Horticultural
Communication
Biosci. Biotech. Res. Comm. 11(3): 451-460 (2018)
Standardization of various factors for production of
adventitious roots in selected varieties of
Withania
somnifera
and estimation of total withanolides by High
Performance Liquid Chromatography
Sindhu Rangaraju, A.N. Lokesha and Chenna Reddy Aswath
Division of Floriculture and Medicinal Crops ICAR-Indian Institute of Horticultural Research, Hesaraghatta
Lake Post, Bangalore-89
ABSTRACT
Withania somnifera (Dunal) popularly known as Ashwagandha, “Winter Cherry” and “Indian Ginseng”. Its roots and
leaves are used in a number of preparations for their anti-in ammatory, anticonvulsive, antitumor properties besides
promoting vigour and stamina. Ashwagandha contains very high concentration of metabolites like steroidal lactones
(Withanolides), alkaloids and avonoids, so it is used in more than 200 commercially ayurvedic formulations. The
annual requirement of Withania sominifera in India is about 9127 MT where as the estimated production in India is
only 5905 MT. This requirement can be met by mass cultivation of adventitious roots using bioreactors. Adventitious
roots induced by this form are considered to be genetically uniform, true to its type that gives rise to mass production
of desired pharmaceutical compound. Seeds of varieties like Jawahar Ashwagandh-20 (JA-20), Arka Ashwagandha
(AA), IIHR WS-48 and IIHR WS-32 have been raised in in-vitro conditions. Adventitious roots were induced from
in-vitro leaves by varying factors. Half strength MS medium yielded more roots than full strength MS medium, com-
bination of IAA and IBA (ranging from 0.025-0.01mg/l) were found to be ideal for adventitious root induction for
each variety. Sucrose concentration (3-4%) in half strength MS media yielded more adventitious roots, with a light
intensity of 16 hours photoperiod than darkness.
KEY WORDS:
WITHANIA SOMNIFERA
(DUNAL), IAA, IBA, HIGH PERFORMANCE LIQUID CHROMATOGRAPHY
451
ARTICLE INFORMATION:
*Corresponding Author: sindhurangaraju63@gmail.co.in
Received 1
st
July, 2018
Accepted after revision 19
th
Sep, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.3/14

452 STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Sindhu, Lokesha and Aswath
INTRODUCTION
Withania somnifera (Dunal) popularly known as Ash-
wagandha, Indian ginseng and Winter cherry belongs
to the family Solanaceae. The plant’s Latin name liter-
ally means, “sweat of a horse” due to the scent of the
roots. The plant is commonly found in Africa, Mediter-
ranian, India and North America. It is an erect branched
under shrub up to 1.25 m in height, minute, smooth
and shiny hairs throughout the plant. Leaves are ovate,
with hairs and soft to touch. Flowers are greenish, the
roots are eshy, papery and whitish brown in color. The
stems are around 3 to 4 feet in height. One plant sur-
vives for up to 4 to 5 years. Its stem contains ber like
texture. The leaves are oval shaped, 2 to 4 inches long
and contain ber. The owers are blooming at the base
of the stems are small, somewhat long with chimney
shape and yellowish green in color. The owers bloom
from the base of the leaves and become red when wipe.
The seeds are small, heart shaped, smooth and at. The
roots are rough, white from within, strong, transparent,
thick and one to one and half feet long, (Geetha et al.,
2018).
In Ayurveda and Unani medications the herb is
majorly used for its high rejuvenating power. It is called
as “The natural stress buster” due to its ability in mak-
ing the human body copes with different kinds of stress
(Rao, 2012). The roots of Ashwagandha help in boost-
ing immunity power of the body. It is commonly pre-
scribed for hiccup, bronchitis, dropsy, rheumatism and
female disorders, the roots of this plant also prescribed
for general sexual weakness in human beings (Kattimani
et al., 2000). Its roots and leaves are used in a number
of preparations for their anti-in ammatory, anticonvul-
sive, antitumor, immuno-suppressive and antioxidant
properties besides promoting vigor and stamina. Ashwa-
gandha is increasingly becoming a popular adaptogenic
herb and is available throughout the western world as
a dietary supplement. Ashwagandha contains very high
concentration of metabolites like steroidal lactones
(Withanolides), alkaloids and avonoids, so it is used
in more than 200 commercially ayurvedic formulations.
Adventitious roots are the roots that are induced at
unusual sites such as roots forming on leaves, which
grow and branch rapidly (Dubrovsky and Rost., 2003).
The roots induced by this form are considered to be
genetically uniform, true to its type, that gives rise to
mass production of desired pharmaceutical compound
(Goel et al ., 2009). Adventitious root cultures provide a
preferred platform to produce commercially important
secondary metabolites (Khan et al 2017). Adventitious
roots can harbor medicinally important compounds
through different strategies like elicitation, temperature
stress etc., (Rani et al., 2017).
The technique of micro propagation is applied with the
objective of enhancing the rate of multiplication. Through
the culture over a million of plants can be grown from a
small piece of plant tissue within 12 months. Such prolif-
erative rate of multiplication cannot be expected by any
in-vivo methods. Large scale production through plant
in-vitro regeneration will provide a means of putting
the plant onto the market at lower prices. In addition,
the technique is cost effective, relatively simple and can
be performed by semi-skilled persons. A sustained sup-
ply of the source material often becomes dif cult due to
the factors like environmental changes, cultural practices,
diverse geographical distribution, labour cost, selection of
the superior plant stock, over exploitation by pharmaceu-
tical industry (Kaur et al 2017)
Optimization of various tissue culture techniques
become very important to explore W. somnifera at
different aspects, as plants obtained from elds fileds
are not enough for all in vitro studies. Therefore, eff-
cient tissue culture techniques like, micropropaogation,
regeneration, organogenesis, hairy root production, etc.
have been established, (Vibha pandey et al. 2017).
The requirement of dried plant material for witha-
nolides drug production in India is estimated to be 9127
tonnes against the annual production of 5905 tonnes
(Sharda et al., 2007). Moreover, eld cultivation is time
consuming, laborious and not able to meet the Ashwa-
gandha global market requirement (Sivanandan et al.,
2012b; 2013a). To improve the commercial cultivation of
Ashwagandha, biological advances must be made that
should either increase yield or reduce time gap to assure
quality (Banerjee et al., 1994).
The provision of alternative sources of Withania
somnifera by encouraging its cultivation will go a long
way in reducing their heavy dependence on the wild
populations and also major diseases of plant like seed
rot and blight can be overcome. The main objective of
this research is to develop a reproducible protocol for
adventitious root induction from in-vitro leaves, and
comparative analysis of withanolides present in in vitro
adventitious roots roots of different varieties and selec-
tion of best variety for mass propagation in bioreactors.
MATERIALS AND METHODS
Selection and establishment of plant material for micro-
propogation.
Seeds of four high yielding varieties of Withania som-
nifera like JA-20 (released variety from MPKVV, Madhya
Pradesh) used as check in AICRP national trials, Arka Ash-
wagandha (released variety from IIHR, Bangalore), IIHR
WS-32 and IIHR WS-48 were selected for the experiment.
The seeds were soaked in 300 ppm of gibberellic acid
for 12 hours and washed with water. The seeds were
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS 453
Sindhu, Lokesha and Aswath
pre-treated using 100 mg Dithane M-45 fungicide for 15
minutes, washed with sterile water followed by 70%(v/v)
ethanol for 1 minute, washed with sterile water and then
with 0.1% (w/v) sodium hypochlorite(5% (w/v) avail-
able chlorine, NICE Kochi Solution) for 4 minutes. Then
seeds were washed with sterile double distilled water
2-3 times to remove traces of sodium hypochlorite and
dried. The seeds were then inoculated into half strength
MS medium (Murashige and Skoog , 1962).
Procedure for adventitious root induction
Leaves from two month old explants were taken for pro-
duction of adventitious roots, before inoculation, leaves
were cut in middle with sterilized scalpel and placed on
MS culture medium with the adaxial surface down. Dif-
ferent factors were varied–
1. Strength of medium: Half strength and full strength
MS medium with selected combination of auxins
were used to study adventitious root induction.
2. Sucrose concentration: Carbohydrate source plays
an important role in maintaining osmoticum in
plant tissue culture. Root initiation and develop-
ment is a high energy process which requires the
expense of available metabolic substrates such as
sugars. Sucrose at different concentrations (2-7%)
were tried.
3. Auxin treatment: In order to determine the opti-
mal conditions for adventitious root induction, we
tested various concentration of auxins (IAA and
IBA) ranging from 0.025-0.01mg/L in 5 different
combinations and compared it with control (with-
out IAA and IBA).
4. Light intensity: The cultures in half strength MS
medium with selected auxin combination were in-
cubated at 25±2°C with 16 hours photoperiod un-
der cool uorescent light and for dark treatment
the bottles were placed in shelves without light.
The adventitious roots were observed after 15 days
and parameters like number of roots per explant
and percentage of explants response for root
induction were studied.
The roots were subjected for HPLC analysis to esti-
mate the total withanolide content.
5. Extraction of bioactive principles from W.somnif-
era
The adventitious roots extracted from in-vitro were
washed twice with milli-Q water to remove the traces
of agar, dried and powder dried using pestle and mortar.
They were assessed for different components that contrib-
ute to total withanolides. The analysis was carried out by
HPLC method (Agarwal and Murali, 2010). Two grams of
dry root powder was extracted with 50 mL of methanol
on boiling water bath for about 20 minutes and transfer
the extract to a 250 mL beaker. Repeat the process 3-4
times till the extract was colorless. Then collected all the
extracts and made up the volume to 100mL with metha-
nol, mixed well and ltered through 0.45 micron mem-
brane lter and these were subjected to analysis by HPLC
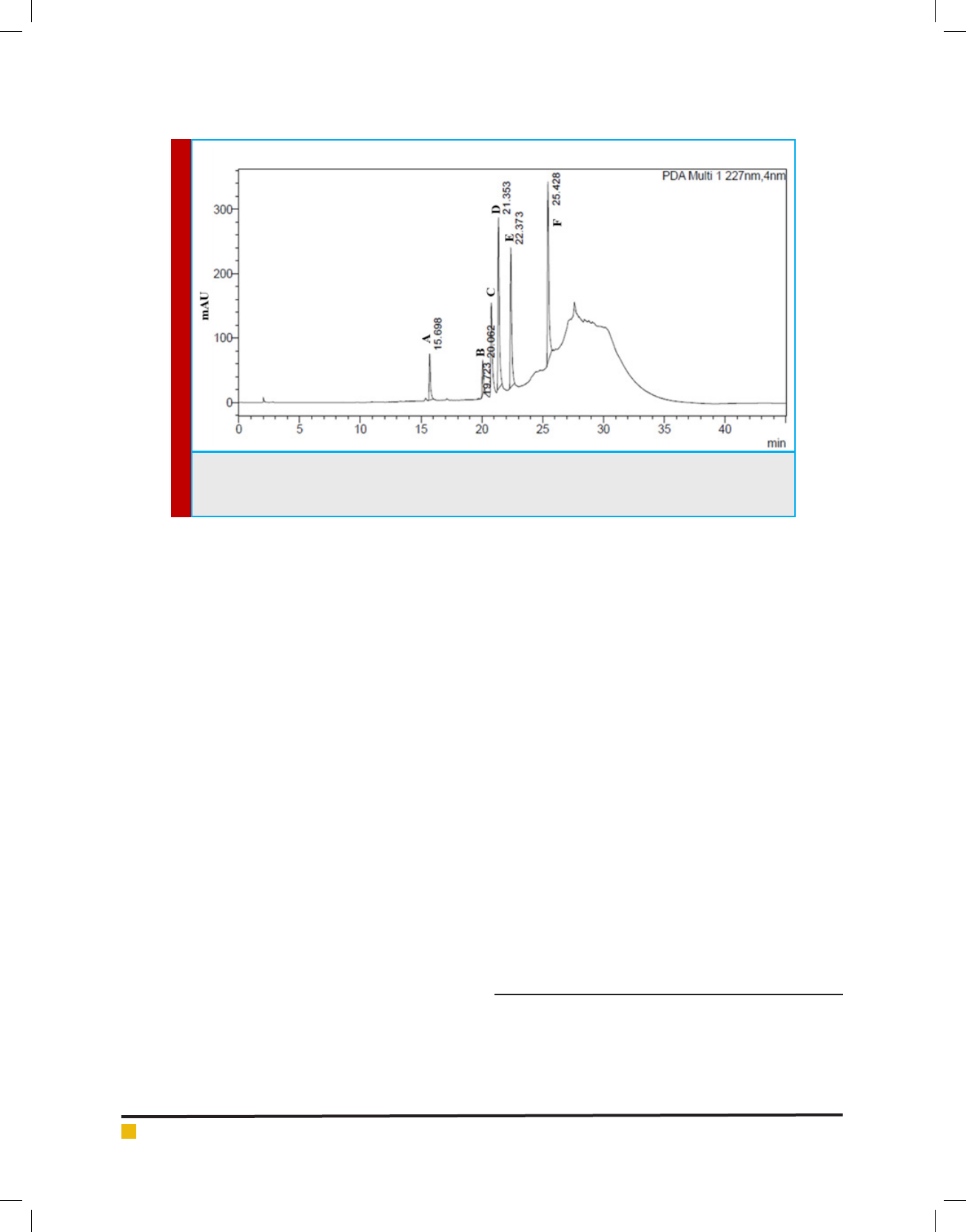
with Photo Diode Array detector. Seven standards such
as Withanoside IV, Withanoside V, Withaferine A, Witha-
nolide A, Withanolide B, 12- deoxy Withanostramolide
and Withanone were used to quantify the amount of vari-
ous withanolides present in the root samples. Chromato-
gram was recorded at 227nm wavelength and later calcu-
late the contents of individual withanolides by the using
the formula and expressed as mg/100g dry weight basis.
Area of the sample
Standard Wt. (mg)
Sample dilution
Purity of standard
--------------------------
100
Area of the standard
Standard dilution
sample weight (mg) 100
RESULTS AND DISCUSSION
The results from the present study demonstrated that
standardization of different factors like strength of the
medium, effect of photoperiod, sucrose concentration
, combinations of auxins at different concentrations
is essential for effective adventitious root induction in
Withania somnifera.
In this study to determine the effects of media
strength half strength MS medium with a combination
of 0.25mg/l IAA and 0.75 mg/l IBA had higher number
of induction response and also higher number of roots
per explants (Table no 1 and 2). The results of the pre-
sent study is similar to results of the previous studies
on adventitious root induction in Withania somnifera
by Wadegaonkar et al (2006) and Praveen and Murthy
(2010), where half strength MS medium was chosen suit-
able for adventitious root induction. It contradicts with
Yin et al (2013) induced adventitious roots in Pseudos-
tellaria heterophylla in full strength MS medium with
3mg/L IBA using root explants.
Highest root induction response (98.2% in JA 20)
and highest number of roots per explant (16 roots in
WS 32) was observed in bottles placed under 16 hours
photoperiod compared to darkness in all the varieties of
Withania somnifera (Table 3 and 4). Explants incubated
under darkness induced profuse callusing which subse-
quently turned brownish and hindered the induction of
roots. Roots initiated were thick and longer in braches
under 16 hours photoperiod compared to thin and brittle
roots in darkness.
Sindhu, Lokesha and Aswath
454 STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
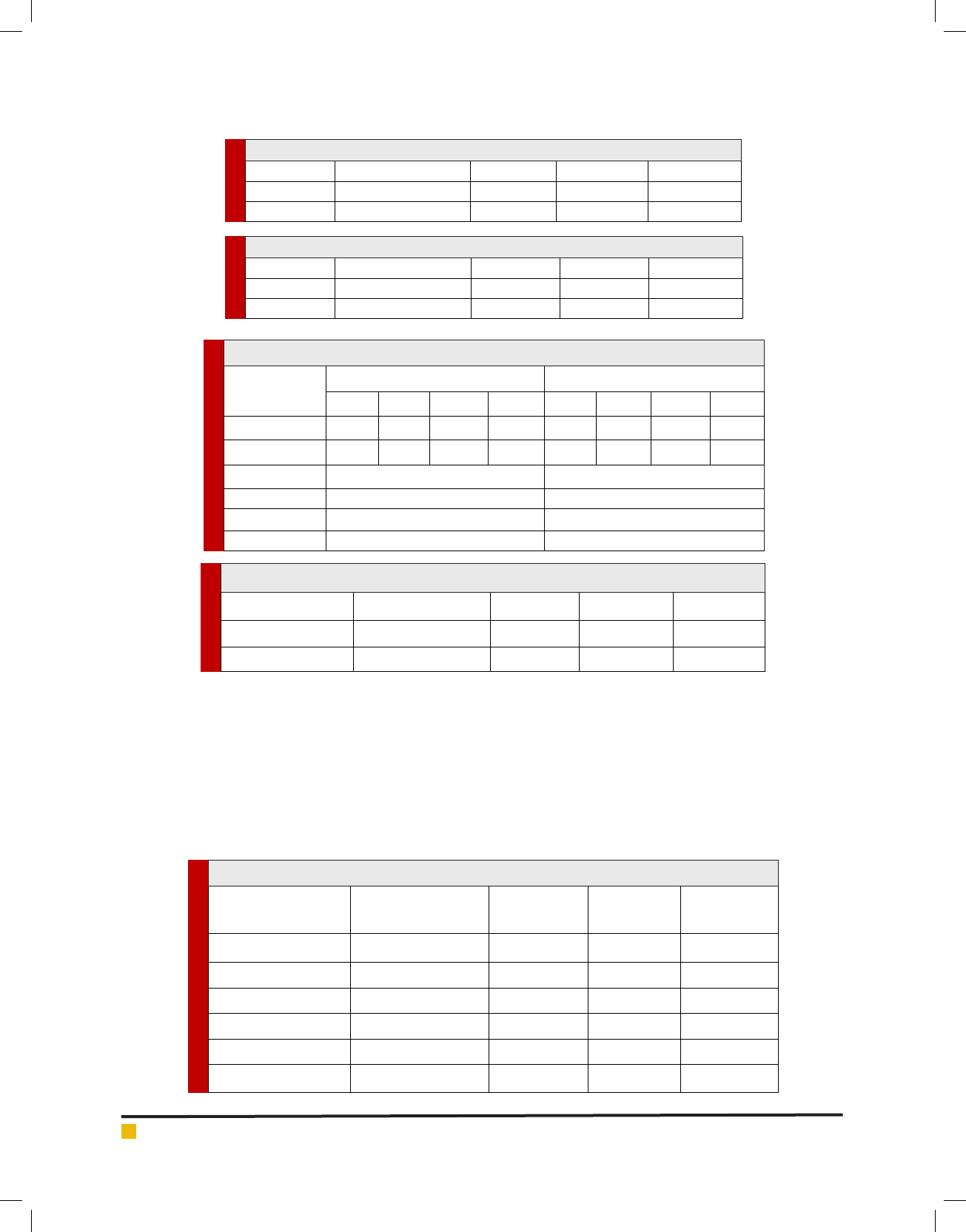
Table 1. Effect of strength of the medium on the induction response (%)
Treatment Arka Ashwagandha JA 20 IIHR WS 32 IIHR WS 48
Half MS 98.00±0.173a 98.00±0.520a 98.60±0.387a 97.40±0.316a
Full MS 95.4±0.173b 96.20±0.520b 96.40±0.387b 96.40±0.316b
Table 2. Effect of strength of the medium on number of roots per explant
Treatment Arka Ashwagandha JA 20 IIHR WS 32 IIHR WS 48
Half MS 13.80±0.070a 13.60±0.173a 14.20±0.173a 12.60±0.141a
Full MS 13.20±0.070b 13.20±0.173b 13.80±0.173b 12.40±0.141b
Table 3. Effect of photoperiod on adventitious root induction
Treatments
Induction Response (%) No of roots per explant
AA JA 20 WS 32 WS 48 AA JA 20 WS 32 WS 48
16 hr photoperiod 97.8 98.2 97.6 97.6 13.5 15.25 16 16.45
Darkness 91.8 91.6 92 91.8 5.1 5.1 5.05 5.6
S.Em 0.2151 0.245
CD 5% 0.619 1.75
CD 1% 0.81 2.36
CV 0.717% 4.69%
Table 4. Effect of photoperiod on number of days taken for adventitious root induction
Treatment Arka Ashwagandha JA 20 IIHR WS 32 IIHR WS 48
16 hours Photoperiod 24.00±0.316a 26.00±0.346a 26.60±0.141a 26.00±0.346a
Darkness 15.00±0.316b 14.80±0.346b 15.80±0.141b 14.80±0.346b
Table 5. Effect of Sucrose concentration on adventitious root induction response (%)
Treatments (Induction
response %)
Arka Ashwagandha JA 20 WS 32 WS 48
2% Sucrose 88.00±0.397b 82.40±0.465 b 80.40±0.389 b 88.20±0.499b
3% Sucrose 96.60±0.397a 86.80±0.465a 85.80 ±0.389a 98.40±0.499a
4% Sucrose 79.40±0.397c 78.60±0.465c 75.40±0.389c 77.00±0.499c
5% Sucrose 61.40±0.397d 61.60±0.465d 61.20±0.389 d 61.00 ±0.499 d
6% Sucrose 33.60±0.397e 35.60±0.465e 31.60±0.389 e 34.60±0.499 e
7% Sucrose 10.20±0.397f 11.00±0.465f 10.20 ±0.389f 10.60 ±0.499f
Praveen and Murthy (2010) also established adventi-
tious roots from leaf segments of Withania somnifera on
half strength MS medium(0.8%) agar with 0.5mg/L IBA,
30g/L sucrose incubated under 16 hours photoperiod
with 100% of explants response for root induction.
Table 1- Values are mean ± standard error of ve
replications in three independent experiments, each
with three explants per treatment. Means followed by
the same letter are not signi cantly different at P<0.05
according to Duncan multiple range test in all tables
Data were scored after15 days of culture
Table 2- Values are mean ± standard error of ve
replications in three independent experiments, each
with three explants per treatment. Means followed by
the same letter are not signi cantly different at P<0.05
according to Duncan Range Multiple Test. Data were
scored after 15 days of culture

Sindhu, Lokesha and Aswath
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS 455
Table 6. Effect of Sucrose concentration on number of adventitious roots per
explant
Treatments Arka Ashwagandha JA 20 WS 32 WS 48
2% Sucrose 11.40±0.319c 11.80±0.331c 10.60±0.294c 11.80±0.261c
3% Sucrose 17.60±0.319a 17.20±0.331a 15.00±0.294a 17.20±0.261a
4% Sucrose 12.80±0.319b 14.00±0.331b 12.40±0.294b 14.20±0.261b
5% Sucrose 8.80±0.319d 10.20±0.331d 9.8 ±0.294c 10.40±0.261d
6% Sucrose 7.40±0.319e 7.60±0.331e 8.60±0.294e 7.40±0.261e
7% Sucrose 5.40±0.319 f 5.60±0.331f 5.20±0.294e 5.40±0.261f
Table 7. Effect of auxins on adventitious root induction response (%)
Treatments Arka Ashwagandha JA 20 WS 32 WS 48
0 IAA+0 IBA 0.000±0.284f 0.000±0.330f 0.000±0.614f 0.000±0.703
0.25 IAA+0.75 IBA 96.60±0.284a 97.20±0.330a 85.80±0.614b 98.40±0.703
0.5 IAA+0.5 IBA 80.80±0.284c 80.60±0.330c 80.40±0.614c 82.20±0.703
0.75 IAA+0.25 IBA 76.80±0.284d 77.40±0.330d 75.60±0.614d 75.00±0.703
0 IAA+1 IBA 83.6±0.284 b 82.40±0.330b 97.6±0.614a 85.60±0.703
1 IAA+0 IBA 72.4±0.284e 68.00±0.330e 67.80±0.614e 65.00±0.703e
Table 8. Effect of auxins on number of adventitious roots per explant
Treatments Arka Ashwagandha JA 20 WS 32 WS 48
0 IAA+0 IBA 0.000±0.257d 0.000±0.245e 0.000±0.238f 0.000±0.371e
0.25 IAA+0.75 IBA 17.60±0.257a 17.20±0.245a 15.00±0.238b 17.20±0.371a
0.5 IAA+0.5 IBA 13.80±0.257b 13.80±0.245b 14.20±0.238c 12.20±0.371c
0.75 IAA+0.25 IBA 11.60±0.257c 11.60±0.245c 11.60±0.238d 13.00±0.371bc
0 IAA+1 IBA 13.60±0.257b 14.40±0.245b 17.40±0.238a 13.60±0.371b
1 IAA+0 IBA 11.40±0.257c 9.20±0.245d 8.80±0.238e 9.80±0.371d
Table 3- Values are mean ± standard error of ve
replications in three independent experiments, each with
three explants per treatment. Data were scored after 15
days of culture. Growth Conditions- Media- Half MS
supplement with selected auxin combination, photoper-
iod-16 hours, culture period-3 weeks at 25±2°C
Table 4- Values are mean ± standard error of ve
replications in three independent experiments, each
with three explants per treatment. Means followed by
the same letter are not signi cantly different at P<0.05
according to Duncan Range Multiple Test. Data were
scored after 15 days of culture. Growth Conditions-
Media- Half MS supplement with selected auxin com-
bination, photoperiod-16 hours and complete darkness,
culture period-3 weeks at 25±2°C
Carbohydrate plays an important role in maintain-
ing osmoticum in plant tissue culture. Sucrose is con-
sidered as an unquestionably important carbon and
energy source which is found in abundance in phloem
sac involved in developmental process. From the obser-
vations of the above study, the optimal condition for
adventitious root induction in Withania somnifera was
half strength MS medium with 3% sucrose concentra-
tion, (Table 5 and 6).
It has been documented earlier that a sucrose concen-
tration (3%) was suitable for hairy root growth, whereas
a too low or too higher a concentration of sucrose
was adverse to adventitious root growth inW. somnif-
era(Sivanandhan et al. 2012 a). A lower concentration
cannot provide enough energy and therefore may not be
able to act as building blocks. However, higher sucrose
concentration exhibited negative effect in growing cells.
Nagella and Murthy (2010) recorded that 3 % sucrose
was suitable for biomass accumulation and withanolide
A production in cell suspension culture ofW.somnifera.
Sucrose at higher concentrations in the nutrient medium
normally reduces cell biomass due to the increase of
osmotic potential which subsequently reduces the
uptake of nutrients. A similar result was obtained by
Zhang et al. (2012) inPeriploca sepiumadventitious root
culture and by Sivanandhan et al. (2012 a) inW. somnif-
eraadventitious root and hairy root cultures.

Sindhu, Lokesha and Aswath
456 STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
The lowest induction response was observed in 7%
sucrose concentration, from this result it was noticed
that the number of roots per explant started decreasing
at a higher root concentration of 5% and above. Higher
amount of sucrose can retard the development of cul-
tured cells (Wu et al., 2006) by causing cessation of the
cell cycle when other nutrients are limited (Gould et al.,
1981).
Table 5- Values are mean ± standard error of ve
replications in three independent experiments, each
with three explants per treatment. Means followed by
the same letter are not signi cantly different at P<0.05
according to Duncan Range Multiple Test. Data were
scored after15 days of culture. Growth Conditions-
Media- Half MS supplement with selected auxin com-
bination, photoperiod-16 hours, culture period-3 weeks
at 25±2°C
Table 6- Values are mean ± standard error of ve
replications in three independent experiments, each
with three explants per treatment. Means followed by
the same letter are not signi cantly different at P<0.05
according to Duncan Range Multiple Test. Data were
scored after15 days of culture. Growth Conditions-
Media- Half MS supplement with selected auxin com-
bination, photoperiod-16 hours, culture period-3 weeks
at 25±2°C.
Development of roots or shoots from explants involved
in organogenesis depends on morphogenetic potentiality
of the cells. Dedifferentiation, induction of organogenesis
pathway and development of organ are the three distinct
stages during organogenesis (de Kler et al., 1997). Supple-
mentation of exogenous auxin is essential for adventitious
root development (Pop et al., 2011). IAA and IBA induced
adventitious roots from leaf explants after 12 days of cul-
ture. Protuberances developed in leaf explants within a
week from the cut ends and adventitious roots directly
developed from these protuberances in another week. The
percentage of explants response for root induction and
number of roots initiated per explants were recorded after
3 weeks of culture.
Table 9. Analysis of bioactive principles from W. sominifera using HPLC.
Variety names Withanoside IV Withaferin A Withanolide A Withanolide B Total Withanolide
Arka Ashwagandha 0.061±0.001 a 0.023±0.000a 0.000±0.000b 0.000±0.000b 0.084±0.001a
JA 20 0.018±0.001d 0.018±0.000c 0.002±0.000a 0.005±0.000a 0.043±0.001d
IIHR WS 32 0.0034±0.001b 0.022±0.000b 0.000±0.000b 0.000±0.000b 0.057±0.001b
IIHR WS 48 0.030±0.001c 0.017±0.000b 0.000±0.000b 0.000±0.000b 0.047±0.000c
FIGURE 1A. 1) 2 month old explant of Withania somnifera , 2) culturing of
leaves for adventitious root induction 3) best hormone treatment for adven-
titious root induction 4) adventitious root induction observed after 7 days
of culture 5) mass production of adventitious roots in bottles 6) adventi-
tious roots are washed and measured. Growth Conditions- Media- Half MS
supplement with selected auxin combination, photoperiod-16 hours, culture
period-3 weeks at 25±2°C

Sindhu, Lokesha and Aswath
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS 457
FIGURE 1B. Best culture conditions for mass production of adventi-
tious roots in bottles. a, a1- Arka Ashwagandha variety with an auxin
concentration of 0.25mg/L IAA and 0.75mg/L IBA, b,b1- JA 20 variety
with an auxin concentration of 0.25mg/L IAA and 0.75mg/L IBA, c,c1
– IIHR WS 32 variety with an auxin concentration of 0.50mg/L IAA
and 0.50mg/L IBA,d,d1 – IIHR WS 48 variety with an auxin concentra-
tion of 0.25mg/L IAA and 0.75mg/L IBA. Growth Conditions- Media-
Half MS supplement with 3% sucrose concentration, photoperiod-16
hours, culture period-3 weeks at 25±2°C.
FIGURE 1C. Data represents mean ± standard error of three replicates; each experi-
ment was repeated thrice.Comparision of total withanolide, withanolide B, withnolide
A, withaferin A, and Withanoside IV among different varieties of Ashwagandha.
Taiz and Zeiger (2002) reported that roots may
require a less concentration of auxin to grow, but root
growth is strongly inhibited by its higher level because
at this level, auxin induces the production of ethylene,
a root growth inhibitor. The adventitious roots were also
induced in leaf explants of Withania somnifera using
a combination of IAA and IBA by Praveen and Murthy
(2010). Combination of IBA and IAA performed better
than individual treatment of auxin upon adventitious
root induction in Withania somnifera (Sivanandan et al
2012a). Wadegoankar et al (2006) reported that a combi-
nation of IAA and IBA was effective in adventitious rot
induction in leaves of Withania somnifera .
Hence the best auxin concentration for adventitious
root induction with 96.6% root induction with 18.25
roots per explant was observed in Arka Ashwagan-
dha variety with an auxin concentration of 0.25mg/L
IAA and 0.75mg/L where as a combination 0.25mg/L
IAA and 0.75mg/L IBA yielded in 97.2% root induction
response and 15.12 roots per explant in JA 20. Induc-
tion response of 97.2% with 18.5 roots per explant was
observed in an auxin concentration 0.25mg/L IAA and
0.75mg/L IBA of in IIHR WS 48 and induction response
of 97.6% with 18.38 roots per explant was observed
in explants inoculated into half strength MS medium
with 0.50 mg/L IAA and 0.50 mg/L IBA for IIHR WS 32.
Sindhu, Lokesha and Aswath
458 STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
(Table 7 and 8), At the end of 15 day after explant inocu-
lation, the length of the root was between 0.5-1.0 cm and
it was observed that the root length steadily increased
with the increase in growth period with was approxi-
mately 4-5 cm after 30 days. These results indicate fast
growing nature of adventitious roots.
Table 7-Values are mean ± standard error of ve rep-
lications in three independent experiments, each with
three explants per treatment. Means followed by the same
letter are not signi cantly different at P<0.05 accord-
ing to Duncan Range Multiple Test. Data were scored
after15 days of culture. Growth Conditions- Media- Half
MS supplement with selected auxin combination, photo-
period-16 hours, culture period-3 weeks at 25±2°C.
Table 8- Values are mean ± standard error of ve
replications in three independent experiments, each
with three explants per treatment. Means followed by
the same letter are not signi cantly different at P<0.05
according to Duncan Range Multiple Test. Data were
scored after15 days of culture. Growth Conditions-
Media- Half MS supplement with selected auxin com-
bination, photoperiod-16 hours, culture period-3 weeks
at 25±2°C.
Variation persist in accumulation of withanolides due
to plant parts, developmental stages (Praveen and Mur-
thy, 2010), plant part obtained from different types of
cultures (Sharada et al., 2007; Singh et al., 2017) of W.
somnifera. These studies establish relationship between
morphology/condition of plant tissue and withanolide
contents. Sivanandhan et al., 2012b, 2013b; Singh et al.,
2017) used in vitro grown plants in different studies to
develop adventitious roots, using different growth con-
ditions. These developed roots were harvested to extract
different combinations of withanolides.
The results for total withanolides analyzed in adven-
titious roots of four genotypes revealed signi cant dif-
ference among them (Table 9).
Among the genotypes, Arka Ashwagandha recorded
high total withanolide content of 0.084% when com-
pared to check JA-20 (0.043%). Among seven witha-
nolides analyzed, withanoside V, 12- deoxy Witha-
nostramolide and Withanone were not detected in all
genotypes and withanoside IV constitutes highest in all
the genotypes. Withanolide A and B were detected only
in JA-20. Arka Ashwagandha and IIHR WS-32 contain
high withaferin A and Withanoside IV when compared
to Check JA-20.
Table 9-Values are mean ± standard error of three
repeated experiments, each experiment was repeated
thrice. Means followed by the same letter are not signif-
icantly different at P<0.05 according to Duncan Range
Multiple Test.
CONCLUSION
From the present research a standard protocol has been
developed for mass production of adventitious roots
from in-vitro leaves in Withania somnifera. A variety
with highest total withanolide content has been identi-
FIGURE 1D. Standard HPLC chromatogram of extracts of W. somnifera roots. A.Withanoside IV (Rt=15.69),
B. Withanoside V (Rt=19.72), C. Withaferin A (Rt=20.06), D. 12-Deoxy Withastramonolide (Rt= 21.35), E.
Withanolide A (Rt=22.37), F. Withanolide B (Rt=25.42)

Sindhu, Lokesha and Aswath
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS 459
ed in comparision to JA 20. The requirement of dried
plant material for withanolides drug production in India
is high. Moreover, eld cultivation is time consuming,
laborious and not able to meet the Ashwagandha global
market requirement. By transferring these roots in to
suspension culture and mass propagating it in bioreac-
tors reduce time gap compared to eld grown roots and
assure good quality Withania somnifera roots with high
total withanolide content to cater the global demand.
Large scale production through plant in vitro regenera-
tion will provide a means of placing the plant onto the
market at lower prices. In addition, the technique is cost
effective, relatively simple and can be performed by
semi-skilled persons.
ACKNOWLEDGEMENTS
The research was conducted in Indian Institute of Hor-
ticulture Research and was supported by centre for Post
Graduation Studies Jain University. The author also
acknowledges Department of Science and Technology
for providing DST-Inspire fellowship for conducting this
research.
REFERENCES
Agarwal A and Murali, B (2010) Estimation of withanolides in
Ashwagandha by HPLC. Quality assessment of selected Indian
medicinal plants. P: 241-243.
Geetha, M. and Rajashekar, S. (2017) Quality assessment of
phytochemical analysis of Withania somnifera. International
Journal of Current Research in Life Sciences Vol. 07, No. 04,
pp.1435-1437.
Gould AR, Everett NP, Wang TL, Street HE (1981) Studies
on the control of cell cycle in cultured plant cells: Effects of
nutrient limitation and nutrient starvation. Protoplasma, 106
(1-2):1-13.
Kamaljit Kaur, Gurpreet Kaur and Ritesh Kaur (2017). Witha-
nia somnifera: An important herb in Ayurveda and Indegi-
nousIndigenous medicinal system. Jour Pl Sci Res 33(2) 209-
222.
Kattimani KN, Reddy YN and Rajeshwar Rao BR In uence
of pre- sowing seed treatment on seedling vigor, root length,
and dry root yield of Ashwagandha (Withania somnifera (L.)
Dunal) under semiarid climate of Hyderabad, J. Medicinal
and Aromatic plant sciences, proceeding of national seminar,
22(4A) and 23(1A) (2000).
Kumar V, Kotamballi N, Chidambara M, Bhamid S, Sudha CG,
Ravishankar GA (2005). Genetically modi ed hairy roots of
Withania sominifera Dunal : A potent source of rejuvenating
principles. Rejuvination Res. A: 37-45.
Murthy HN, Dijkstra C, Anthony P, White DA, Davey MR,
Power JB, Hahn EJ, Paek KY (2008). Establishment of Withania
somnifera hairy root culture for the production of withanolide
A. J. Int. Plant Biol.50: 975-981.
Pandey V, Ansari WA, Misra P and Atri N (2017) Withania
somnifera: Advances and Implementation of Molecular and
Tissue Culture Techniques to Enhance Its Application. Front.
Plant Sci. 8:1390
Pawar PK, Maheshwari VL (2004). Agrobacterium rhizogenes
mediated hairy root induction in two medicinally important
members of family Solanaceae. Indian J. Biotechol. 3: 414-417
Nagella P, Murthy HN (2011) Effects of macroelements and
nitrogen source on biomass accumulation and withanolide-A
production from cell suspension cultures ofWithania somnif-
era(L.) Dunal. Plant Cell Tissue Organ Cult 104: 119–124.
Praveen N, Murthy HN (2011). Synthesis of Withanolide A
depends on carbon source and medium Ph in hairy root cul-
tures of Withania somnifera. Ind. Crop Prod. 35: 241-234.
Rao S, Teesta VK, Bhattrai A, Khushi K and Bhatt S (2012)
In-vitro propogation of Withania sominifera and estimation
of withanolides for neurological disorders. Journal of Pharma-
cognosy. 3(2): 85-87.
Rani , R., Khan, M.A Khayani, W.K,Ullah,S., Naeem I.,
Mirza.B.,2017. Metabolic signatures altered by in vitro tem-
perature stress in Ajuga bracteosa Wall.ex.Benth.Acta physiol.
Plant 39(4)97.
Ray S,Ghosh B, Sen S, Jha S (1996) Withanolide production by
root cultures of Withania somnifera transformed with Agro-
bacterium rhizogenes. Planta Med. 62: 571-573.
Sharada M, Ahuja A, Suri KA, Vij SP, Khajuria RK, Verma V,
Kumar A (2007). Withanolide production by in-vitro cultures
of Withania somnifera and its association with differentiation.
Biol. Plant 51: 161- 164.
Singh, P., Guleri, R., Angurala, A., Kaur, K., Kaur, K., Kaul,
S. C., et al. (2017). Addressing challenges to enhance the bio
actives of Withania somnifera organ, tissue, and cell culture
based approaches. Biomed Res. Int. 2017:3278494
Sivanandhan G, Arun M, Mayavan S, Rajesh M, Jeyraj M, Kapil
Dev G, Manickavasagam M, Selvaraj N, Ganapathi A (2012b).
Optimization of elicitation condition with methyl jasmonate
and salicylic acid to improve the productivity of withanolides
in the adventitious root culture of Withania somnifera (L.)
Dunal. Appl. Biochem. Biotechnol. 168: 681-696.
Sivanandhan G, Kapil Dev G, Jeyraj M, Rajesh M, Arjunan A,
Muthuselvam M, Manickavasagam M, Selvaraj N, Ganapathi A
(2013b). Increase production of withanolide A, withanone and
withaferrin A in hairy root culture of Withania somnifera (L.)
Dunal elicited with methyl jasmonate and salicylic acid. Plant
Cell Tissue Org. Cult. 114: 121-129.
Sivanandhan G, Rajesh M, Arun M, Jeyraj M, Kapil Dev G,
Arjunan A, Manickavasagam M, Muthuselvam M, Selvaraj N,
Ganapathi A (2013a). Effect of culture conditions ,cytokinin-
ins, methyl jasmonates and salicylic acid on the biomass accu-
mulation and production of withanolides in multiple shoot
culture of Withania somnifera (L.)
Dunal using liquid culture.
Acta Physiol. Plant. 35: 715-728.

Sindhu, Lokesha and Aswath
460 STANDARDIZATION OF VARIOUS FACTORS FOR PRODUCTION OF ADVENTITIOUS ROOTS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Sivanandhan G, Rajesh M, Arun M, Jeyraj M, Kapil Dev G,
Arjunan A, Manickavasagam M, Muthuselvam M, Selvaraj N,
Ganapathi A (2012a). Optimization of carbon source for hairy
root growth and withaferrin A and Withanone production in
Withania somnifera. Nat.Prod. Commun. 7:1271-1272.
Tariq Khan, Bilal Hyder Abbasi, Mubarak Ali Khan, Muhammed
azim (2017) Production of biomass and useful compounds
through elicitation in adventitious root cultures of Fagona
indica. Industrial crops & products. 108(2017)451-457.
Wu Ch, Dewir YH, Hahn ej and Peak KY (2006) Optimization of
culturing conditions for the production of biomass and pheno-
lics from adventitious roots of Echinacea angustifolia. Journal
of Plant Biology, 49(3):193-199.
Yin S, Liang Y, Gao W, Wang J, Jing S, Zhang Y and Liu H
(2013) In uence of medium salt strength and nitrogen source
on biomass and metabolite accumulation in adve4ntitious root
cultures of Pseudostellaria heterophylla. Acta physiologiae
Plantarum.
Zhang J, Yuan Gao W, Wang J, Li X (2012) Effects of sucrose
concentration and exogenous hormones on growth and peri-
plocin accumulation in adventitious roots ofPeriploca sepium
Bunge. Acta Physiol Plant 34:1345–1351.