee (Approval No.–
RIMS.171/IAEC/2011) prior to the experiments. Animals
were acclimatized in laboratory condition for 7 days in
polypropylene cages lined with husk under standard
environmental conditions. Animals had free access to
water and were fed on pelleted diet.
Acute oral toxicity study :The acute toxicity study
was performed as per Organisation for Economic Co-
operation and Development (OECD) guideline no. 423
(Annexure-2c) adopted by the Committee for the pur-
pose of control and supervision of experiments on ani-
mals (CPCSEA), Government of India (Veeraraghavan,
2000). Mice were kept under observation for 14 days
with special attention for the rst 24 h after dosing.
Induction of diabetes: Diabetes was induced in mice
by single intraperitoneal (i.p.) injection of nicotinamide
(120mg/kg i.p.) and then 100 mg/kg b.w. of streptozo-
tocin (freshly dissolved in 0.1M citrate buffer, pH 4).
Mice showing marked hyperglycemia (fasting blood glu-
cose ≥ 250 mg/dl) at 48 h after streptozotocin treatment
were considered as diabetic and selected for the study,
(Nakatsu et al., 2017)
Effect of rosmarinic acid on postprandial hyperglyce-
mia :Both normal and diabetic mice were fasted over-
night and divided into ve groups (n=6). Group I served
as normal control and received vehicle, group II served
as positive control and received acarbose (10 mg/kg
b.w.). Group III, IV and V received rosmarinic acid (5,
10 and 20 mg/kg) suspended in gum acacia (2% w/v)
(Chandramohon et al., 2015). All groups received 2 g/kg
b.w. sucrose orally along with their respective test sam-
ples (Miura et al., 2004). Blood glucose levels were esti-
mated from the tail vein from each group before (0 min)
and at 30, 60 and 120 min after sucrose load. Blood glu-
cose was measured by using Accu-Check Active, Roche
Diagnostic Mannheim, Germany. AUC was calculated by
using the software, GraphPad Prism 5.
In silico structure prediction of -glucosidase: S. cer-
evisiae -glucosidase protein sequence (MAL12, Acces-
sion Number: P53341) was retrieved from UniProt (www.
uniprot.org). NCBI CDD
(Marchler-Bauer et al., 2015)
was
used to nd out the conserved domain and other impor-
tant catalytic sites. Structural 3D model for the protein
was built using automated comparative protein mod-
eling server SWISS-MODEL (https://swissmodel.expasy.
org). Homology based search was performed by the
server in Protein Data Bank (PDB) and SWISS-MODEL
template library (SMTL) repositories for template struc-
tures (Biasini et al., 2014). Suitable template with similar
biological property and highest similarity was selected
for creating the 3D model. Further checking accuracy of
Maibam B. Chanu et al.
650 BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
the newly generated structure was done by using PRO-
CHECK (Laskowski et al.,1993) and ProSA-web (Sippl,
1993; Wiederstein and Sippl, 2007) for evaluation of
Ramachadran plot.
Docking study: 3D structure of rosmarinic acid
(PubChem CID: 5281792) was obtained from NCBI
PubChem database (https://pubchem.ncbi.nlm.nih.gov/).
Surface topology was calculated using CASTp server
(Dundas et al., 2016) for potential binding pockets in
the structural model of -glucosidase. Initially whole
protein was considered for docking. Polar hydrogen
atoms and Gasteiger charge were added to the protein
molecule. Grid size of 96 Å × 120 Å × 110 Å with a
spacing of 0.619 Å was set to cover the entire protein.
Lamarckian genetic algorithm search was considered to
generate 100 conformations and rest of the parameter
was set to default. Later, a cluster analysis was carried
out by considering the root mean square deviation of
conformations to identify major occupied binding sites
in the protein. On the basis of major CASTp pockets and
top AutoDock clusters information, a smaller grid box,
62 Å × 60 Å × 70 Å with a spacing of 0.464 Å was
determined for the second set of docking simulation. All
other parameters and procedureof the docking program
were kept as earlier.
The best docked conformation of rosmarinic acid
was selected based on lowest binding energy. Minimiza-
tion of the docked complex was then performed using
CHARMm force- eld with smart minimizer (2000 steps)
by considering Generalized Born with Molecular Volume
(GBMV) implicit solvent model. Binding free energy (∆G)
of the docked complex was calculated by using the same
GBMV model. Implicit Distance-Dependent Dielectric
model was used to calculate total and individual inter-
action energy (IE) possessed by the amino acid residues.
Molecular graphics, analysis and depiction were per-
formed using UCSF Chimera
(Pettersen et al., 2004) and
BIOVIA Discovery Studio Visualizer Version 4.5
(http://
www.3dsbiovia.com).
Statistical analysis: Results were expressed as Mean ±
S.D., where n=6. Differences among data were deter-
mined using one way ANOVA followed by Tukey’s Mul-
tiple Comparison test (Graph Pad Prism software, ver-
sion 7).
*
p<0.05 was considered statistically signi cant.
RESULTS AND DISCUSSION
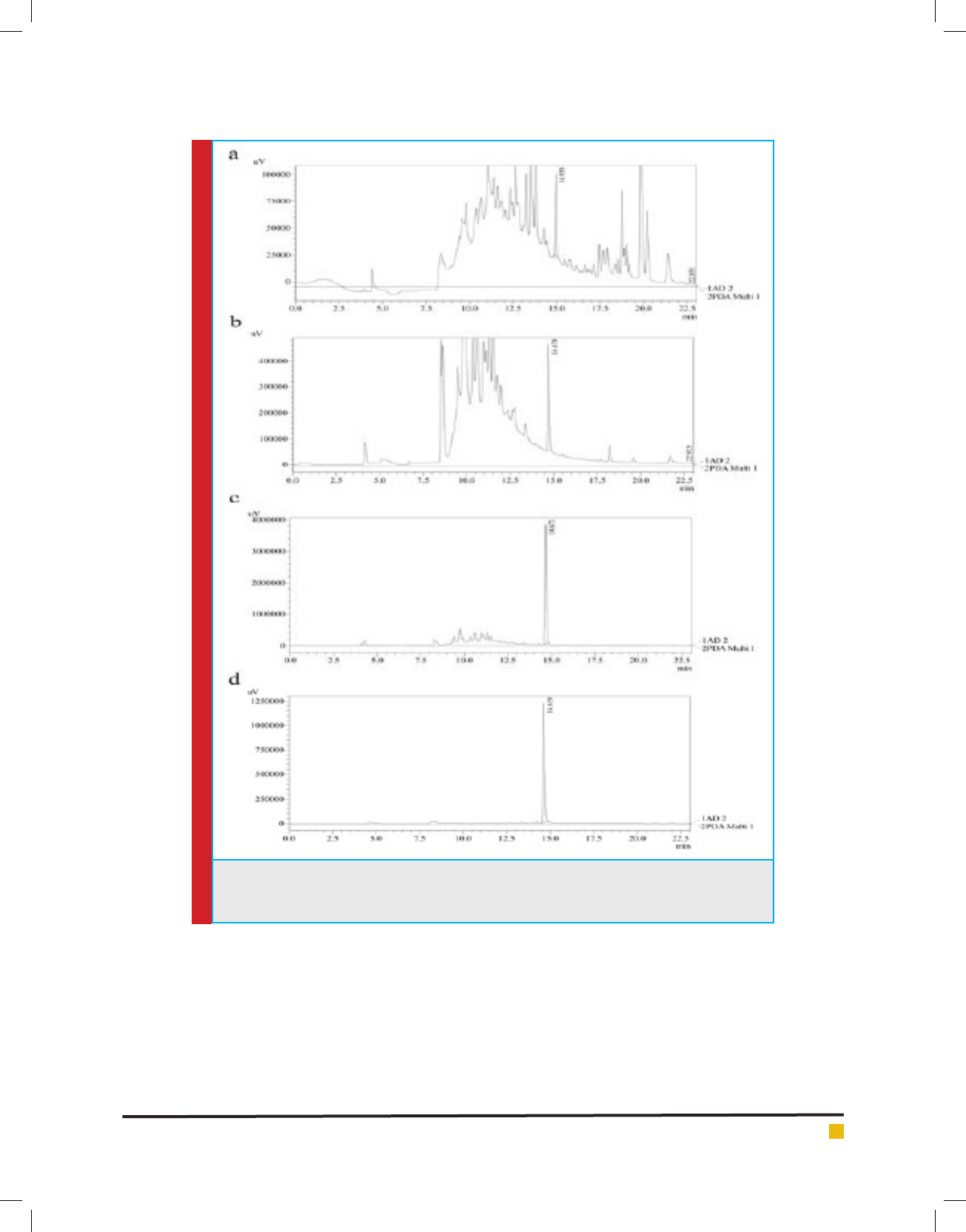
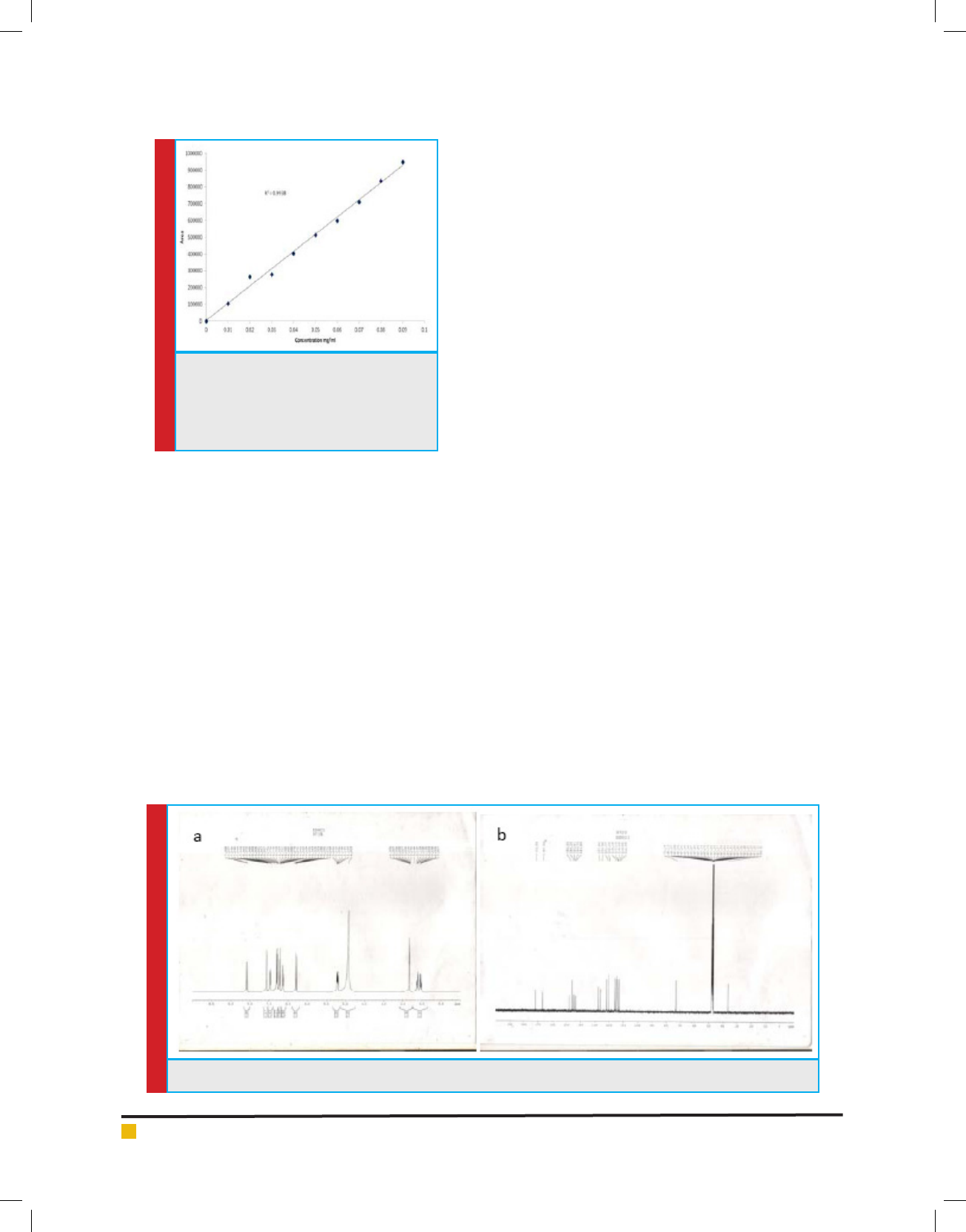
Quantitative HPLC analysis: Rosmarinic acid (Fig. 1)
was found to be the major compound present in the
enriched fraction Sfr 6. Fig. 2 showed the HLPC chro-
matogram of rosmarinic acid in methanolic extract,
n-butanol fraction, enriched fraction (SFr 6) as well as
that of isolated rosmarinic acid. The calibration curve
(Fig. 3) of the standard rosmarinic acid was found to be
linear (r
2
=0.993) in the concentration range of 0.01 to
0.09 mg/ml. 54.28±4.14 mg, 239±1.21 mg and 426±8.36
mg of rosmarinic acid was present per 1gm each of
methanol extract, n-butanol fraction and enriched frac-
tion (SFr 6) respectively.
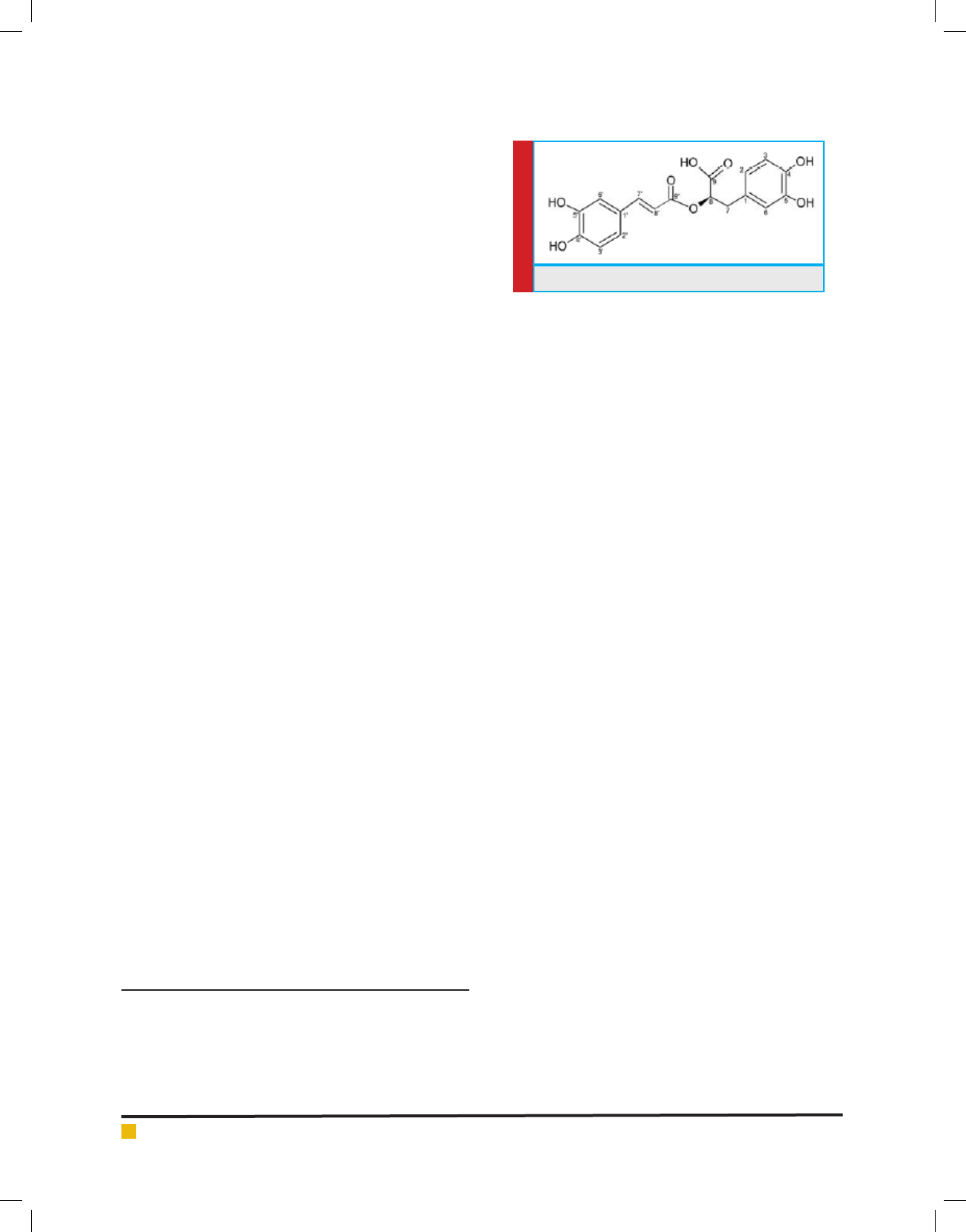
Rosmarinic acid NMR interpretation:Compound 1 (Fig.
1) gave a molecular ion peak in HRMS at m/z = 360.3212
[M+H]
+
corresponding to the molecular formula C
18
H
16
O
8
.
The
13
C NMR in CD
3
OD indicated the presence of signals
attributed to all 18 carbon atoms. One ester carbonyl
at = 167.06 displayed HMBC correlation with H-7’ (
7.58), coupled to a doublet of J = 14.5 Hz at 6.34
indicative of a cinnamoyl ester. Another carbonyl at
172.06 showed HMBC correlation with signal of H-7 (
3.13, H-7
a
and 3.05, H-7
b
), coupled to a methene signal
at 5.20, agreeing with the presence of a phenyl lactic
acid unit. Two ABX systems were noted in the aromatic
region of the
1
H NMR vouching for 3,4-dihydroxylation
pattern of both the rings. The linkage of the cinnamoyl
ester to the phenyl lactic acid moiety was inferred from
HMBC correlations displayed by the H-8 signal to both
the carbonyl peaks. Signals at 6.98 (1H, d, J = 2.0 Hz,
H-6’) and 7.06 (1H, dd, J = 8.5, 2.0 Hz, H-2’) show-
ing meta coupling between them and ortho coupling
between H-2’ and H-3’ with signal at 6.79 (1H, d, J =
8.5 Hz). Another ABX system with signal at 6.64 (1H,
d, J = 2.0 Hz, H-2), 6.70 (1H, dd, J = 9.0, 2.0 Hz, H-6)
and 6.69 (1H, d, J = 9.0 Hz, H-5) is observed. All sig-
nals of
1
H and
13
C, and the observed HMQC,
1
H-
1
H COSY
and HMBC correlations are in good agreement with lit-
erature reports on rosmarinic acid.
1
H NMR (500 MHz, CD
3
OD, ppm): 7.58 (1H, d, J =
14.5 Hz, H-7
/
), 7.06 (1H, dd, J = 2.0, 8.5 Hz, H-2
/
), 6.98
(1H, d, J = 2.0 Hz, H-6
/
), 6.79 (1H, d, J = 8.5 Hz, H-3
/
),
6.70 (1H, dd, J = 2.0, 9.0 Hz, H-6), 6.69 (1H, d, J = 9.0
Hz, H-5), 6.64 (1H, d, J = 2.0 Hz, H-2), 6.34(1H, d, J =
14.5 Hz, H-8
/
), 5.20 (1H, m, H-8), 3.13 (1H, m, H-7
a
) and
3.05 (1H, m, H-7
b
).13C NMR (125 MHz, CD
3
OD, ppm):
172.06 (C-9), 167.06 (C-9
/
), 148.34 (C-4
/
), 146.35 (C-7
/
),
145.41 (C-5
/
), 144.76 (C-4
/
), 143.89 (C-3), 127.84 (C-1),
126.25 (C-1
/
), 121.77 (C-2), 120.40 (C-6
/
), 116.18 (C-6),
FIGURE 1. Structure of Rosmarinic acid
Maibam B. Chanu et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND 651
FIGURE 2. HPLC chromatogram of (a) methanol extract (b) n-butanol fraction (c) enriched frac-
tion SFr6 and (d) isolated rosmarinic acid, showing the peak of rosmarinic acid along with the
retention time.
115.10 (C-5), 114.89 (C-3
/
), 113.81 (C-8
/
), 112.99 (C-2
/
),
73.18 (C-8) and 36.51 (C-7). (Supp Fig. 1a, 1b)
Oral acute toxicity study: No fatality and ethological
changes were observed when administered a dose of
2000 mg/kg b.w. of the methanolic extract. No changes
in behavioral pattern were observed in the mice in oral
toxicity study. So, Quercus serrata leaves were non toxic
upto a dose of 2000mg/kg.
In vitro -glucosidase inhibitory effect: Blood glucose
levels are highly affected by the saccharides contained
in food which are converted into glucose by the actions
of digestive enzymes like -glucosidase. Carbohydrates
like sucrose are hydrolysed to monosaccharides (glu-
cose and fructose) by -glucosidase thereafter caused an
increased in blood glucose. The methanolic extract of
Quercus serrata leaves, ethyl acetate fraction, n-butanol
Maibam B. Chanu et al.
652 BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 3. Calibration curve of standard
rosmarinic acid as obtained from HPLC
chromatogram. The graph is plotted, Area
against Concentration (mg/ml) from the
range 0.01 to 0.09mg/ml.
SUPP FIG. (1a) 1H NRM of rosmarinic acid (1b) 13C NMR of rosmarinic acid
fraction, enriched fraction (SFr 6) and rosmarinic acid
potentially inhibited -glucosidase, and the result is
shown as table 1. The above result signi es Quercus ser-
rata as a promising plant for postprandial management
of diabetes mellitus type 2. Moreover, rosmarinic acid
showed greater potential in inhibition of -glucosidase
in vitro when compared to that of acarbose.
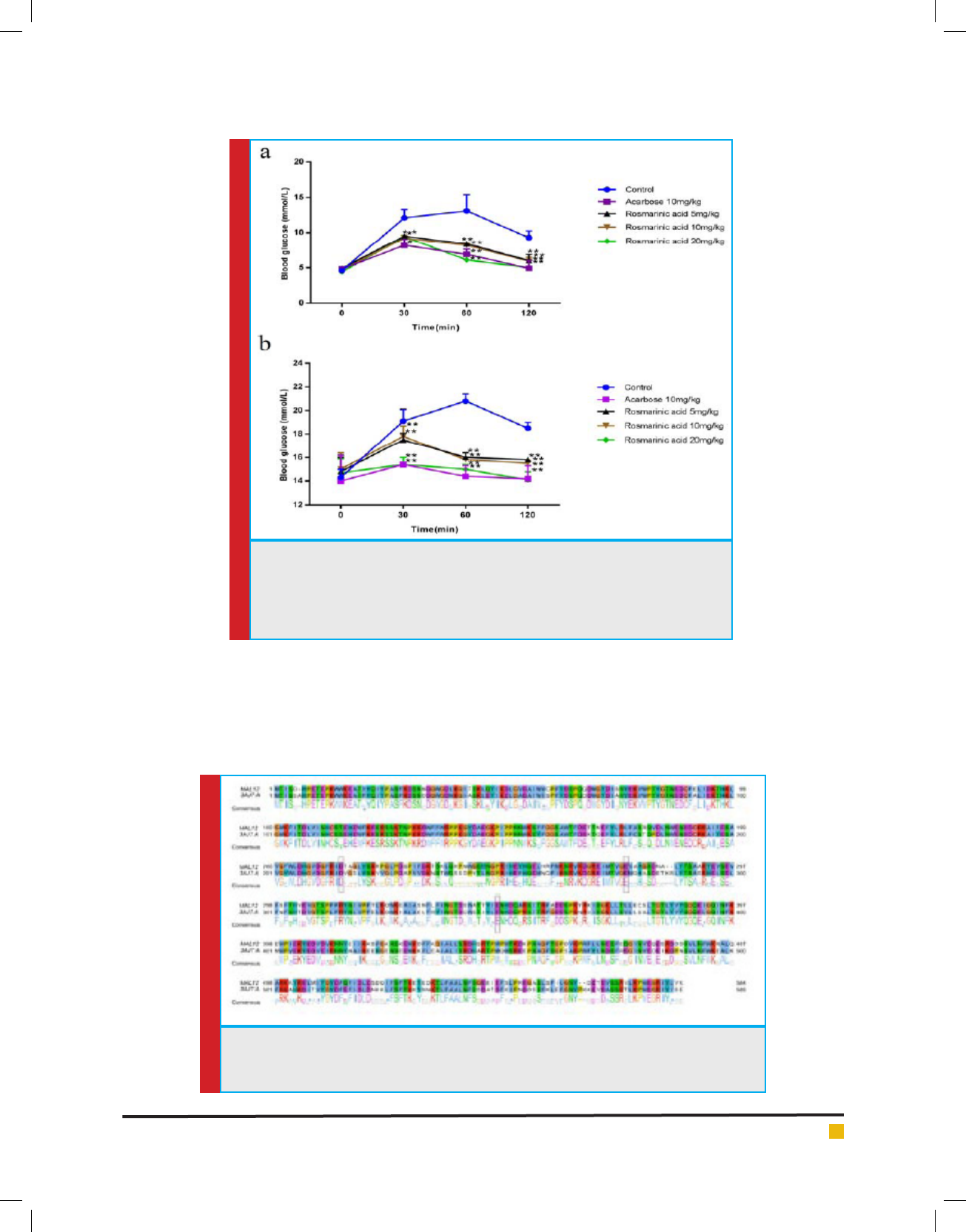
Postprandial antihyperglycaemic effect of rosmarinic
acid: Rosmarinic acid is a polyphenol compound. It is
an ester of caffeic acid and 3,4-dihydroxyphenyllactic
acid (Petersen and Simmonds, 2003). The effect of ros-
marinic acid on postprandial blood glucose level after
sucrose load in overnight fasted normal and diabetic
mice were presented in the Fig 4A & B. In diabetic mice
postprandial blood glucose increased from 19.14±1.01 at
30 min to 20.81±0.62 mmol/L at 60 min and decreased
to 18.50±0.51 mmol/L at 120 min. Rosmarinic acid
at a dose of 20 mg/kg b.w signi cantly reduced the
postprandial blood glucose to 15.43±0.64, 15.01±0.48
and 14.19±0.74 mmol/L at 30 min, 60 min and 120
min which value was comparable to that of acarbose
15.45±0.23, 14.42±4.01 and 14.21±1.12 mmol/L at 30
min, 60 min and 120 min respectively. Rosmarinic acid
at all the doses caused signi cant fall in AUC (Table 2)
when compared to diabetic control (56.31±1.50 mmol.
min/L) indicating its effectiveness in lowering postpran-
dial glucose absorption. There are studies which reported
that medication which can attens the peak blood glu-
cose level postprandial can reduce the AUC (Inoue et
al.1997). Postprandial blood glucose and AUC in normal
rats was in consistent with that of diabetic groups.
Docking: To understand the binding interaction between
rosmarinic acid and -glucosidase, molecular docking
was performed. Yeast and human glucosidase are similar
in their substrate speci city, pH optimum, and inhibitor
sensitivity. Thus, the yeast enzyme besides its affordabil-
ity serves as a good experimental model to learn more
about the structure, substrate speci city, and enzymatic
mechanism of human glucosidase (Brito-Arias et al.,
2018).
AutoDock 4.2.6 (Morris et al., 2009) incorporated in
MGL Tools version 1.5.6 (The Scripps Research Insti-
tute) was used to execute the docking simulations. In
this docking study -glucosidase was treated as rigid
molecule and rosmarinic acid as exible. Due to non
availability of three-dimensional crystal structure of S.
cerevisiae -glucosidase, homology study is done. Based
on homology study , the protein structure of S. cerevi-
siae isomaltase (PDB ID: 3AJ7, Chain A at 1.3Å resolu-
tion) which got 72% similarity and 99% query coverage
when compare to S. cerevisiae -glucosidase, MAL12
Maibam B. Chanu et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND 653
FIGURE 4. Effect of rosmarinic acid on postprandial blood glucose concentration
after sucrose load. Graph showed plasma blood glucose concentration (mmol/L)
on Y axis against time (min) on X axis. (a) on normal mice, (b) on diabetic mice.
Values are the Mean ± SD, n=6, *P < 0.05 statistically signi cant when comparing
to control.
FIGURE 5. Target-template alignment of MAL12 (-glucosidase protein sequence of S. cerevi-
siae) and 3AJ7 (Isomaltase protein sequence of S. cerevisiae (PDB ID: 3AJ7, Chain A)), showing
high sequence homology of important catalytic site residues.
was chosen for our study as the best template to build
the 3D structure for S. cerevisiae -glucosidase. (Fig 5)
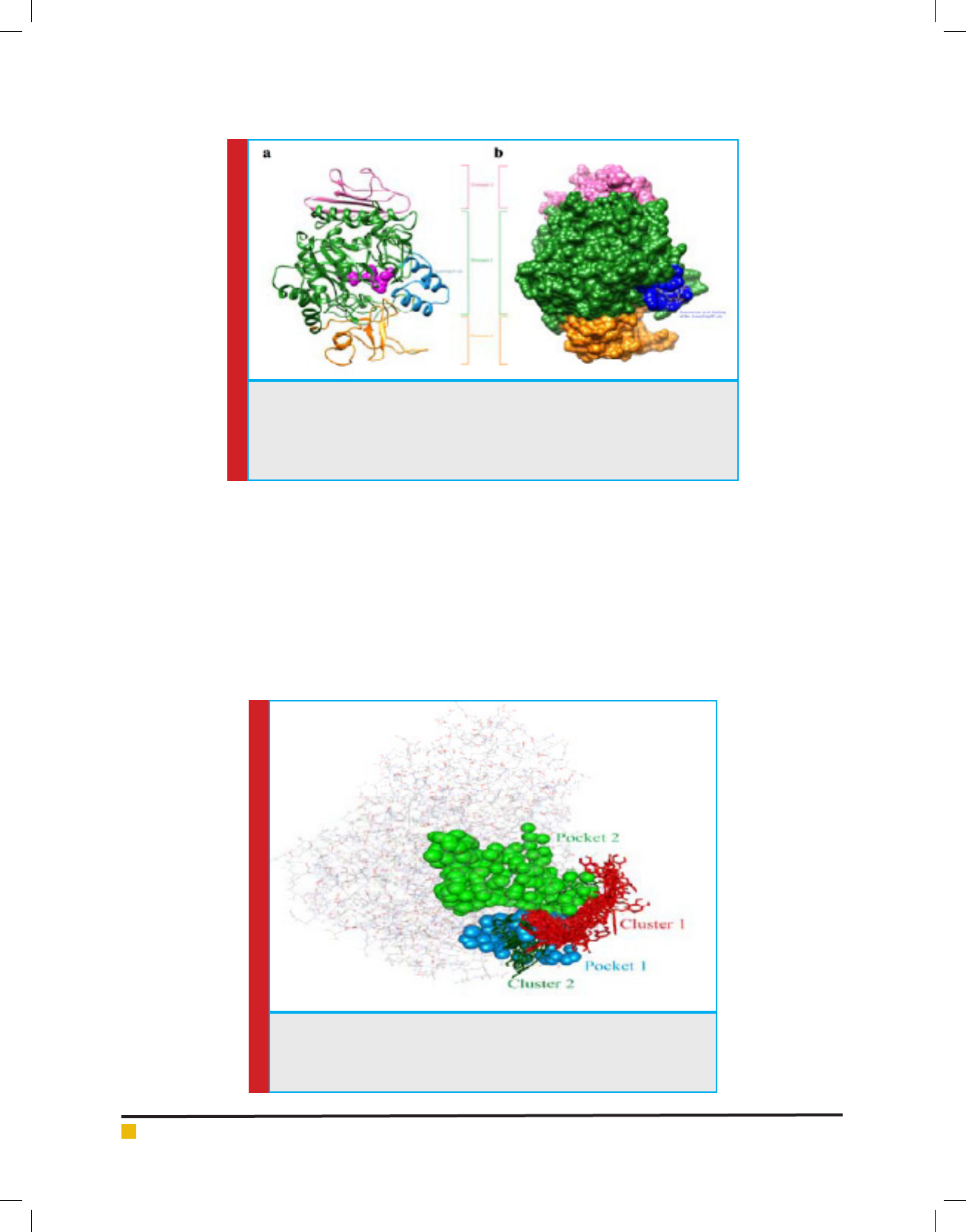
Molecular docking study found that Domain 1 of
-glucosidase resembled -amylase catalytic domain
with a gap of 42 amino acids, named here as AamyGap42
(Fig. 6a). These AamyGap42 site residues are shaped
as -helices near the entry point amino acid residues
(HIS279, THR307, SER308, PRO309, PHE310, PHE311,
and ARG312)
of the active site pocket of the enzyme
(Yamamoto et al., 2010).
Maibam B. Chanu et al.
654 BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 6. (a) 3D homology model predicted by the SWISS-MODEL program presented
in rounded ribbon with designated three domains and AamyGap42 site. Catalytic
residues are represented in magenta spheres. (b) Rosmarinic acid and -glucosidase
docked complex in solid surfaces, representing AamyGap42 interacting residues in
blue color.
FIGURE 7. CASTp pockets and AutoDock conformation clusters at
-glucosidase. Major two CASTp identi ed pockets represented in spheres.
Top two AutoDock conformation clusters (stick representation) coinciding
with Pocket 1.
According to Benkert et al 2009, GMQE score which is
expressed between 0 and 1 is a score where higher value
indicate increased stability of the predicted structure.
GMQE score for our nal 3D model was found to be 0.92
which imply good model accuracy. The evaluation of the
obtained models was done using the PROCHECK pro-
gram through Ramachandran plot. Ramachandran plot
revealed 88.8% residues were within the most favoured
regions, 10.6% of residues were in additional allowed
regions, 0.4% residues were in generously allowed
regions, and only 0.2% residues were found in the dis-
allowed regions. The percentage of residues in most
favorable regions showed the quality of protein mod-
els. The above result obtained indicated that the model
developed was quali ed to be used for molecular dock-
ing process.
Surface topology analysis, was done to know the
potential binding pockets in the structural model of
-glucosidase. Surface topology analysis on the protein
have identi ed two major pockets, named here as Pocket

Maibam B. Chanu et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND 655
FIGURE 8. Docking analysis: (a) 3D surface representation of rosmarinic acid and -glucosidase interactions,
representing hydrogen bonding sites (light green), Pi-interactions (pink), and van der Waals interactions (blue). (b)
Schematic representation of the interaction in detail with covalent and non-covalent bonds.
Table 1. Half maximum inhibitory concentration (IC
50
)
of the tested samples on -glucosidase
Samples IC
50
(μgmL
-1
)
MeOH extract 1.84 ± 0.11
Ethylacetate fraction 2.83 ± 0.07
n-butanol fraction 1.15 ± 0.06
Water fraction
No inhibition up to 500
gmL
-1
n-butanol Sub fraction (SFr 6) 0.66 ± 0.01
Rosmarinic acid
0.23 ± 0.02(0.636
µmolmL
-1
)
Acarbose 78.2 ± 0.17
Each value is the mean ± SD (n=3).
Table 2. AUC of postprandial glucose after sucrose
load in normal and diabetes mice
Group (mg/kg.bw)
AUC mmol.min/L
Normal rats Diabetic rats
Control 32.15±0.23 56.31±1.50
Acarbose(10) 19.95±0.41** 43.90±0.94*
Rosmarinic acid (5) 23.35±0.43* 48.82±0.57*
Rosmarinic acid (10) 22.71±0.25* 48.85±1.21*
Rosmarinic acid (25) 20.20±0.21** 44.80±1.33*
Each value is the mean ± SD (n=6). *p< 0.05 statistically signi cant
comparing to control.
performed, rst by involving the whole protein, result-
ing in multiple conformations of the rosmarinic Acid.
Root mean square deviation cluster analysis of these
docked conformations showed two most populated clus-
ters, Cluster 1 and Cluster 2 (Fig. 7). Auxiliary observa-
tions have shown that all the pockets and clusters were
surrounding the AamyGap42 site with Cluster 1 directly
falling under it. The catalytic and active sites of the pro-
tein were falling under Pocket 2. CASTp pockets (Pocket
1 and Pocket 2) along with AutoDock major clusters
(Cluster 1 and Cluster 2) lead the target area in the pro-
tein to perform second set of docking experiment. This
targeted docking study showed that rosmarinic acid did
not directly bind at the active site present in Pocket 2 of
the protein, instead it attached to the AamyGap42 site of
Domain 1 with the lowest binding energy of -7.72 kcal/
mol (Fig. 6b).
Binding details showed that rosmarinic acid inter-
acted with 12 amino acids in the AamyGap42 site form-
ing total 6 hydrogen bonds, two each with amino acid
residue GLU402 and TYR404, and one each with LYS417
and LYS422. This interaction substantially increased the
binding af nity as these amino acid residues were also
found to be the major contributor of electrostatic inter-
action energy (IE). Moreover, the residues TYR413 and
LYS410 were involved in Pi-Pi T-shaped and Pi-alkyl
interactions respectively (Fig. 8a,8b). Pi-Pi T-shaped
interaction between TYR413 and rst benzene ring of
the rosmarinic acid, Pi-Alkyl interaction with LYS410,
and van der Waals interaction with PRO400, ILE401,
GLU405, ASP406, VAL409, GLU414, strengthened the
stability of the enzyme and rosmarinic acid complex.
Binding free energy (∆G) between the receptor and ligand
was found to be -19.14 kcal/mol. The energy value sug-
1 and Pocket 2 with volume of 431.6 Å
3
and 384.7 Å
3
respectively (Fig. 7). Two sets of docking study were

Maibam B. Chanu et al.
656 BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
gested the possibility of inhibition of -glucosidase by
rosmarinic acid. The interaction energies (IE) between
the protein and ligand analysed for each amino acid
residues and overall residues showed electrostatic IE as
-44.70 kcal/mol and van der Waals IE as -31.22 kcal/mol
which showed good binding energy with the enzyme.
For the rst time we have reported the anti-diabetic
effect of Quercus serrata leaves extract and the dominant
active constituent rosmarinic acid. Further, this study
reported the in silico binding analysis of rosmarinic acid
with the enzyme -glucosidase of S. cerevisiae.
Con ict of interest: The authors declare no competing
interest.
ACKNOWLEDGEMENTS
Authors are thankful to the Director, IBSD, Imphal and
Department of Biotechnology (DBT), Govt. of India and
the Director, Institute of Advanced Study in Science
and technology for providing necessary infrastructure
to carry out this work. Authors are also thankful to Dr.
Basudeb Achari and Dr. Bikash C Pal for their valuable
suggestions.
REFERENCES
Benkert, P., Künzli, M. and Schwede, T. (2009) QMEAN server
for protein model quality estimation. Nucleic Acids Research,
37, W510–W514. http://doi.org/10.1093/nar/gkp322.
Biasini, M., Bienert, S., Waterhouse, A., Arnold, K., Studer,
G., Schmidt, T., Kiefer, F., Gallo, C.T., Bertoni, M., Bordoli,
L. and Schwede, T. (2014) SWISS-MODEL: modelling protein
tertiary and quaternary structure using evolutionary infor-
mation. Nucleic Acids Reserch, 42, W252-W258. http://doi.
org/10.1093/nar/gku340.
Brito-Arias, M., Ramírez-Hernández, P.L., García-Barrera,E.C.,
Perez- Venegas,M., Montiel-Valenciana, C. and Rodríguez-Pas-
cual, L.P. (2018) Synthesis,
-Glucosidase Enzymatic Inhibi-
tion and Docking Studies of some Fused Heterocycles. Chemi-
cal & Pharmaceutical Research, 1(1)
Chandramohan, R., Pari, L., Rathinam, A. and Sheikh, B., A.
(2015) Tyr osol, a phenolic compound, ameliorates hyperglyce-
mia by regulating key enzymes of carbohydrate metabolism in
streptozotocin induced diabetic rats. Chemico-biological Inter-
actions. 229 44-54. https://doi.org/10.1016/j.cbi.2015.01.026
Dundas, J., Ouyang, Z., Tseng, J., Binkowski, A., Turpaz, Y.
and Liang, J. (2006) CASTp: computed atlas of surface topog-
raphy of proteins with structural and topographical mapping
of functionally annotated residues. Nucleic Acids Research, 34,
W116-W118. http://doi.org/10.1093/nar/gkl282.
Inoue, I., Takahashi, K., Noji, S., Awata, T., Negishi, K. and
Katayama, S.(1997) Acarbose controls postprandial hyper-pro-
insulinemia in non-insulin-dependent diabetes mellitus. Dia-
betes Research and Clinical Practice, 36, 143-151. https://doi.
org/10.1016/S0168-8227(97)00045-4.
International Diabetes Federation, (2017) Diabetes Atlas.
8th Edition. https://www.worlddiabetesfoundation.org/sites/
default/ les/Atlas-8e-Global-factsheet.pdf/ (accessed on 03
October 2018).
Kaveeshwar S.A.and Cornwall J. (2014) The current state of
diabetes mellitus in India. Australasian Medical Journal, 7(1),
45-48. http//dx.doi.org/10.4066/AMJ.2014.1979.
Laishram, S., Sheikh, Y., Moirangthem, D.S., Deb, L., Pal, B.C.,
Talukdar, N.C. and Borah, J.C. (2014) Anti-diabetic molecules
from Cycas pectinata Griff. traditionally used by the Maiba-
Maibi. Phytomedicine, 22 (1), 23-26. https://doi.org/10.1016/j.
phymed.2014.10.007.
Laskowski, R.A., MacArthur, M.W., Moss, D.S. and Thornton,
J.M. (1993) PROCHECK - a program to check the stereochemi-
cal quality of protein structures. J. App. Cryst. 26, 283-291.
https://doi.org/10.1107/S0021889892009944.
Marchler-Bauer, A., Derbyshire, M.K., Gonzales, N.R., Lu,
S.,Chitsaz, F.,Geer, L.Y.,Geer, R.C.,He, J.,Gwadz, M.,Hurwitz,
D.I.,Lanczycki, C.J.,Lu, F.,Marchler, G.H.,Song, J.S,Thanki,
N.,Wang, Z.,Yamashita, R.A.,Zhang, D.,Zheng, C. and Bry-
ant, S.H. (2015) CDD: NCBI’s conserved domain database.
Nucleic Acids Research, 43, 222–226. http://doi.org /10.1093/
nar/gku1221.
Miura, T.,Itoh, C.,Iwamoto, N.,Kato, M.,Kawai, M.,Park, S.R.
and Suzuki, I. (2001) Hypoglycemic activity of the fruit of the
Momordica charantia in type 2 diabetic mice. Journal of Nutri-
tional science and Vitaminology. 47(5), 340-4.
Morris, G.M., Huey, R., Lindstrom, W., Sanner, M.F., Belew,
R.K., Goodsell, D.S. and Olson, A.J (2009) Autodock4 and
AutoDockTools4: automated docking with selective receptor
exiblity. Journal of Computational Chemistry. 30 (16), 2785-
91. http://doi.org/10.1002/jcc.21256.
Nakatsu,Y., Kokubo, H., Bumdelger, B., Yoshizumi, M., Yama-
motoya, T., Matsunaga, Y., Ueda, K., Inoue,Y., Inoue, M-K.,
Fujishiro, M., Kushiyama A., Ono, H., Sakoda H., and Asano,
T. (2017) The SGLT2 inhibitor Luseogli ozin rapidly normal-
izes aortic mRNA levels of in ammation-related but not lipid-
metabolism-related genes and suppresses atherosclerosis in
diabetic ApoE KO Mice, International Journal of molecular
science, 18, 1704. doi:10.3390/ijms18081704.
Pettersen, E.F., Goddard, T.D., Huang, C.C., Couch, G.S., Green-
blatt, D.M., Meng, E.C., Ferrin, T.E. (2004) UCSF Chimera-a
visualization system for exploratory research and analysis.
Journal of Computational Chemistry, 25(13), 1605-12. http://
doi.org/10.1002/jcc.20084.
Petersen, M. and Simmonds, M.S.J. (2003) Rosmarinic acid.
Phytochemistry. 62, 121-125. https://doi.org/10.1016/s0031-
9422(02)00513-7.
Polonsky, K.S., (2012) The Past 200 Years in Diabetes. The New
England Journal of Medicine, 367, 1332-1340. https://doi:
10.1056/NEJMra1110560.

Maibam B. Chanu et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS BIOASSAY GUIDED ISOLATION OF -GLUCOSIDASE INHIBITORY COMPOUND 657
Sheikh, Y., Chanu M.B., Biswas, D., Laishram, S., Deb, L.,
Talukdar , N.C. and Borah, J.C. (2015) Anti-diabetic potential
of selected ethno-medicinal plants of north east India. Journal
of Ethnopharmacology, 171, 37-41. https://doi.org/10.1016/j.
jep.2015.05.030.
Sheikh, Y., Maibam, B.C., Talukdar, N.C., Deka D.C. and Borah,
J.C. (2016) In vitroandin vivoanti-diabetic and hepatoprotective
effects of edible pods ofParkia roxburghiiand quanti cation of
the active constituent by HPLC-PDA. Journal of Ethnopharma-
cology, 191, 21-28. https://doi.org/10.1016/j.jep. 2016.06.015.
Sippl, M.J. (1993) Recognition of Errors in Three-Dimensional
Structures of Proteins. Proteins, 17(4), 355-362. http://doi.
org/10.1002/prot.340170404.
Veeraraghavan, P., Expert Consultant, CPCSEA, OECD Guide-
line No. 423. (2000) https://ntp.niehs.nih.gov/iccvam/sup-
pdocs/feddocs/oecd/oecd_gl423.pdf/ (accessed on 14 March
2014).
Wiederstein, M. and Sippl, M.J. (2007) ProSA-web: interactive
web service for the recognition of errors in three-dimensional
structures of proteins. Nucleic Acids Research, 35, W407-W410.
http;//doi.org/10.1093/nar/gkm290.
Yamamoto, K., Miyake, H., Kusunoki, M. and Osaki, S. (2010)
Crystal structures of isomaltase from Saccharomyces cerevi-
siae and in complex with its competitive inhibitor maltose.
The FEBS Journal, 277(20), 4205-4214. http://doi.org/10.1111/
j.1742-4658.2010.07810.x.
Zimmet, P.Z., Magliano, D.J., Herman, W.H. and Shaw J.E.
(2014) Diabetes: a 21st century challenge. The Lancet Diabe-
tes and Endocrinology, 2(1), 56-64. https://doi.org/10.1016/
S2213-8587(13)70112-8.