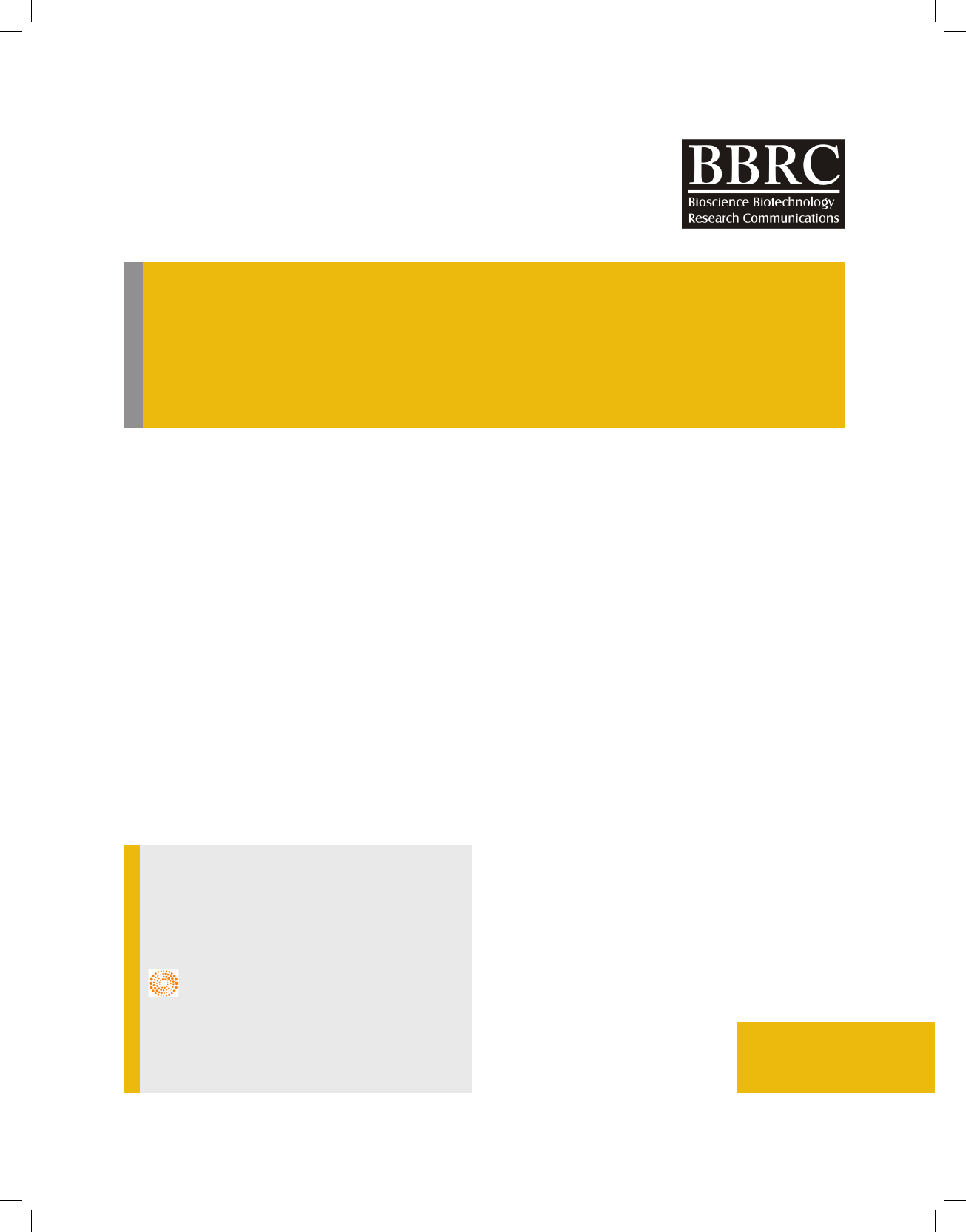
Differentiation of human fat mesenchymal stem cells
using electromagnetic waves
Nariman Gharari
1
and Mahsa Laleh
2
1
School of Biology, University of Tehran, Tehran, Iran
2
Department of Biomedical Engineering, Azad University of Science and Research Branch, Tehran, Iran
ABSTRACT
In orthopedic sciences, cartilage regeneration and repairing is an underlying issue investigated by many studies in tis-
sue engineering. Bioactive growth factors, cell implantation and biocompatible scaffolding are the new developments
in this eld. Keeping the proliferation and differentiation ability of cultured cells is essential in producing extracellular
matrix and cartilage. Adult stem cells can be used due to their high ability to be differentiated in several cell types in
tissue engineering. Till recently hematopoietic stem cells and fat tissue stem cells have been used to repair the tissues.
Studies indicates that using fat tissue stems cells is preferred because of easy access to adipose tissue and maintain-
ing the ability of proliferation and differentiation to cartilage in high passages compared to hematopoietic stem cells.
Therefore, this study has been conducted to analyze the differentiation of human fat mesenchymal stem cells using
electromagnetic waves. In this work simultaneous effect of platelet-rich plasma and electromagnetic waves have been
studied on ef ciency of differentiation of fat mesenchymal stem cells to cartilage. Mesenchymal stem cells have been
extracted from human fat tissue and then, the cells have been differentiated to fat, bone and cartilage tissue cells using
different treatments. Cartilage differentiation was done in 2-D form and single-layer cell in bottom of 6-bed and 3-D
cells in cell falcons. Required tests to estimate ef ciency of cartilage differentiation were done in two general examina-
tions for analysis of evaluation of speci c and pathologic markers such as osteogenicity, angiogenesis and in ammation
parameters by measuring the amount of TNF and VEGF with ELISA technique. The results obtained from this study
showed reasonable use of platelet-rich plasma (PRP) and electromagnetic waves in medicine and tissue engineering.
Although PRP enables cartilage differentiation, it cannot reduce pathologic symptoms.
KEY WORDS: DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS, ELECTROMAGNETIC WAVES, PRP
577
Biomedical
Communication
Biosci. Biotech. Res. Comm. 11(4): 577-586 (2018)
ARTICLE INFORMATION:
Corresponding Authors: narimangharari@gmail.com
Received 1
st
Oct, 2018
Accepted after revision 21
st
Dec, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
Mono of Clarivate Analytics and Crossref Indexed
Journal Mono of CR
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at:
http//www.bbrc.in/
DOI: 10.21786/bbrc/11.4/7

Nariman Gharari and Mahsa Laleh
578 DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
INTRODUCTION
The aim of tissue engineering is normalizing tissue per-
formance through transferring living factors in body
of patients, (Kocan et al. 2017, Bondarava et al. 2017).
Continuous efforts of cell biologists, engineers, mate-
rial engineers, mathematicians, geneticists and physi-
cians should be in way of achievement and success in
new tissue production, (Alonso-Goulart et al. 2017, Klar
et al. 2017).
Nowadays, tissue engineering is being used as a tech-
nique for tissue or organ transplant. The lost and injured
tissues and organs can be treated by engineered biologi-
cal transplant (Zhai et al. 2016). Engineered products and
components should have high performance and have
the ability to form expected functional tissue (Zajdel
et al. 2017). Mesenchymal stem cells (Skeletal Stem Cells
or Bone marrow stromal stem cells) are plastic sticky
non-hepatocytes cells placed in bone marrow stro-
mal vessels (Seong et al. 2014 Ross et al. 2015 and Liu
et al. 2017) and have multi-generation differentiation
with self-renewal capacity (Heo et al. 2016 and Mobini et
al. 2017). Deciding which type of cell should be applied
to repair injured tissue is essential which can be adult
brucellosis, mesenchymal stem cells or protozoal cells
from hypothyroidism, pericardium or cells manipulated
genetically, (Tan et al. 2017). Cartilage is non-vascular
brous connective tissue having resistant extracellular
matrix, which is stiffer than other connective tissues and
includes intra-matrix chondrocytes, (Choi et al. 2018).
The rst function of cartilage is protection of soft tis-
sues and helping evolution and growth of long bones.
Fat tissue stem cells are similar to bone marrow stromal
cells in terms of physical form and can be appropriate
replacement. These cells have common features com-
pare with mesenchymal stem cells such as self-renewal
ability, powerful cells, broblast appearance, ability to
attach to plastic dishes for cell cultivation and ability
to be changed into other mesenchymal types, (Dubois
et al. 2008 and Tan et al. 2017).The differentiation of
these cells from fat, bone, cartilage, skeletal muscular
and cardiovascular cells has been reported in recent
years by different researchers. Fat tissue stem cells, same
as bone marrow stromal cells, can be converted to ecto-
dermal types (like neural and glial cells) by the media-
tion of neural inducers, (Ivan et al. 2017).
Several studies have been conducted in fat tissue stem
cells, especially for treatment of neural system lesions
and revealed that direct injection of fat stem cells to
brain ischemic models can convert these cells to quasi-
neural cells. These cells can improve laboratory models
in the place of lesion with (injured place by) secretion
of neural growth factors (Gugjoo et al. 2016).In order to
prepare mesenchymal cell, fat tissue is a considerable
source, since it is available in large amount (liposuc-
tioned) fat tissue or in fat tissue section
and contains
large amount of stem cells called fat-derived stem cells.
The property has made it be considered as a good can-
didate for 1-step treatment of cartilage defects (Bruder
et al. 1997).
In the past decade, PRP or platelet-rich plasma has
gained many attentions, especially in regenerative medi-
cine, (Liu et al. 2017). PRP can be considered as a part of
blood plasma with platelet density higher than base level
(Xu et al. 2017 Qian et al. 2017). Importance of PRP with
appropriate density of platelet is the abundant growth
factors and proteins used in tissue engineering, (Anitua,
et al. 2007). In 2012, a study was done in China for
regenerating cartilage, which evaluated and compared
mesenchymal stem cells, extracted from bone marrow
and fat tissue placed on the PRP-derived scaffolding.
Findings showed that rich plasma is appropriate bioac-
tive scaffolding with ability of secreting growth factor,
bone marrow stem cells and fat mesenchymal stem cells
-placed on PRP were differentiated from cartilage and
ef cient to repair cell-based cartilage, (Lee et al. 2012).
In a study, the effects of platelet-rich plasma on his-
tological, biochemical and biomechanical properties of
cartilage tissue engineering were studied. Cultured cells
in PRP created 20% thicker cartilage tissue compared
with other cultured cells. Hence, platelet-rich plasma in
culture medium can cause creation of cartilage in vitro
cartilage formation with increased content of glycosa-
minoglycan and more mechanical compressive proper-
ties at the same time with maintaining Phenotype Fea-
tures of Hyaline cartilage (Petrera, et al. 2013). In 2014,
a research team from Spain studied effects of PRP on
human mesenchymal stem cells, which can increase or
limit clinical uses of these cells. PRP can preserve dif-
ferentiated immune of mesenchymal stem cells and can
apparently postpone ageing phenomenon. They also
provide data on exact molecular relation and its mecha-
nisms, (Rubio-Azpeitia et al. 2014).
In 2014, an economic method was proposed to pre-
pare PRP. PRP-derived growth factor-BB was measured
under conditions of using Anticoagulant dextrose solu-
tion A (ACD-A), along with or in absence of Prosta-
glandin E1 (PGE1) (as Platelet aggregation inhibitor).
The new method was successful in analysis of PRP with
growth factor and high BB under all growth conditions
and high volume of PRP is obtained using ACD-A and
PGE1(Fukaya et al. 2014).
In 2014, the effect of PRP on cartilage cells differ-
entiated from rabbit fat-derived stem cells was studied
in vitro. Stem nature of rabbit cells were studied with
differentiation of fat, bone and cartilage types in vitro.
Collagen type 2 expression and agrican expression in
PRP-treated cells was increased to 10% compared to
Nariman Gharari and Mahsa Laleh
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES 579
control group. Hence, the ndings showed that PRP of
rabbit can take cartilage differentiation in mesenchymal
stem cells extracted from rabbit fat (Elder et al. 2014).
Analysis of the simultaneous effect of PRP and elec-
tromagnetic waves on cartilage differentiation ef ciency
in fat mesenchymal cells has been studied. Mesenchymal
stem cells have been extracted from human fat tissue
and then, the cells were differentiated to types of fat
tissues, bone and cartilage using different treatments
Therefore, these experiment have been conducted to
analyze differentiation of mesenchymal human fat stem
cells using electromagnetic waves.
MATERIALS AND METHODS
Extraction of mesenchymal stem cells from fact
tissue:To transfer fat tissue, fat pieces were placed in
DMEM medium containing Penicillin and streptomycin
3X , amphotericin 1x , without FBS and were transferred
to laboratory in ice. After each time of adding buffer to
2-3 times of tissue volume, centrifuge was taken with
speed of 1200rpm for 20min and buffer solution was
disposed. A thin layer of fat was formed on the solution,
which had to be removed, since it could cause problems
such as choking on cells due to lack of oxygen while
cultivation. In this step, suspended cells were transferred
to asks 75 containing 10ml culture medium with FBS
and were then heated in incubator.
To make cells face electromagnetic waves, after
counting cells using Neubauer lam method, a part of
culture medium containing 200.000 cells was removed
from culture asks and was transferred to sterile falcons.
Then, 1ml of culture medium without differentiation fac-
tors and containing Penicillin - Streptomycin and FBS
was added and maintained for 1 day. After the heating
in incubator, cells were accumulated in bottom of the
falcon in rounded form and were prepared to continue
the examination and to expose to electromagnetic radi-
ation. To apply electromagnetic waves on cell culture,
wave winding machine was used. The winding machine
was placed inside the incubator and culture falcon was
placed there to be exposed to electromagnetic waves for
6 hours per day. Frequency of the machine was set in
range 171-173Hz and according to power about 30%,
ultimate and real time power about 50Hz was obtained.
Cells were exposed to such conditions in 14-day period
and the culture medium was changed every 3 days. 5
falcons containing culture medium with 5% PRP were
exposed to electromagnetic waves and other 4 falcons
were not. Measurement of VEGF distributed in differ-
entiated cells was done using supernatant of cultures
in days 7, 14 and 21. The phosphorylation of this factor
was measured by kit based on Sandwich ELISA method.
The Sandwich ELISA method was used to measure the
amount of TNF propagated from differentiating cells.
To this end, the supernatant collected from cell culture
was examined in the days 7, 14 and 21. The nal results
were analyzed statistically at the con dence level of 95%
to test signi cance of the difference of different groups
using Prism software and using one-way ANOVA and
T-test. Moreover, the diagrams were drawn using Excel
and SPSS software. The results obtained from analysis of
cartilage differentiation gene expression were analyzed
using REST software by normalization with reference
beta-actin gene. Each test was replicated 3 times and the
signi cance was considered lower than 0.05.
RESULTS
Analysis of differentiation of stem cells extracted from
fat tissue to cartilage :In this study, differentiation to
cartilage was analyzed using different staining methods
and Immunocytochemistry test during 14 days of cell
differentiation. Expression of some genes relevant to
cartilage was also studied at the end of the differentia-
tion period.
Proof of cartilage differentiation: The Immunocyto-
chemistry method was used to analyze expression and
production of collagens types 2 and 10. Collagen type 2
was expressed during cartilage differentiation and colla-
gen type 10 was one of the bone and hypertrophy mark-
ers expressed by chondrocytes. Figure 1 has illustrated
expression of these collagens in treated cells in the day
14. The blue color in the gure is DAPI staining to stain
cell core and red color shows presence of studied col-
lagens in differentiating cells.
Analysis of bone markers during differentiation of
stem cells to cartilage: At the time of applying differ-
entiation factors such as using PRP as a bioactive sub-
stance or electromagnetic radiation to stimulate carti-
lage differentiation in mesenchymal stem cells of fat
tissue, non-speci ed differentiation to bone is also pos-
sible. With taking tests such as measurement of Alka-
line Phosphatase Activity and Calcium sedimentation
measurements, the non-speci ed differentiation was
also examined.
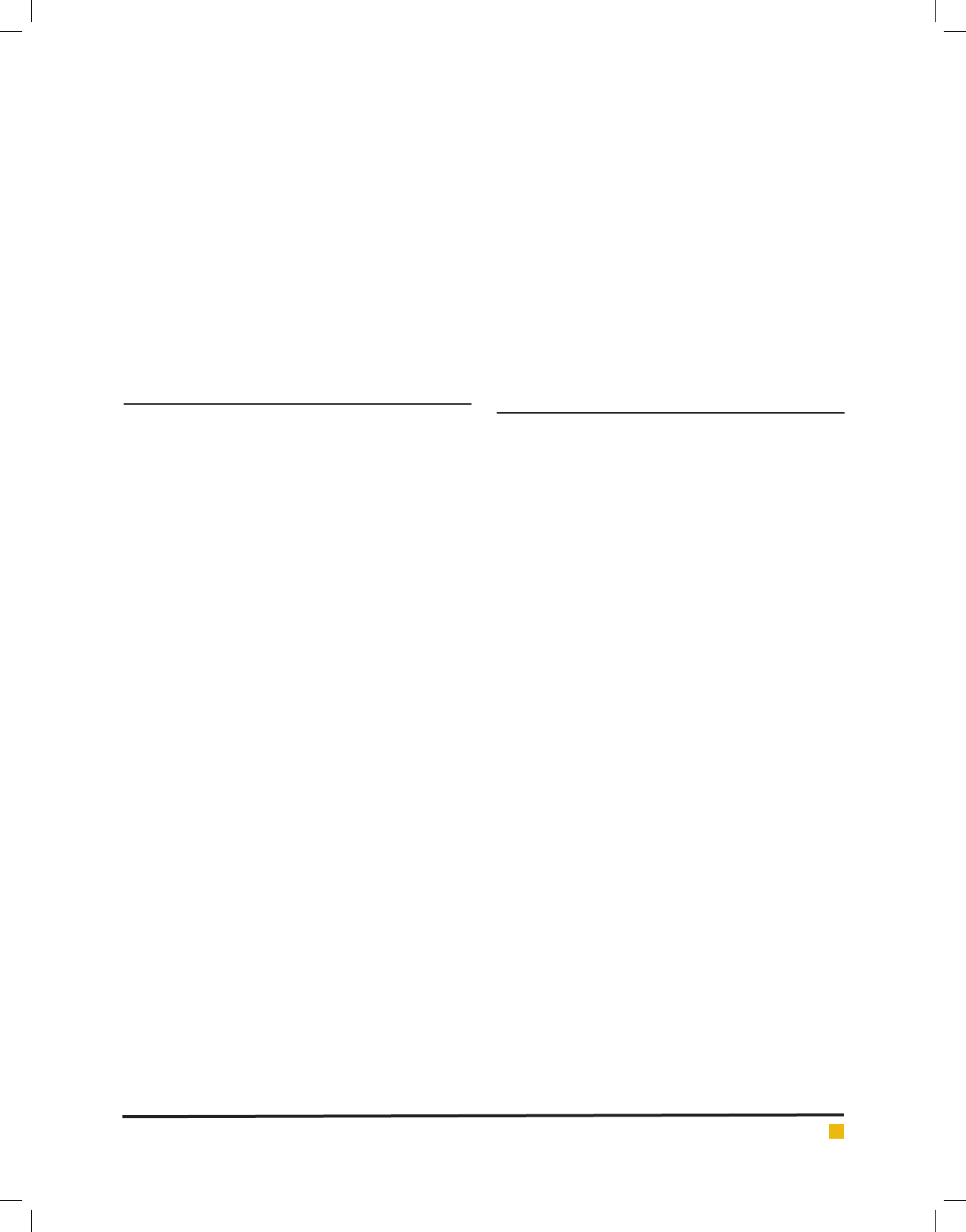
Measurement of Alkaline Phosphatase Activity: To
determine non-speci ed differentiation towards bones,
measurement of alkaline phosphatase enzyme was activ-
ity was done. As it is clear in diagram 1, the amount of
alkaline phosphatase in treatment PRP.W is the highest
level measured equal to 2 units of optical absorption per
mg protein. Then, treatment PRP a with value of 1.44
unit absorption per mg protein shows highest alkaline
phosphatase activity. The lowest level of phosphatase
Nariman Gharari and Mahsa Laleh
580 DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 1. Immunocytochemistry staining of collagens type 2
and 10 in treated cells; A) collagen type 2 in treatment Dif.a; B)
treatment Dif.W and c) collagen 10 in treatment PRP.a (the upper
part is staining core by DAPI and the lower part is collagen stain-
ing); blue color shows cell core and red color refers to surface
place of collagens; the line illustrates size of 0.26mm and magni-
cation of image ×4.
DIAGRAM 1. Phosphatase alkaline activity per unit optical absorption per mg protein in different treat-
ments
alkaline was observed in treatment base.w at 0.32
unit absorption per mg protein. According to obtained
results, treatments PRO.W and PRP.a motivate differ-
entiation towards bones more than others and this can
be inferred based on activity of alkaline phosphatase in
these treatments. Despite to them, treatment base.w has
caused lowest stimulation towards boning.
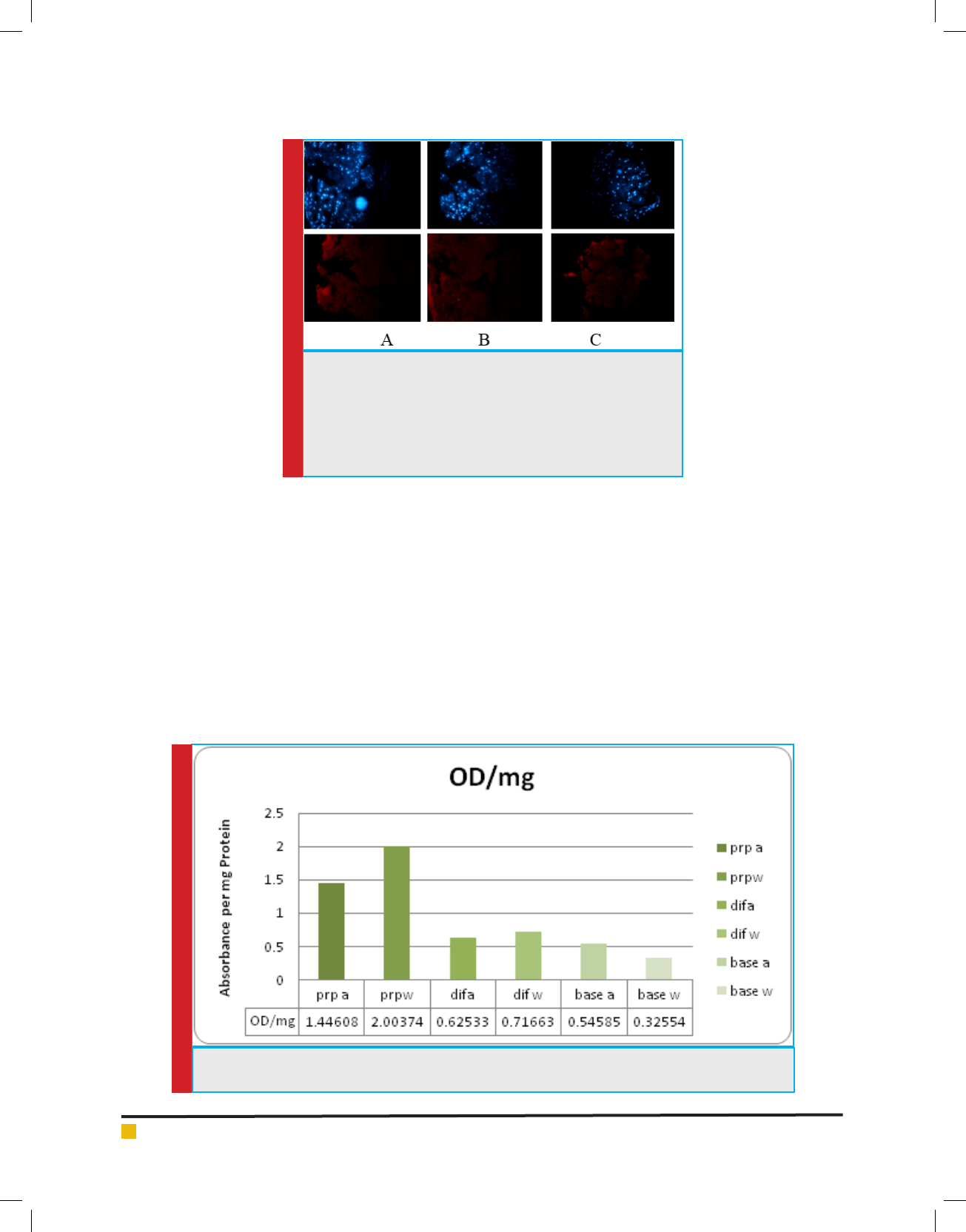
VEGF factor secretion : According to diagram 2, highest
secretion level of VEGF was observed in treatments with
base environment with PRP. In the base treatment with
PRP.a, higher level of Endothelial Angiogenesis Growth
Factor was observed compared to base environment in
combination with PRP.w. The lowest secretion level was
detected in base.w and it seems that the effect of electro-
magnetic wave treatments and TGF factor varies due to
medium culture used. For example, in environment with
Dif base, secretion in wave treatment is higher than oth-
ers; although the results differ in base environment and
secretion has been in higher level using TGF treatment.
The effects of PRP have been also con icting. This factor
has shown different effects in combination with differ-
ent culture media exposed to wave and TGF treatments.
However, it seems that it has led to increased secretion
of VEGF by itself, since secretion in treatments with PRP
has been higher than others.
Nariman Gharari and Mahsa Laleh
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES 581
DIAGRAM 2. Secretion of VEGF in different treatments
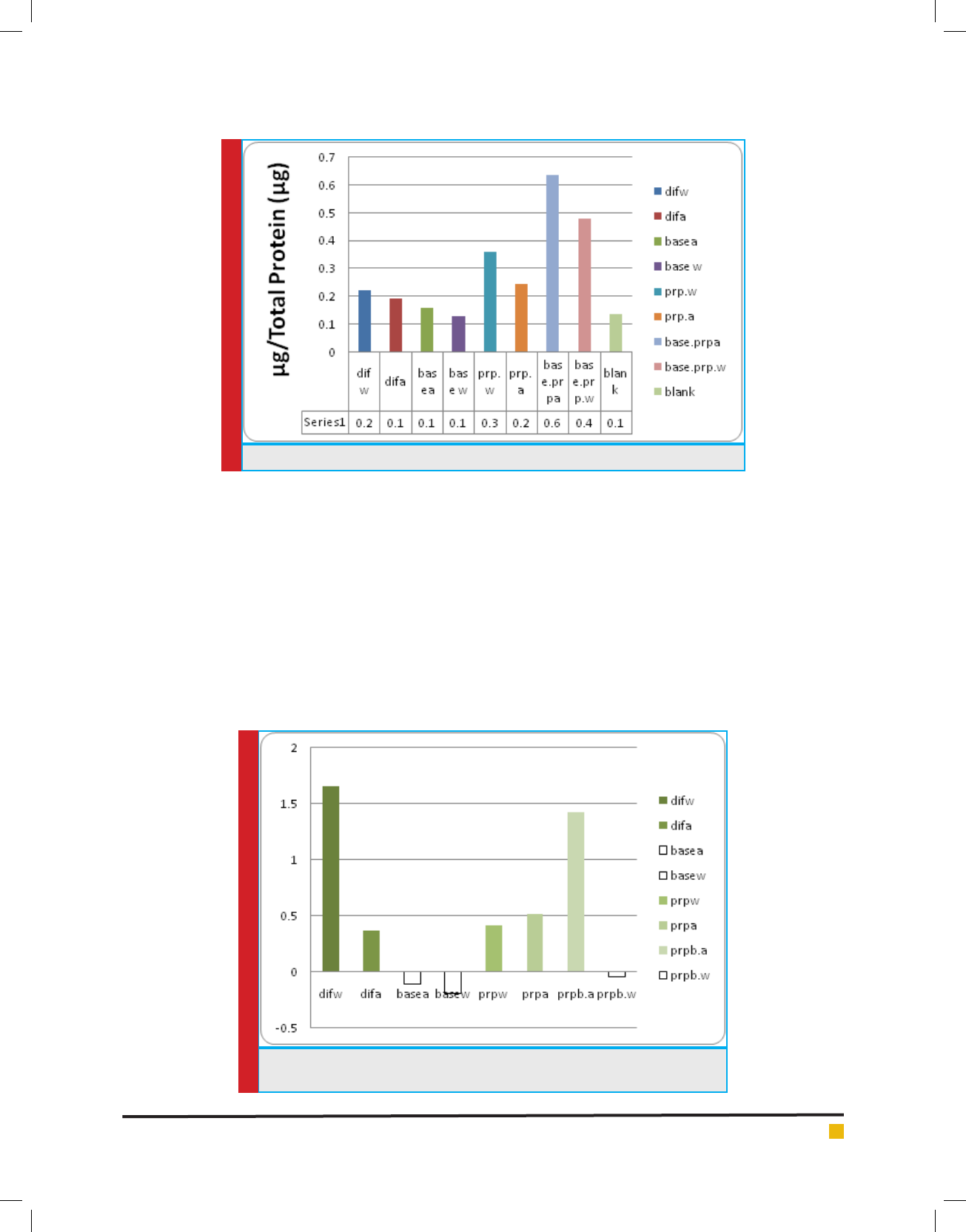
DIAGRAM 3. TNF level in different treatments of induction of differentiation
to cartilage
Analysis of in ammation with TGF measurement:
Another symptom of cartilage-related articular diseases
is in ammation. According to existing reports, PRP
can cause in ammation in some cases and may inten-
sify that. Moreover, it can inhibit and reduce in am-
matory reactions. The con ict is because of wide range
of in ammatory factors in PRP. In ammatory potential
of engineered tissue has been examined by measure-
ment of TNF. The results have been presented in dia-
gram 3. According to these results, treatments Dif.w and
PRPb.a have shown highest level of in ammatory activ-
ity. On the contrary, treatments Base.w and Base.A and
PRP.W have shown low level of TNF even lower than
ELISA control level. Treatment Dif showed highest level
of in ammation; although same factor showed lowest
level of in ammatory activity in combination with Base
medium. PRP.b factor has also shown different behav-
ior in different combinations of treatment with TGF and
electromagnetic radiation, so that it has shown high
in ammatory activity in combination with TGF and
in ammatory activity lower than control level in com-
bination with electromagnetic radiation. The con icting
responses can be attributed to complexity of compounds
in culture media and PRP compounds, since controlling

Nariman Gharari and Mahsa Laleh
582 DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 2. Agarose gel produced by
PCR reaction for ALK5 M 1--bp marker
gene
FIGURE 3. Agarose gel pro-
duced by PCR reaction for
Pleotrophin M gene 100bp
marker
DIAGRAM 4. Sox9 gene expression during cartilage differentiation
these complex compounds and inhibition of interfering
effects of factors in the combinations is dif cult.
Measurement of cartilage and bone gene expression :
Analysis of cartilage differentiation by gene expression
using RT-PCR
Expression of Pleotrophin and ALK5 genes as markers
in cartilage differentiation samples
and collagen status
type 2 gene were analyzed. Collagen type 2 includes 2
isoforms called ColllA and ColllB created as a result of
alternative array of exon 2. Expression of these markers
during Chondrogenic differentiation caused differentia-
tion ef ciency detection. The image of gels produced by
reaction of these genes is presented in gures 2 and 3.
Emergence of 250bp band showed expression of
ALK5 gene in samples, which can show cartilage differ-
entiation in these samples. 111bp band emergence also
showed expression of Pleotrophin in cells and con rms
cartilage formation processes. However, no band was
observed in proliferation of isoforms in collagen type
2 and the result of this gene proliferation was negative,
which shows lack expression of this gene in this differ-
entiation steps.
Analysis of speci c cartilage genes expression using Real
Time PCR
In order to analyze expression of speci c cartilage genes
such as sox9, colll and colX using b2m reference gene,
Real Time PCR method was used.
Sox9 cartilage marker: Sox9 marker is the transcription
factor required for expression of cartilage matrix genes
Nariman Gharari and Mahsa Laleh
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES 583
DIAGRAM 5. Colll gene expression during differentiation process
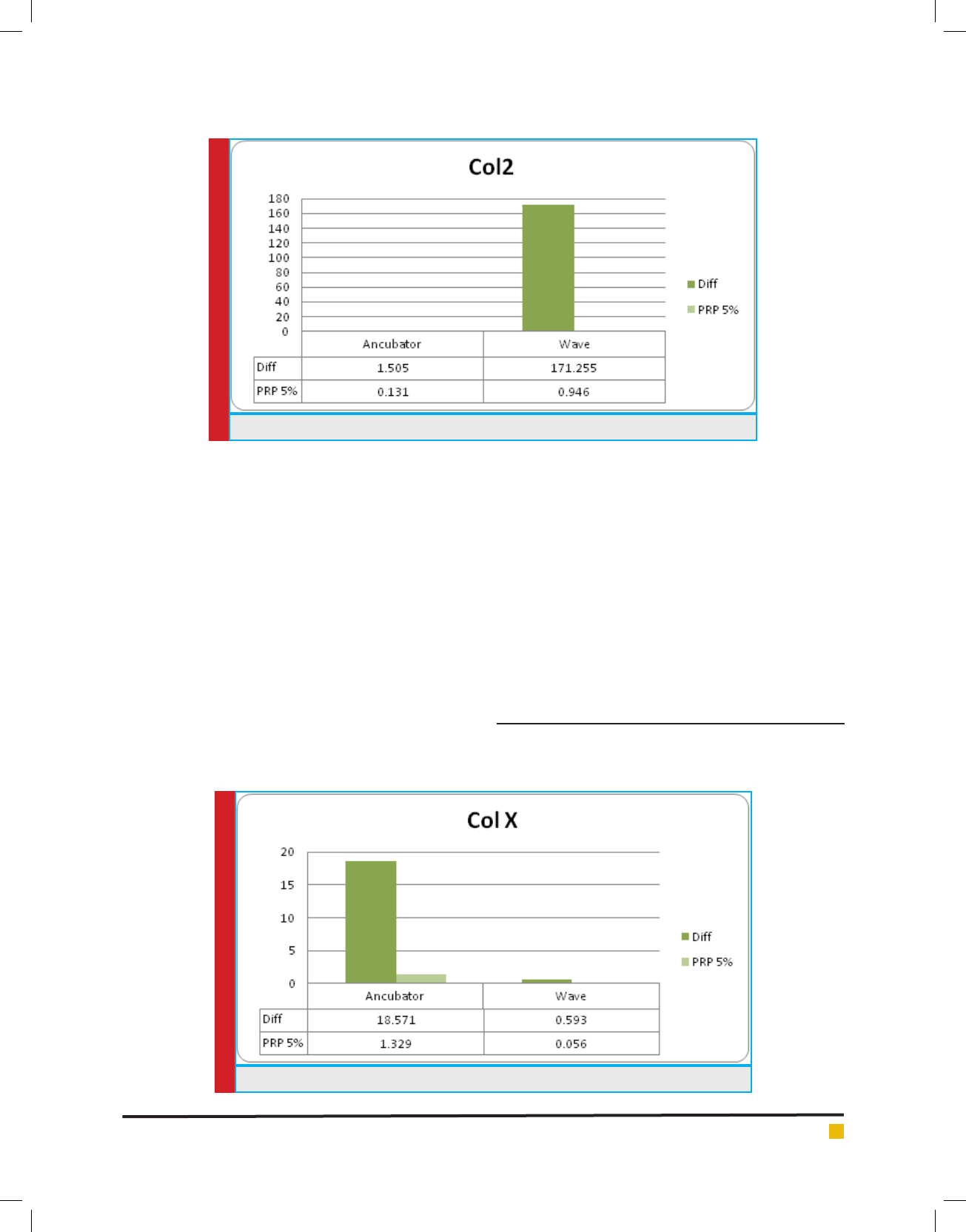
DIAGRAM 6. ColX gene expression during differentiation of stem cells to cartilage
such as collagen type 2. Diagram 4 illustrates sox9 gene
expression during cartilage differentiation process. In
the day 14 of treatment, expression of this gene in Dif
medium exposed to electromagnetic waves was signi -
cantly high; although its expression in medium contain-
ing 5% PRP was not tracked. Moreover, expression of
this gene was not observed or was insigni cant in treat-
ments without wave.
Collagen type 2: The products of this gene were
observed in hyaline cartilage in form of bril. The pro-
teins can’t form ber. In this study, proalpha-1 chain has
been detected, which has interfered in partial production
of collagen type 2. According to diagram 5, colll gene
expression has been signi cantly increased under effect
of electromagnetic waves. Moreover, the results show
that medium containing 5% PRP has been unable to
induce expression of this gene and has also had inhibi-
tory effects on wave induction capacity on the other
hand.
Collagen type 10: Alpha-1 collagen chain was coded by
COL10A1 gene. This gene can encode collagen type-10
alpha chain, which is expressed by hypertrophic chon-
drocytes during Endocardal bone formation.
Diagram 6 has illustrated adjustment of existing data
of electromagnetic wave and 55 PRP on both inhibitory
effects on expression of this gene. When the two factors
are applied in same treatment on cells, no expression
can be observed; although colX gene expression is in
high level in presence of the two factors.
DISCUSSION
Mature stem cells can be used for tissue engineering
purposes due to high differentiation ability to several

Nariman Gharari and Mahsa Laleh
584 DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
cell types. Recently, fat tissue stem cells have been used
to repair tissues. The study conducted by Zul et al. (2001)
using stem cell extraction methods based on using col-
lagenase could extract these cells from fat tissue of adult
people, (Hunziker et al. 2002). Moreover, Estes Research
Team used this method to extract stem cells to obtain fat
tissue stem cells to create cartilage phenotype, (Jonstone
et al. 1998).
According to ndings of Pena et al. (2011), spindle
cells with ability of adherence to bottom of culture dish
and the ability of differentiation to mesodermic cell lines
can be considered as mesenchymal stem cells (Yoo et al.
1998). Adherence ability of cells to plastic surfaces has
been used for early separation of stem cell populations.
Similar results were also observed in study of Tappe
et al. (2009) and Aust et al. (2004), in which nature of
fat tissue was analyzed using this type of staining, (Sol-
chaga et al. 2006, Diduch et al. 2000).
In this study, to analyze the differentiation of stem
cells extracted from fat tissue to cartilage, expression
and production of collagens type 2 and 10 has been
applied using Immunocytochemical method. Similar
results were also obtained by Ogawa, (Williams et al.
2003). In this study, expression of collagen type 2 was
observed during cartilage differentiation and type 10 as
bone and hypertrophy markers in chondrocytes. It seems
that Dif medium and electromagnetic wave treatment
have induced cartilage differentiation and bone mark-
ers have been observed in PRP-contained treatment and
it seems that the treatment has the ability of inducing
hypertrophy and boning. Mishra et al. (2009) showed the
effect of PRP on cartilage differentiation. Further, con-
icting effects of PRP on cartilage and bone differentia-
tion were revealed (Bosnakovski et al. 2006, Wakitani
et al. 2002).
Alkaline phosphatase enzyme activity can be con-
sidered as one of the most underlying factors of hyper-
trophy and bone formation and when the cartilage cells
are differentiated, they gain hypertrophic mode and start
production of alkaline phosphatase enzyme, (Bruder
et al. 1997). According to the results of this study, treat-
ments PRP.W and PRP.a have stimulated differentiation to
bone more than others and lowest alkaline phosphatase
activity has been observed in treatment Base. W on the
other hand. Measurements showed that highest secretion
of VEGF was observed in treatments with base medium
containing PRP. Lowest secretion level was also detected
in Base. W medium. It is expected that PRP can induce
such reaction because of nature rich of growth factors.
However, there are some reports on inhibitory effect
depended on PRP dose in VEGF secretion (Clin Pediatr
Endocrinol 2014). The con icts can be because of differ-
ence in type of treatments, PRP preparation method and
its source and also type of cells under treatment.
According to results obtained from this study, treat-
ments Dif.W and PRP.a have shown highest level of
in ammatory activity. On the contrary, treatments
Base.W, Base.A and PRPb.W have even shown TNF
level even lower than ELISA control level. It seems that
combination of base medium lays key role in reduction
of in ammatory activities regardless of other inductive
factors.
The effects of electromagnetic radiation or using PRP
showed different results. For example, radiation in com-
bination with Dif medium showed highest in ammation
level; although same factor showed lowest in ammation
activity in combination with base medium. In 3 out of 4
treatments containing electromagnetic wave, TNF level
was low and insigni cant and it could be inferred that
electromagnetic wave can probably reduce in amma-
tory activity regardless of culture medium compounds.
Controlling expression of cartilage matrix gene like
collagen type 2 was taken by transcription factor called
sox9. During cartilage differentiation process, the tran-
scription factor played key role and its expression was
increased in chondrocytes and Chondroprogenitor cells.
According to obtained results, expression of this gene
in Dif medium under electromagnetic radiation was
signi cantly increased; although its expression was
not detected in medium containing 5% PRP. Moreover,
the expression of this gene was not observed or was
insigni cant in treatments without wave. As a result of
increased expression of sox9 gene, its downstream gene
(colll) was also increased in terms of expression. Moreo-
ver, the results showed that medium containing 5% PRP
has not the ability to induce expression of this gene and
has had also inhibitory effects on wave induction capac-
ity on the other hand. Colll expression pattern under
treatment has been signi cantly similar to expression
pattern of control gene (sox9).
Adjustment of data of electromagnetic wave and 5%
PRP has shown that both of them have inhibitory effects
on colX gene expression.
CONCLUSION
The results of the present study showed the ef cient and
logical use of PRP and electromagnetic waves in medi-
cine and tissue engineering. However PRP allow carti-
lage differentiation in the right format, this method need
further study and cannot decrease pathologic symptoms.
ACKNOWLEDGMENT
This research was an independent bachelors project sup-
ported by research affairs University of Tehran, Tehran,
Iran with cooperation of Mrs. Mahsa Laleh at Azad Uni-
versity of Science and Research Branch, Tehran, Iran.

Nariman Gharari and Mahsa Laleh
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES 585
REFERENCES
Agnes S. Klar, Jakub Zimoch, and Thomas Biedermann, Skin
Tissue Engineering: Application of Adipose-Derived Stem
Cells, BioMed Research International, vol. 2017, Article ID
9747010, 12 pages, 2017. doi:10.1155/2017/9747010
Alicja Zajdel, Magdalena Kałucka, Edyta Kokoszka-Mikołaj,
Adam Wilczok (2017). Osteogenic differentiation of human
mesenchymal stem cells from adipose tissue and Wharton’s
jelly of the umbilical cord. Acta Biochimica Polonica. 2017 no.
64 Issue 2 pp 365-369
Anitua, E., et al., (2007) The potential impact of the prepara-
tion rich in growth factors (PRGF) in different medical elds.
Biomaterials, 2007. 28(31): p. 4551-60.
Beata Kocan, Aleksandra Maziarz, Jacek Tabarkiewicz, (2017)
Trophic Activity and Phenotype of Adipose Tissue-Derived
Mesenchymal Stem Cells as a Background of Their Regenera-
tive Potential, Stem Cells International, Article ID 1653254, 13
pages doi:10.1155/2017/1653254
Bosnakovski, D., et al., (2006) Chondrogenic differentiation of
bovine bone marrow mesenchymal stem cells (MSCs) in dif-
ferent hydrogels: in uence of collagen type II extracellular
matrix on MSC chondrogenesis. Biotechnology and Bioengi-
neering, 93(6): p. 1152-1163.
Bruder, S.P., N. Jaiswal, and S.E. Haynesworth (1997), Growth
kinetics, self‐renewal, and the osteogenic potential of puri ed
human mesenchymal stem cells during extensive subcultiva-
tion and following cryopreservation. Journal of cellular bio-
chemistry, 1997. 64(2): p. 278-294.
Choi JR, Yong KW, Choi JY (2018). Effects of mechanical load-
ing on human mesenchymal stem cells for cartilage tissue
engineering. J Cell Physiol. 233(3):1913-1928. doi: 10.1002/
jcp.26018. Epub 2017 Jun 12. DOI: 10.1002/jcp.26018
Diduch, D.R., et al., (2000) Marrow stromal cells embedded in
alginate for repair of osteochondral defects. Arthroscopy: The
Journal of Arthroscopic & Related Surgery, 16(6): p. 571-577.
Elder, S. and J. Thomason (2014) Effect of Platelet-Rich Plasma
on Chondrogenic Differentiation in Three-Dimensional Cul-
ture. The Open Orthopaedics Journal. 8: p. 78-84.
Fukaya, M. and A. Ito, (2014) A New Economic Method for
Preparing Platelet-rich Plasma. Plastic and Reconstructive
Surgery Global Open, 2(6): p. e162.
Gugjoo, M. B., Amarpal, Sharma, G. T., Aithal, H. P., & Kin-
javdekar, P. (2016). Cartilage tissue engineering: Role of mes-
enchymal stem cells along with growth factors & scaffolds. The
Indian Journal of Medical Research, 144 (3), 339–347. http://
doi.org/10.4103/0971-5916.198724
Heo JS, Choi Y, Kim HS, Kim HO (2015). Comparison of molec-
ular pro les of human mesenchymal stem cells derived from
bone marrow, umbilical cord blood, placenta and adipose
tissue. Int J Mol Med. 2016 Jan;37(1):115-25. doi: 10.3892/
ijmm.2015.2413. Epub 2015 Nov 19.
Hunziker, E., Articular cartilage repair: basic science and clin-
ical progress. A review of the current status and prospects.
Osteoarthritis and cartilage, 2002. 10(6): p. 432-463.
Johnstone, B., et al. (1998) In Vitro Chondrogenesis of Bone
Marrow-Derived Mesenchymal Progenitor Cells. Experimental
cell research, 238(1): p. 265-272.
Judit Iván, Evelin Major, Adrienn (2017). The Short-Chain
Fatty Acid Propionate Inhibits Adipogenic Differentiation of
Human Chorion-Derived Mesenchymal Stem Cells Through
the Free Fatty Acid Receptor 2. Stem Cells Dev. December 1;
26(23): 1724–1733.Doi: 10.1089/scd.2017.0035
Lee, H.R., et al., (2012) Platelet-rich plasma loaded hydrogel
scaffold enhances chondrogenic differentiation and matura-
tion with up-regulation of CB1 and CB2. J Control Release,
159(3): p. 332-7.
Lei Tan, Bin Zhao, Fu-Tao Ge, Da-Hui Sun & Tiecheng Yu
(2017). Shockwaves Inhibit Chondrogenic Differentiation of
Human Mesenchymal Stem Cells in Association with Adeno-
sine and A2B Receptors. Scienti c Reports 7, Article number:
14377 (2017). doi:10.1038/s41598-017-14875-y
Liu Z, Yuan X, Fernandes G, Dziak R, Ionita CN, Li C, Wang
C, Yang S (2017). The combination of nano-calcium sulfate/
platelet rich plasma gel scaffold with BMP2 gene-modi ed
mesenchymal stem cells promotes bone regeneration in rat
critical-sized calvarial defects. Stem Cell Res Ther. 2017 May
25;8(1):122. doi: 10.1186/s13287-017-0574-6. DOI: 10.1186/
s13287-017-0574-6
Liu, X., Ren, W., Jiang, Z., Su, Z., Ma, X., Li, Y.,Yang, X. (2017).
Hypothermia inhibits the proliferation of bone marrow-
derived mesenchymal stem cells and increases tolerance to
hypoxia by enhancing SUM Oylation. International Journal of
Molecular Medicine, 40(6), 1631–1638. http://doi.org/10.3892/
ijmm.2017.3167
Maryna Bondarava, Chiara Cattaneo, Bin Ren, Wolfgang E. Tha-
sler, Volkmar Jansson, Peter E. Müller & Oliver B. Betz (2017).
Osseous differentiation of human fat tissue grafts: From tissue
engineering to tissue differentiation. Scienti c Reports volume
7, Article number: 39712 (2017). doi:10.1038/srep39712
Mobini, S., Leppik, L., Thottakkattumana Parameswaran, V., &
Barker, J. H. (2017). In vitro effect of direct current electrical
stimulation on rat mesenchymal stem cells. PeerJ, 5, e2821.
http://doi.org/10.7717/peerj.2821
Petrera, M., et al., (2011) Supplementation With Platelet-Rich
Plasma Improves the InVitro Formation of Tissue-Engineered
Cartilage With Enhanced Mechanical Properties. Arthroscopy.
29(10): p. 1685-1692.
Qian, Y., Han, Q., Chen, W., Song, J., Zhao, X., Ouyang, Y. Fan,
C. (2017). Platelet-Rich Plasma Derived Growth Factors Con-
tribute to Stem Cell Differentiation in Musculoskeletal Regen-
eration. Frontiers in Chemistry, 5, 89. http://doi.org/10.3389/
fchem.2017.00089
Ross, C. L., Siriwardane, M., Almeida-Porada, G., Porada, C. D.,
Brink, P., Christ, G. J., & Harrison, B. S. (2015). The effect of
low-frequency electromagnetic eld on human bone marrow
stem/progenitor cell differentiation. Stem Cell Research, 15(1),
96–108. http://doi.org/10.1016/j.scr.2015.04.009
Rubio-Azpeitia, E. and I. Andia, (2014) Partnership between
platelet-rich plasma and mesenchymal stem cells: in vitro

Nariman Gharari and Mahsa Laleh
586 DIFFERENTIATION OF HUMAN FAT MESENCHYMAL STEM CELLS USING ELECTROMAGNETIC WAVES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
experience. Muscles, Ligaments and Tendons Journal 4(1): p.
52-62.
S.G. Dubois et al. (2008) Isolation of Human Adipose-derived
Stem Cells from Biopsies and Liposuction Specimens 20 8
Seong Y, Moon J, Kim J. Life Sci. 2014 Apr 25; 102(1):16-27.
Epub 2014 Mar 3.
Solchaga, L.A., et al., (2006) A rapid seeding technique for
the assembly of large cell/scaffold composite constructs. Tissue
Engineering 12(7): p. 1851-1863.
Tan, A. R., & Hung, C. T. (2017). Mesenchymal Stem Cells for
Functional Cartilage Tissue Engineering: Taking Cues from
Chondrocyte-Based Constructs. Stem Cells Translational Medi-
cine, 6(4), 1295–1303. http://doi.org/10.1002/sctm.16-0271
Toshimi Michigami (2014) Current Understanding on the
Molecular Basis of Chondrogenesis Clin Pediatr Endocrinol.
2014 Jan; 23(1): p,1–8.
Vivian Alonso-Goulart, Lorraine Braga, Ferreira, (2017).
Mesenchymal stem cells from human adipose tissue and
bone repair: a literature review. Biotechnology Research and
Innovation. Available online 14 November 2017. https://doi.
org/10.1016/j.biori.2017.10.005
Wakitani, HJ et al. (2002) Human autologous culture expanded
bone marrow mesenchymal cell transplantation for repair of
cartilage defects in osteoarthritic knees. Osteoarthritis and car-
tilage 2002. 10(3): p. 199-206.
Williams, C.G., et al., (2003) In vitro chondrogenesis of bone
marrow-derived mesenchymal stem cells in a photopolymer-
izing hydrogel. Tissue Engineering, 2003. 9(4): p. 679-688.
Xu Q, Li B, Yuan L, Dong Z, Zhang H, Wang H, Sun J, Ge S,
Jin Y (2017). Combination of platelet-rich plasma within peri-
odontal ligament stem cell sheets enhances cell differentiation
and matrix production. J Tissue Eng Regen Med. 11(3):627-
636. doi: 10.1002/term.1953. Epub 2014 Sep 4. DOI: 10.1002/
term.1953
Yoo, J.U., et al., (1998) The Chondrogenic Potential of Human
Bone-Marrow-Derived Mesenchymal Progenitor Cells The
Journal of Bone & Joint Surgery 80(12): p. 1745-
Zhai L, Ma XL, Jiang C, Zhang B, Liu ST, Xing GY (2016)
Human autologous mesenchymal stem cells with extra-
corporeal shock wave therapy for nonunion of long
bones. Indian J Orthop [serial online] 2016 [cited 2018 Jan
28;50:543-50. Available from:http://www.ijoonline.com/text.
asp?2016/50/5/543/189602