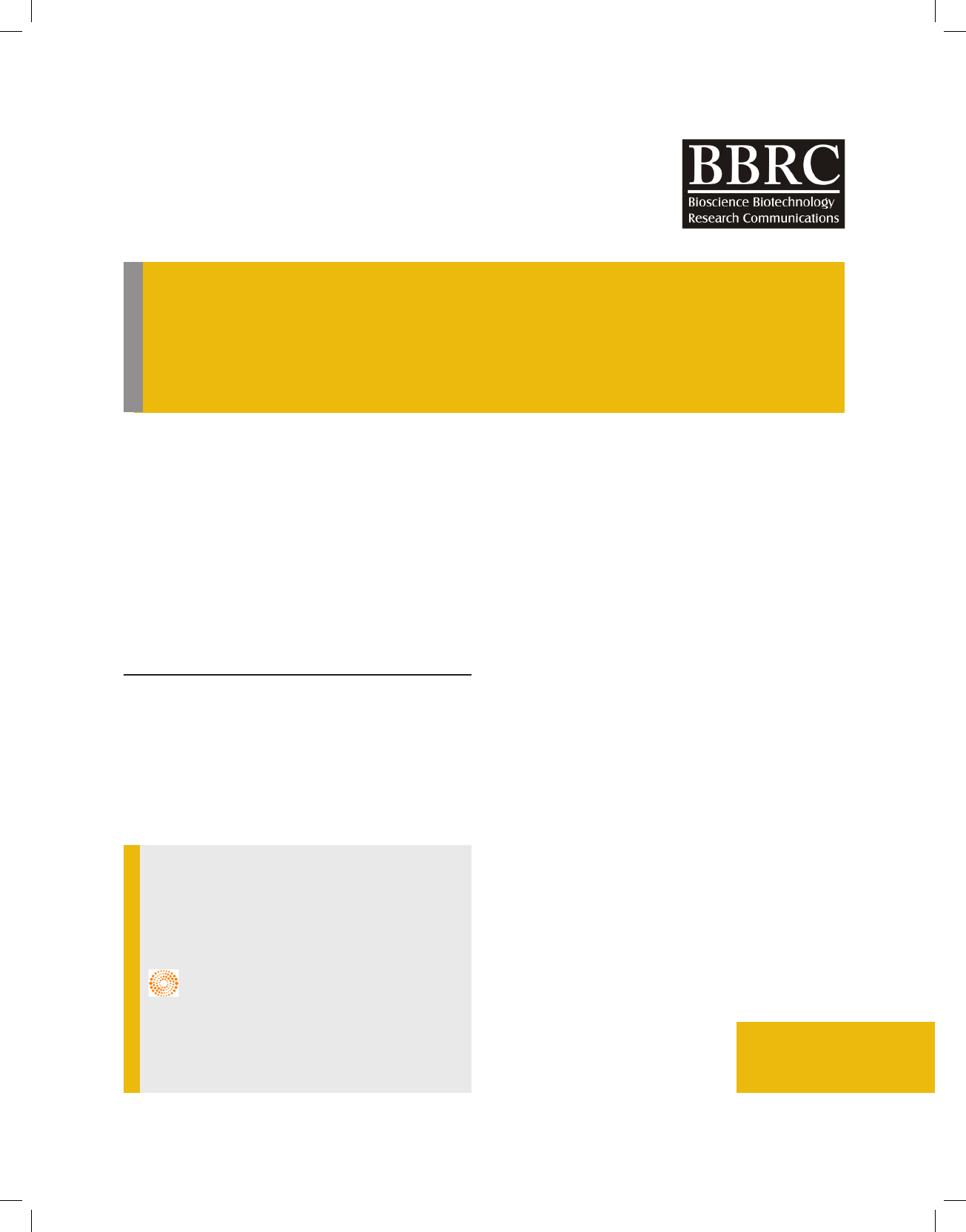
Characterization and continuous production of ethanol
using immobilized yeast cells
Kethineni Chandrika, S. F. Choragudi, Ravipati Srimukhi and Rusumdar Salma
Department of Biotechnology, Koneru Lakshmaiah Education Foundation, Guntur, India
ABSTRACT
In this study, characterization of the immobilized yeast cells for ethanol production by varying the initial sugar and
yeast cell concentration was performed. The results have indicated that the effective fermentation can be achieved
using 1% of the immobilized yeast cells with 10-12% of initial total reducing sugar concentration. The role of plant
and fungal based chemicals as activators were studied and the studies indicated that Chitin and Rhizopus Oryza bio-
mass has shown signi cant effects of increase in fermentation rate (47% and 23.94%) in free and immobilized cell
–activator systems respectively. Continuous ethanol production studies with immobilized yeast cells suggested that
the productivity can be improved up to 100% by reducing retention time. Also, these studies con rmed the reusability
of beads for up to 16 days without losing activity.
KEY WORDS: ETHANOL FERMENTATION, MOLASSES, IMMOBILIZATION, ACTIVATORS, REDUCING SUGAR
571
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(4): 571-576 (2018)
INTRODUCTION
The gradual depletion and the environmental deteriora-
tion resulting from the over consumption of petroleum
derived transportation fuels have gained great attention
across the world. Hence, it is necessarily important to
develop alternatives that are both renewable and envi-
ronmentally friendly. Ethanol is one of the most exotic
synthetic oxygen-containing organic chemicals. It has
unique properties as a fuel, a solvent, a beverage, a ger-
micide, an antifreeze that reduces greenhouse gas (GHG)
emission, lessens the dependence on fossil fuels, and
improves vehicle performance. It is generally manufac-
tured by three types of agricultural raw materials viz.,
saccharine materials, starchy materials and lignocel-
lulosic biomass using bacteria and yeast. Fermentation
using yeast and sugarcane molasses is one of the widely
used and ancient fermentation systems.
Despite the great advantages and importance, fuel
ethanol is not competing to cheaper oil derivatives
ARTICLE INFORMATION:
Corresponding Authors: kkchandrika@kluniversity.in
Received 1
st
Nov, 2018
Accepted after revision 21
st
Dec, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA
Mono of Clarivate Analytics and Crossref Indexed
Journal Mono of CR
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at:
http//www.bbrc.in/
DOI: 10.21786/bbrc/11.4/6

Kethineni Chandrika et al.
572 CHARACTERIZATION AND CONTINUOUS PRODUCTION OF ETHANOL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
because of its high production costs due to low pro-
ductivity. Hence, attempts cells, immobilization of whole
cells, vacuum fermentation and optimization of operat-
ing parameters to reduce production costs. Several stud-
ies show that ethanol productivity can be increased by
the addition of ergosterol (Patil, 1985, Kanellaki 1989,
Patil 1990, Jozef 2003, Giuliano, 2004, Kara. 2005) chi-
tin and polysaccharides (Neetu 2006), mineral kissiris
skim powder and fungal mycelium
Different working parameter condition shows the
optimal condition of initial sugar concentration, pH,
temperature, dilution rate and bead size on maximum
production of ethanol (Bardi,1994). Using continuous
immobilized yeast fermentation, microalgal hydrolysate
was converted into ethanol at a yield of 89% (Kyoung
2014) conversion of biomass into ethanol varies consid-
erably depending on the nature of feedstock primarily
due to the variation in biochemical composition, and so,
only a few feedstocks have been exploited commercially
(Zabed 2017).
In the present study, the effect of initial sugar concen-
tration, yeast cell concentration on an immobilized cell
system and the effect of different activators (saw-dust,
chitin and Rhizopus-Oryzae) on free and immobilized
cell system is studied. Continuous production of Ethanol
was done to study the effect of ow rate and the reus-
ability study. The reduction of bead size increased mass
transfer of substrates from the liquid to the immobilized
cells accelerating sugar consumption and ethanol pro-
duction (2018). Fermentation of sugar for production of
ethanol was carried out using Saccharomyces cerevisiae
cells immobilized in calcium alginate lms, (Leal 2018).
MATERIALS AND METHODS
MICROORGANISM
Commercially available fresh baker’s yeast was used for
experimentation. The culture was obtained as a cake.
The same was stored under 0-4
O
C temperature till it was
further used.
PREPARATION OF IMMOBILIZED YEAST BEADS
About 4g of sodium alginate was weighed and dissolved
in 100mL of boiling distilled water and made slurry. In
another beaker 8g of fresh baker’s yeast was dissolved in
distilled water. The two solutions were mixed and made
slurry. The nal volume was adjusted to 200mL with dis-
tilled water. The slurry was extruded drop wise into 0.2M
CaCl
2
solution with the help of a micro-tip connected to
silicone tubing, which passes through peristaltic pump.
The beads were left in calcium chloride solution over
night for curing. The beads were then taken out, washed
and stored till they were further used.
PREPARATION OF MOLASSES SOLUTIONS
Pre-activated yeast beads of 1% concentration were
taken in four conical asks (250mL), each containing
different concentrations of molasses solution (i.e., 6%,
10%, 12%, 16% and 20%). The activity of immobilized
yeast was observed for different time interval like 24,
48, 72, 96 and120 hours. The amount of total reducing
sugars was measured at each time interval.
PREPARATION OF YEAST CELL
CONCENTRATION
A series of batch experiments was conducted to test
the effect of yeast bead concentration on alcohol yield.
Yeast beads of various concentrations were taken (i.e.,
0.5%, 1%, 2%, 3%, 4%, and 5%) and activated for over-
night in the medium separately. Each of the beads was
then transferred to 12 percent molasses solution and the
reduction of total reducing sugars was estimated.
FERMENTATIONS WITH THE USE OF
ACTIVATORS
Experiment on external activator compounds were con-
ducted by using 1% of activators (i.e., chitin, sawdust
and Rhizopus-Oryzae biomass) with 1% free and immo-
bilized yeast cells in conical asks (250mL). The fermen-
tation was allowed for 10 hours. The amounts of total
reducing sugars were measured after every two hours.
EXPERIMENTAL SETUP FOR REACTOR
Initially, the fermentation was carried out batch wise
using immobilized yeast cells until the substrate was
depleted and was then switched to a continuous mode
with the feeding of molasses. Fresh molasses was contin-
uously supplied to the fermenter with a peristaltic pump
and at the same time cell free liquid was removed from
the bio-reactor through the lter module. As a whole,
the volume of broth in the fermenter was controlled at 5
liters by making the total ltrate ow (outlet) rate coun-
ter balance the total feed ow rate. The fermentation
was carried out at a room temperature.
ANALYTICAL METHODS FOR SUGAR AND
ETHANOL ESTIMATION
Amount of total reducing sugar was estimated by the
DNS method (Milller,1985). The ethanol concentration
was measured by gas chromatography (Thermo sher,
8610) using a chromosorb 101 column (80 to 100 mesh
of packing material, 1/8 inch outer diameter and 6 ft
long stainless steel tube) with ame ionization detector.
Temperatures of the detector, injector and column oven
Kethineni Chandrika et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS CHARACTERIZATION AND CONTINUOUS PRODUCTION OF ETHANOL 573
were 300
o
C, 200
o
C, and 150
o
C respectively. Nitrogen was
used as a carrier gas. The data reported for sugar con-
centration and percent alcohol were the mean value of
at least two independent samples analyzed in duplicate.
RESULTS AND DISCUSSION
EFFECT OF SUGAR CONCENTRATION OF
ETHANOL FERMENTATION RATE
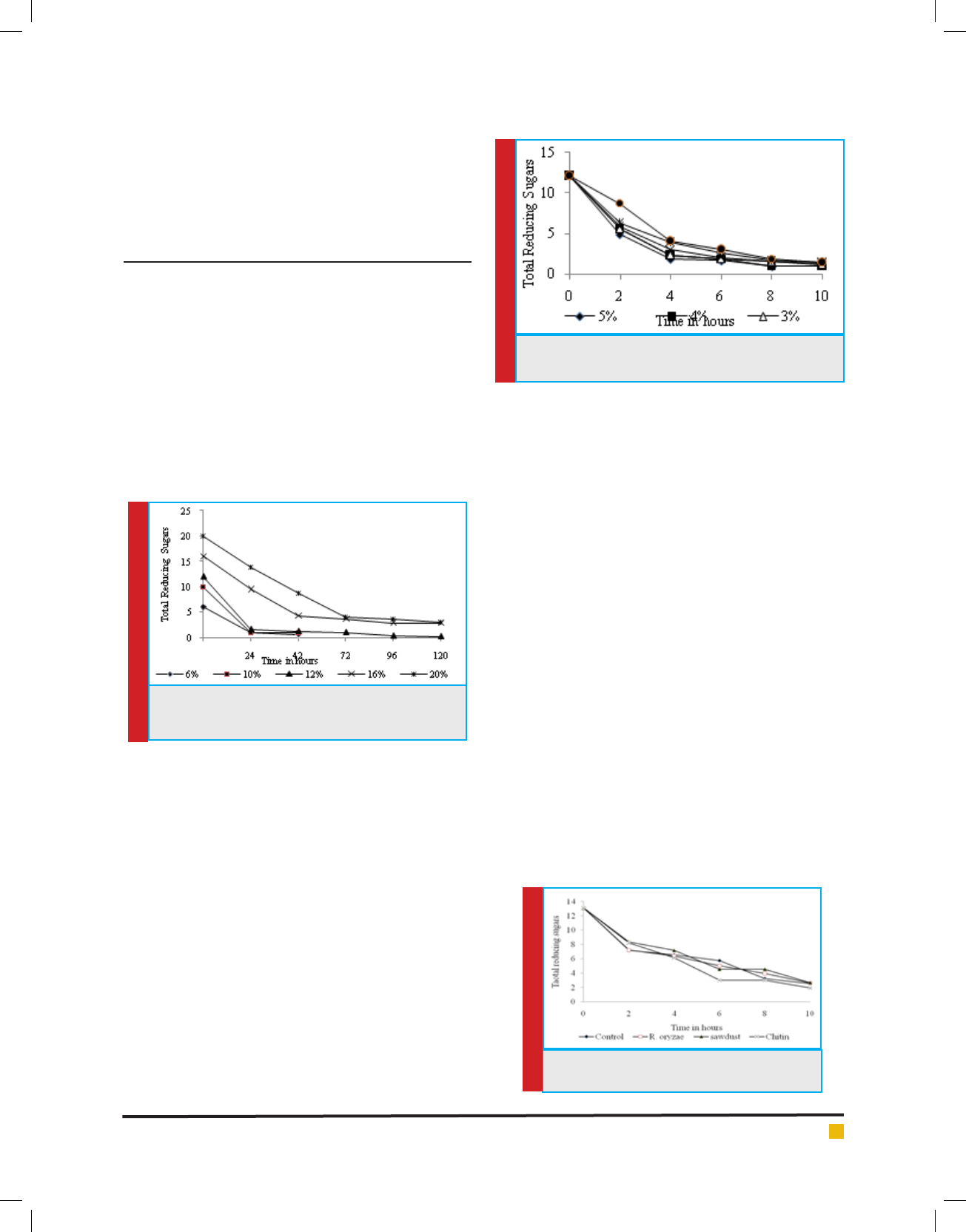
It was evident from the Fig.1 that total reducing sugar
level in the fermentation broth has in uence on the fer-
mentation rate. The maximum reduction in the amount
of total reducing sugar was observed after 24 hours of
the fermentation in the broth with 12 % TRS. As this
total reducing sugar level increased beyond 12 percent,
the ef ciency of the yeast fermentation has reduced,
indicating the high sugar concentration decreased the
fermentation rate.
observed to be increasing with decrease in amount of
immobilized yeast crystal concentration. In case of fer-
mentation with the 5 % immobilized yeast, the ferment-
able sugars were completely utilized within 4 h of fer-
mentation time.
The data also indicated that the effective fermenta-
tion can be achieved using 1% of immobilized yeast
crystals with 12% TRS level in the fermentation broth.
However, the economic feasibility in ethanol fermenta-
tion can be achieved with 5 percent yeast crystals. The
data acknowledge that 12% concentration broth is more
productive for ethanol production.
EFFECT OF ACTIVATORS ON THE
FERMENTATION RATE
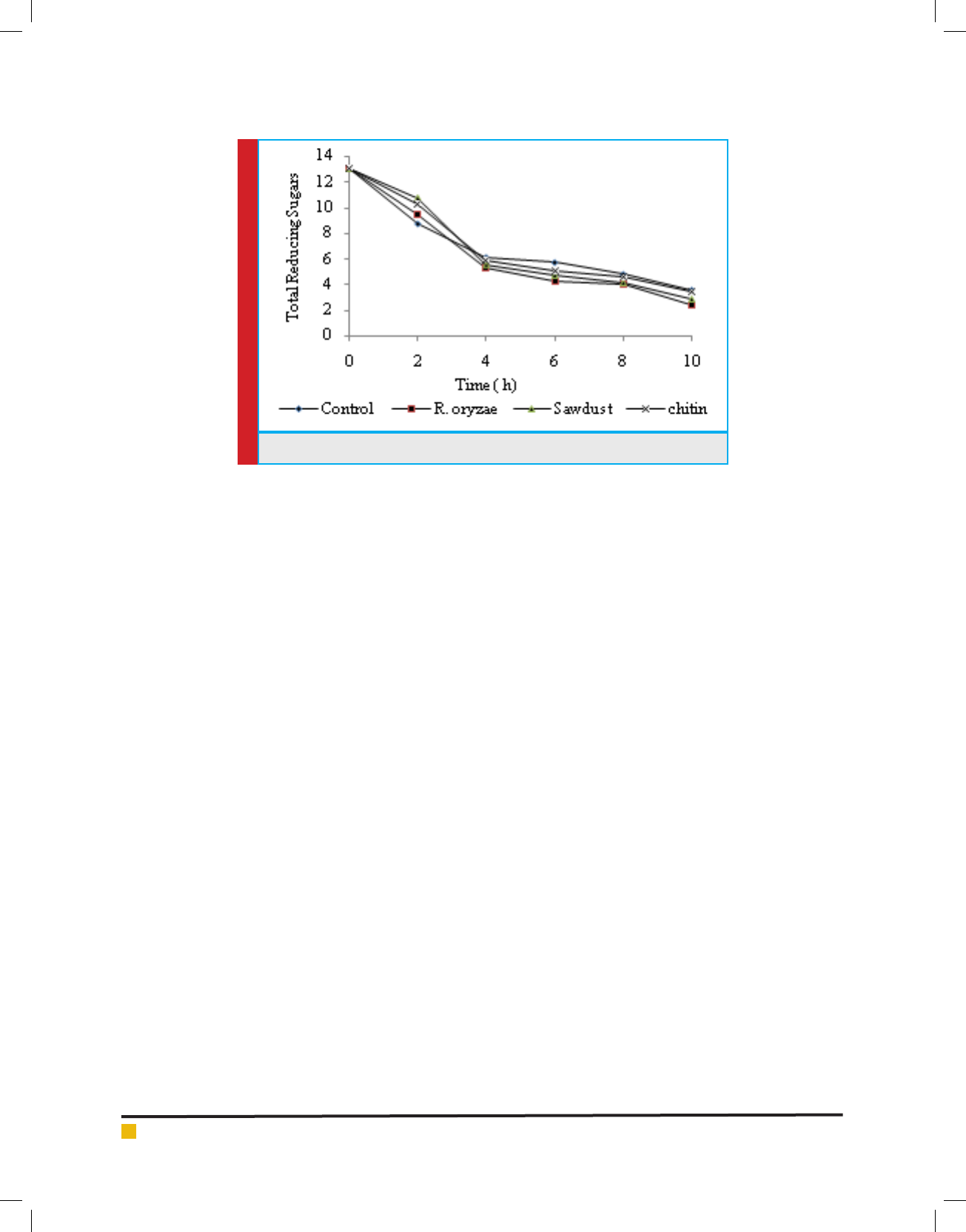
As evidenced from the ( gure 3), in the free cell sys-
tem it was observed that control has shown more effect
than activators for the initial 2 hours of fermentation
time however , the activator effect was observed start-
ing from 4 hours which was pronounced only at the
end of 6 hours of fermentation time where chitin sup-
plemented broth showed 47% increase in fermentation
rate followed by Sawdust (20.86%) and Rhizopus Ory-
zae (12.5%) over control where maximum sugars were
degraded.
FIGURE 1. Effect of initial sugar concentration on
ethanol fermentation rate.
FIGURE 2. Effect of immobilized yeast concentration on
ethanol fermentation rate.
The reduced sugar conversion with the higher con-
centration of molasses is probably due to osmotic effects
(Roukas 1994), decreased water availability and plas-
molysis of cells that leads to the growth inhibition of
cells. Though the initial fermentation rate was less in
16 and 20% broth than in 12 percent broth, it increased
with increase in fermentation time. The amount of TRS
left after 120 hours of fermentation was higher at 16%
and 20% broth than in 12% broth. This suggests that
the fermentation rate decreased with increase in alcohol
concentration.
EFFECT OF IMMOBILIZED YEAST
CONCENTRATION ON ETHANOL
FERMENTATION RATE
As evident from the Fig.2, the rate of fermentation
increased with increase in immobilized yeast concen-
tration. However, the left-over sugar concentration was
FIGURE 3. Effect of activators on the free cell
fermentation rate
Kethineni Chandrika et al.
574 CHARACTERIZATION AND CONTINUOUS PRODUCTION OF ETHANOL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
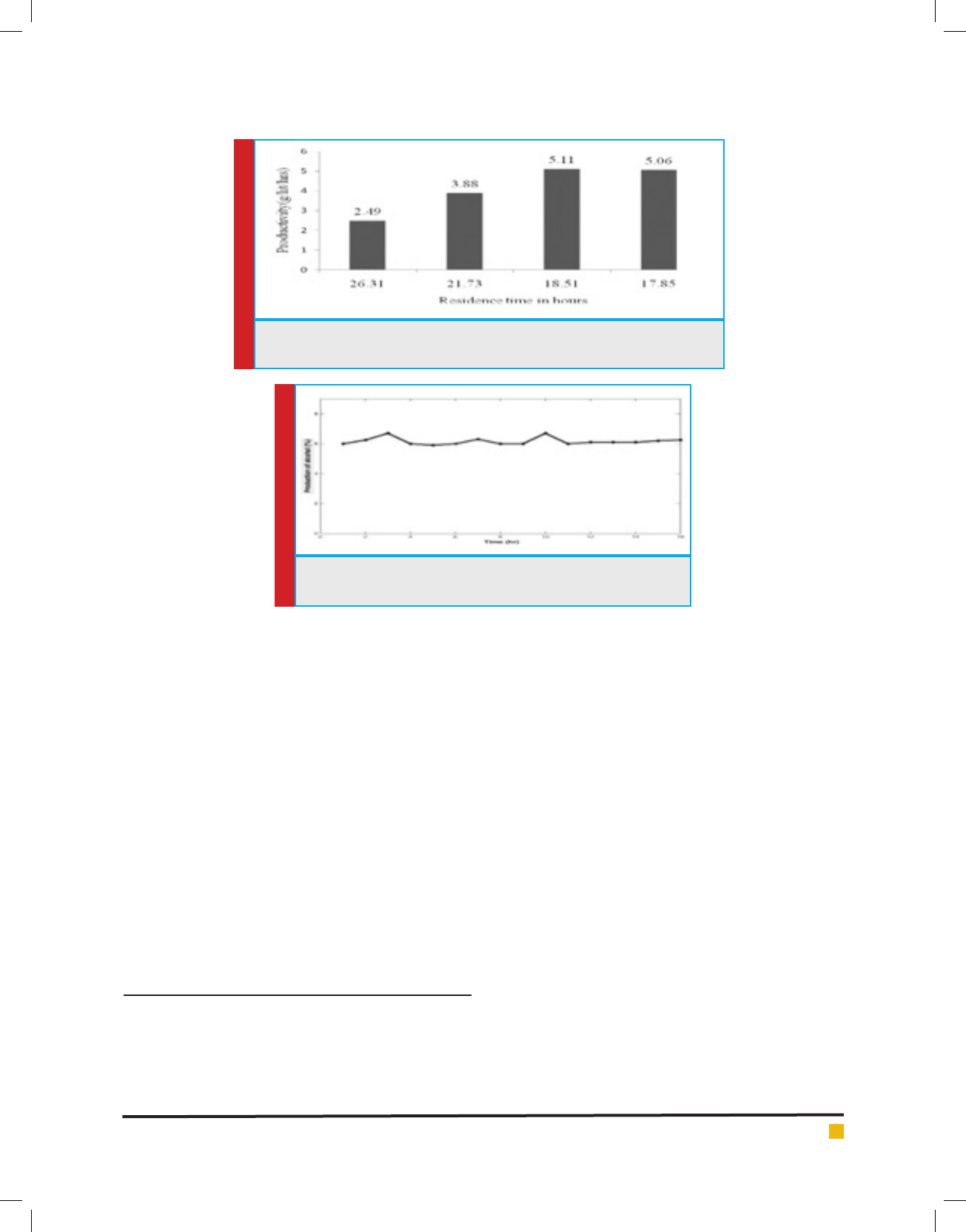
The effect of activators (Fig.4) on immbobilized cell
system shows a similar trend of free cell system was
repeated where control showed more effect than activa-
tors for initial 2 hours while the effect of activators was
observed starting after 4hours , however, unlike the free
cell system where chitin showed much effect here in this
case Rhizopus oryzae has shown signi cant effect, i.e., a
23.94 % increase in fermentation rate followed by saw
dust (16.37%) and Chitin (12.32%) with respect to control
after 6h where maximum sugars have been degraded
Chitin, a cell wall component of yeast increased the
ethanol production, which is probably not contributing
as a nutritional factor needed by yeast cells, but may
have released some nutritional factors that lead to the
partial hydrolysis of complex substances present in the
molasses or may be due to the removal of inhibitory
compounds. The increased in ethanol productivity due
to Sawdust is due to cells immobilized on diligni ed cel-
lulosic material that lead to an active bio-catalyst reduc-
ing the activation energy (Bardi, 1994). Besides, Saw-
dust may have enhanced the catalytic action of some
enzymes involved in the fermentation pathways or any
undesirable substances in molasses may be excluded by
their adsorption on the diligni ed materials.
Patil and Patil found the acceleration of ethanol pro-
duction activity of yeast in cane molasses fermentation
by the addition of fungal mycelium (Patil 1990). Besides,
brewery yield of ethanol was increased by the addition
of Proteo-lipid from Aspergillus and Oryzae promote
yeast growth and yeast durability against ethanol and
salt concentration.In the present work we hypothesize
that the activators in common may have released some
nutritional factors from other complex polysaccharides
(as molasses contain different polysaccharide beside
sugars) which degraded the sugar. This activator effect
is seen more pronounced only at lower, reducing sugar
concentrations (after 50% of initial sugar degradation)
which is clearly re ected in our work, where the increase
in fermentation activity has started at 4 hours of fer-
mentation time both in free and immobilized activator
system. This may probably due to less mass transfer
effect of activators in high sugar concentration.
CONTINUOUS PRODUCTION OF ETHANOL
USING IMMOBILIZED YEAST
To evaluate the sodium alginate- yeast beads for con-
tinuous production of ethanol, the experiments were
planned using 5-L reactor with an inlet and outlet. The
reactor was equipped with an overhead stirrer so as to
provide uniform mixing.
Initially the reactor was run in batch mode for 24h
there after the inlet feeding was initiated at a ow rate
of 190mL h-1 having residence time of 26.3 hours. The
productivity was observed to be 2.49g L-1 h-1. Once
the stabilization was achieved at this ow rate it was
increased so as to achieve the residence time of 21.73 h
( ow rate-230mL h-1). It was noticed that at this resi-
dence time the productivity was increased in the out-
let at the tune of 50%. The same was re ected by the
decrease in sugar concentration in the outlet. To evalu-
ate further, experiments was conducted at ow rates of
270mL h
-1
and 280mL h
-1
with residence time of 18.51 h
and 17.85 h respectively.
As evidenced from the gure 5, the productiv-
ity increased with increasing ow rate up to a certain
level (270mL h
-1
) beyond which the increased ow-rate
resulted in the decrease of productivity.
FIGURE 4. Effect of activators on immobilized cell fermentation rate
Kethineni Chandrika et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS CHARACTERIZATION AND CONTINUOUS PRODUCTION OF ETHANOL 575
FIGURE 5. Continuous production of ethanol using immobilized yeast cells at
different ow rates
FIGURE 6. Continuous production of ethanol using immobilized
yeast cells at a xed ow rate
Data indicated that critical dilution was achieved at
around (270mL h
-1
) having maximum speci c growth,
increase in ow rate might have led to washing out of
cells resulting in decreased productivity.
Based on the above data, the continuous production
of alcohol using immobilized yeast beads in a CSTR was
continued using at a ow rate of 190mL h
-1
(residence
time of 26.31h) to access the reusability of immobilized
yeast breads. The data were presented in the graph ( g-
ure 6). The results indicated that production of ethanol
ranged from 6-6.8%, depending on the initial TRS load-
ing.On an average, consistent ethanol production was
observed for more than 16 cycles (maximum cycles
studied). It was observed that the productivity levels
were varied as the fermentation time increased. A visual
observation indicated that a slower rate of fermentation
was observed in the molasses vessel because the medium
is not sterilized.
C ONCLUSION
Effective Fermentation can be made with 1% of Yeast
cell concentration and 12% of initial TRS loading. Plant
and Fungal based Activators were shown to signi -
cantly increase the fermentation Rate. Continuous pro-
duction of Ethanol showed an increase in productivity
up to 270mL h-1 beyond which showed reversed trend.
Continuous production of ethanol at a xed ow rate
showed the beads can be reused up to 16 cycles.
REFERENCES
Atiyeh, H., and Duvnjak, Z (2002) Production of fructose and
ethanol from sugar beet molasses using Saccharomyces Cerevi-
siae ATCC3658. Biotechnol. Progrs Vol.18: Page234.
Bardi, E., and Koutinas, A.A (1994) Deligni ed cellulosic mate-
rial supported Biocatalyst for room and low temperature wine
making. J. Agric. Food Chem. Vol.42: Pages 221- 226.
David Orrego (2018) Ethanol production from coffee mucilage
fermentation by S. cerevisiae immobilized in calcium-alginate
beads. Bioresource Technology Reports Vol. 3: Pages 200-204.
Eduardo Leal (2018) A novel method for bioethanol produc-
tion using immobilized yeast cells in calcium-alginate lms
and hybrid composite pervaporation membrane. Bioresources
Technology Vol. 247:Pages 165-173
Giuliano, D., Daniel, P. S., and João B. de Almeida e Silva
(2004) Factors in uencing ethanol production rates at high-
gravity brewing., Lebensmittel- Wissenschaft und-Technologie
Vol.37 No 7:Pages 797- 802.
Józef, S.N., and Ali, Al-K.Production (2003) Properties, and
Some New Applications of Chitin and Its Derivatives., Crit.
Rev. in Food Sci. and Nutr. Vol 43: Pages 145-171

Kethineni Chandrika et al.
576 CHARACTERIZATION AND CONTINUOUS PRODUCTION OF ETHANOL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Kanellaki,M., (1989) Ethanol production by Sacchromyces
Cerevisiae promoted by Y-alumina. Biotechnol. Bioeng Vol.
34:Pages 121-125.
Kara, J. T., David, E. B and Marjorie, L. L (2005) Elasticity
and Phase Behavior of DPPC Membrane Modulated by Choles-
terol, Ergosterol, and Ethanol., Biophys. J Vol. 89 No 4:Pages
2481–2493..
Kourkoutas, (2006) Effect of storage of immobilized cells at
ambient temperature on volatile by-products during wine-
making. J. FoodEng Vol.74 No 2:Pages 217-223.
Kyoung H G (2014) Bioethanol production from the nutri-
ent stress-induced microalga Chlorella vulgaris by enzymatic
hydrolysis and immobilized yeast fermentation, Bioresource
Technology,Volume 153,Pages 47-54.
Milller, G.L (1985) Use of DNS reagent for the determination of
reducing sugars. Anal. Chem. Vol.31: Pages 426-428
Neetu JL et al (2006) Biotechnological aspects of chitino-
lytic enzymes: A review. Appl.Microb.and Biotech Vol.71:
Pages773-782.
Patil, S.G., and Patil B.G (1990) Acceleration of ethanol pro-
duction activity of yeast in cane molasses fermentation by the
addition of fungal mycelium.Enzyme Microb. Technol Vol.12:
Pages 141-148.
Patil, S.G., Gokhale, D.V. and Patil, B.G (1985) Enhancement
in ethanol production from cane molasses by skim milk sup-
plementation. Enzy Microb. Technol Vol. 8 No8:Pages 481- 486
Roukas, T (1994) Continuous ethanol production from carob pod
extract by immobilize Saccharomyces Cerevisiae in a packed bed
Reactor. J.Chem. Technol.and Biotechnol. Vol. 59: Page 387
Zabed LM (2017) Bioethanol production from renewable sources:
Current perspectives and technological progress. Renewable and
Sustainable Energy Reviews,Vol. 71: Pages 475-501