
Nanobiotechnological
Communication
Biosci. Biotech. Res. Comm. 11(3): 442-450 (2018)
Formulation of detection bioconjugates of gold coated
iron oxide nanoparticles for a atoxin M1 in milk
Jayesh H Kabariya
1
and Vimal M Ramani
2
*
1
Senior Research Fellow, Postgraduate Institute of Dairy Education and Research, Kamdhenu University,
Amreli, Gujarat
2
Principal and Dean, College of Dairy Science and Postgraduate Institute of Dairy Education and Research,
Kamdhenu University, Amreli, Gujarat, India
ABSTRACT
Mycotoxins particularly a atoxins are gaining increasing importance due to their deleterious effects on human
and animal health and also due to ubiquitous presence of a atoxigenic fungi in all the agricultural products. Corn,
groundnuts and other plants were infected by Aspergillus avus & Aspergillus parasiticus and secrete the mycotox-
ins. A atoxin B1 transmitted to cow by feeding these infected plants and a atoxin B1 transformed into its hydroxy-
lated product such as a atoxin M1 and M2 and such a atoxin secreted in cow milk. In the present study, we explored
the nanobiotechnology approach to prepare detection bioconjugates of gold coated iron oxide nanoparticle for the
detection of a atoxin M1 in milk. Gold coated iron oxide nanoparticles (Au-Fe3O4 NPs) ranging between 10-20
nm were synthesized by co-precipitation method and functionalized by EDC-NHS then labeled with FITC-labeled
streptavidin (FITC-STV). Biotinylated a atoxin M1 (biAFM1) speci c to monoclonal anti-a atoxin M1 antibodies
(mAFM1) were prepared, separately. The uorophore FITC-STV-Au-Fe
3
O
4
NPs and bimAFM1 antibodies were allowed
to interact to obtain Au-Fe
3
O
4
NPs-FITC-STV-bimAFM1 antibody and AFM1 bioconjugates. The bioconjugates were
characterized by Transmission Electron Microscope (TEM), Scanning Electron Microscope with Energy Dispersive
X-ray (SEM-EDAX), Fourier Transform Infrared (FTIR), Particle Size Analyzer with zeta potential (PSA) and Fluores-
cence Microscopy. The bioconjugation formation was con rmed by pinkish red color in bright ied microscopy same
eld observed by uorescence microscopy shows the green uorescence which con rm the aggregation between
Au-Fe3O4 NPs-FITC labeled STV-bimAFM1 antibody and AFM1.
KEY WORDS: AFLATOXIN M1, BIOCONJUGATES, GOLD COATED IRON NANOPARTICLE, MONOCLONAL ANTIBODY AFM1
442
ARTICLE INFORMATION:
*Corresponding Author: vimamramani@gmail.com
Received 1
st
July, 2018
Accepted after revision 19
th
Sep, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.3/13

Jayesh H Kabariya and Vimal M Ramani
INTRODUCTION
From the last decade iron oxide nanoparticles (Fe
3
O
4
NPs) and their combinations with gold (core/shell) has
become more attractive because of their vast applica-
tions in different elds such as biosensor, medical eld
and drug delivery etc. The surface binding of various
chemical and biological molecules onto gold particle
was due to thiol chemistry of gold surface for attach-
ment of functionalized compounds (Kouassi et al., 2007).
Gold coated iron oxide nanoparticle (Au-Fe
3
O
4
NPs) were
synthesized by reduction of metal with sodium boro-
hydride through sonication method (Baniukevic et al.,
2013). Using hydroxylamine as a reducing agent gold
(shell) coated iron oxide (core) nanoparticle also syn-
thesized by iterative reduction method and it analyze
by transmission electron microscope (TEM) and super-
conducting quantum interference device (SQUID) mag-
netometer reported (Lyon et al., 2004). The measurement
of uorescent and optical properties of Au-Fe
3
O
4
NPs has
been reported by (Baniukevic et al., 2013 Carrasco et al.,
2018).
Corn, groundnuts and other plants were infected
by Aspergillus avus and Aspergillus parasiticus and
secrete the mycotoxins which is responsible for the food
born disease. Corn, groundnuts and other plants were
infected by Aspergillus avus and Aspergillus parasiti-
cus and secrete the mycotoxins. A atoxin B1 transmit-
ted to cow by feeding these infected plants and a atoxin
B1 transformed into its hydroxylated product such as
a atoxin M1 and M2 and such a atoxin secreted in cow
milk which quite stable during storage, pasteurization
and milk product preparation (Stroka & Anklam, (2002).
The a atoxin is high temperature resistant and is not
inactivated after milk treatment processes such as pas-
teurization, sterilization and others. Mycotoxins particu-
larly a atoxins are gaining increasing importance due
to their deleterious effects on human and animal health
and also because of ubiquitous presence of a atoxigenic
fungi in all the agricultural products. Animals contact
to mycotoxins by consumption of infected food, it may
be harmful to their health as well as humans, who are
consumers of the animal products such as milk (Gacem
and Hadj-Khelil 2016, Ketney et al., 2017).
A atoxin is a kind of mycotoxin that was discovered
from the mass poisoning of turkeys in Britain in 1960,
and has strong carcinogenicity. A atoxin M1 (AFM1)
is hydroxylated product of a atoxin B1. Numerous
diagnostic methodology like, chromatographic method
includes, TLC, HPLC and OPLC, FT-NIR and enzyme-
linked immunosorbent assay (ELISA) were available for
the detection of a atoxins but it is time consuming and
requiring sophisticated instruments as well as trained
manpower (Espinosa et al., 2011). Diagnostic immu-
noassay have been use with speci c antigen antibody
interaction for the detection of many molecules such as
AFM1 because of their sensitivity, easy to handle and
quantitatively measurements. In uorescence micro-
scopic technique antibody labeled with so many uo-
rescent dyes such as Fluorescein isothiocyanate (FITC),
Rhodomine, Alexa uor and other are used as an indi-
cator which provide optical contrast for better analysis
(Adarsha et al., 2015).
Till now, the use of gold coated iron oxide nanoparti-
cle was comparatively less because it require more time
to synthesis, require high temperature for the synthesis
of nanoparticle and very dif cult to prevent aggregation
without chemical modi cation or use of surfactant. Here
in this study, we have used simple and rapid method for
the synthesis of gold coated iron oxide nanoparticle at
room temperature by chemical co-precipitation, reduc-
tion of sodium borohydride and sonication method. In
rst step, synthesis of iron oxide nanoparticle as a core
by chemical co-precipitation and then it coated with
gold as shell by reduction of sodium borohydride with
sonication to enhanced particle monodispersity. Then
prepared particles were characterized by Transmission
Electron Microscope (TEM), Scanning Electron Micro-
scope with Energy dispersive X-ray (SEM-EDAX), Fou-
rier Transform Infrared (FTIR), Particle Size Analyzer
with zeta potential (PSA) and Fluorescence Microscopy.
Surface charges and further modi cations with mercap-
topropionic acid and EDC-NHS also characterization by
the same.
Our research effort is in direction of the detection
of AFM1 with biotin-streptavidin binding approach by
labeling of uorescence dye with gold coated iron oxide
nanoparticle. The bioconjugation formation between
streptavidin-AFM1 and biotinylated monoclonal anti-
body of AFM1 was con rmed by uorescence micros-
copy shows the uorescence compounds which con rm
the interaction. Mycotoxins particularly a atoxins are
gaining increasing importance due to their deleterious
effects on human and animal health and also due to
ubiquitous presence of a atoxigenic fungi in all the
agricultural commodities under eld and storage condi-
tions. Common established methodologies for a atoxin
detection include thin-layer chromatography (TLC),
(Flores and Gonzalez 2017) and high performance liquid
chromatography (HPLC) (Carrasco et al., 2018).
These techniques have excellent sensitivities but they
require skilled operators, extensive sample pre-treat-
ment and expensive equipments. The present investiga-
tion was an attempt to develop basic mechanism of new
nanotechnology based detection system with a minimal
size, weight and real low cost and rapid detection will
have signi cantly impact the practice of monitoring
program for a atoxin.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS FORMULATION OF DETECTION BIOCONJUGATES OF GOLD COATED IRON OXIDE NANOPARTICLE 443

Jayesh H Kabariya and Vimal M Ramani
MATERIAL AND METHODS
All chemicals were obtained from commercial source
and used as received. FeSO
4
.7H
2
O, FeCl
3
, HAuCl
4
, FITC-
streptavidin, biotin and NHS-biotin, a atoxin M1
(AFM1) were purchased from Sigma Aldrich (USA).
Sodium hydroxide, perchloric acid, sodium borohydrate,
cetyltrimetyl–ammonium bromide (CTAB), ethylene di-
amine tetra-acetic acid (EDTA), 3-mercaptopropionic
acid, N-hydroxysuccinimide (NHS), N-Ethyl-N-(3-di-
methyl-aminopropyl)-carbodimide, (EDC), streptavidin
(STV), Dimethyl Sulfoxide (DMSO), phosphate buffer
solution (PBS) at pH 7.4 HPLC grade pure water, were
purchased from HiMedia (India). A atoxin M1 mono-
clonal antibody (mAFM1-Ab) were purchased from
MyBiosource (USA). All chemicals materials were used
as received.
The synthesis of Fe
3
O
4
NPs were carried out by modi-
ed Massart’s co-precipitation method. The ratio of iron
salt (Fe(III)/Fe(II) was kept as 2:1 in an alkaline solu-
tion. Brie y, 0.64M FeCl
3
and 0.32M FeSO
4
.7H
2
O were
dissolved in 40ml of deionized water and the solution
was stirred (TARSON SPINOT DIGITAL) until the iron
salt completely dissolved. Subsequently add 40ml of
1M NaOH solution drop wise into stirred mixture and
stirred it for 20minutes. The formation of Fe
3
O
4
(mag-
netite) nanoparticle by indicating the color change from
yellowish brown to black. The chemical reaction of mag-
netite precipitation may be written as follows (Tamer
et al., 2010).
Fe
+2
+ 2Fe
+3
+ 8OH
Fe
3
O
4
+ 4H
2
O
Yellowish color Black color
The black color precipitate was separated by permanent
magnet and washed with deionized water. To obtain oxi-
dized magnetite nanoparticles, separated black precipi-
tate was rst washed with 2M perchloric acid and waited
for 2-3 hours to oxidized iron salt to magnetite until the
color was change from black to brown under inert con-
dition. Then particle was centrifuged (REMI CM-12plus)
at 10,000 rpm for 20 minutes. After centrifugation, dis-
card the supernatant and washed with deionized water.
Repeat he washing procedure for 2-3 times or until pH
was reduced to neutral pH. The gold coating procedure
was carried out in the presence of CTAB to encapsulate
the synthesized Fe
3
O
4
NPs by gold as a shell. Brie y,
10mg Fe
3
O
4
NPs were add into 5ml 0.27M EDTA solution
which prepared in 1M NaOH. The mixture was stirred
in sonicator (LABMAN) at 30 amplitude for 5 minutes.
Then resulting solution was centrifuge at 10,000 rpm
for 10 minutes. Supernatant was discarded and pellet
was washed with deionized water. Washing procedure
was repeated for 3 times. Then add 7ml of 0.1M CTAB,
3ml 0.01M HAuCl
4
and 300μl 1M NaOH into resulting
precipitation and stirred vigorously, subsequently add
150mg sodium borohydrate into stirred mixture and
stirred vigorously for 3minutes. The color was change
from yellow to dark red indicated the gold was coated
into core (iron oxide nanoparticles (Fe
3
O
4
NPs) as a shell
(Tamer et al., 2010).
SYNTHESIS OF GOLD COATED IRON OXIDE
NANOPARTICLES WITH FITC LABELED
STREPTAVIDIN AS A CARRIER MOLECULE
10mg gold coated iron oxide nanoparticles were added
in 1ml of 20 mM 3-mercaptopropionic acid and soni-
cated for 4 hours. After sonication particles were washed
with pure water. This procedure createdf the carboxyl
group onto surface of gold coated iron oxide nanopar-
ticles with EDC and NHS linked and it was used as a
carrier material for streptavidin binding. For cross link
of carboxylated nanoparticles to EDC-NHS, rst 5mg of
nanoparticle was added into 2ml EDC (0.05mg/ml) solu-
tion containing 0.2% NHS prepare in cold water then it
sonicate for 5 minutes at 4
o
C, after sonication particles
were separated out by using permanent magnet. After
this process, add 1ml of 2mM streptavidin prepared in
PBS (pH-7.4) in separated nanoparticle, mix it properly
then incubate it for 2 hours.
Streptavidin uorescently labelled with Fluorescein
isothiocyanate (FITC) at a stock concentration of 1 mg/
mL. This dye has an absorption peak at 495 nm and
an emission peak of 525 nm. The resultant FITC labeled
streptavidin binded gold coated iron oxide nanoparti-
cles were separated by permanent magnet from reac-
tion mixture, then air dried for 12 h and resuspended
in water. The procedure was repeated for three times to
remove impurities (Eivari and Rahdar (2013). Anti-AFM1
monoclonal antibody having sulfosuccinimidyl group
was prepared separately by covalently binding primary
amines of antibody (Gretch (1987). 10mg (10mM) NHS-
biotin solution in 1ml DMSO was added and dissolved in
it and was prepared prior to use. Then was added mon-
oclonal AFM1 antibody with 80μg of NHS-biotin/mg
ratio, mixed immediately and was incubated for 3 hours
in shaking condition. After incubation period dialysis
procedure of NHS-biotin binded antibody was carried
out at 4
o
C for overnight to remove unbinded NHS-biotin
molecules. Subsequently, store the biotin labeled AFM1
antibody at 2-8
o
C.
The procedure was started with 50μl biotinylated
monoclonal AFM1 antibody and 20μl streptavidin con-
jugated gold coated iron oxide nanoparticles were mix
together and incubated at 4
o
C for 1 h in dark condition.
The biotinylated antibody had bound non-covalently to
streptavidin and had formed bridge for direct sensing of
a atoxin M1. After the formation of nanoparticle bio-
444 FORMULATION OF DETECTION BIOCONJUGATES OF GOLD COATED IRON OXIDE NANOPARTICLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Jayesh H Kabariya and Vimal M Ramani
conjugate add 10 μl AFM1, it was incubated at 4
o
C for
30 minutes in dark condition. Characterization of bio-
conjgates aggregation was done by different techniques
(Adarsha et al., 2015).
RESULTS AND DISCUSSION
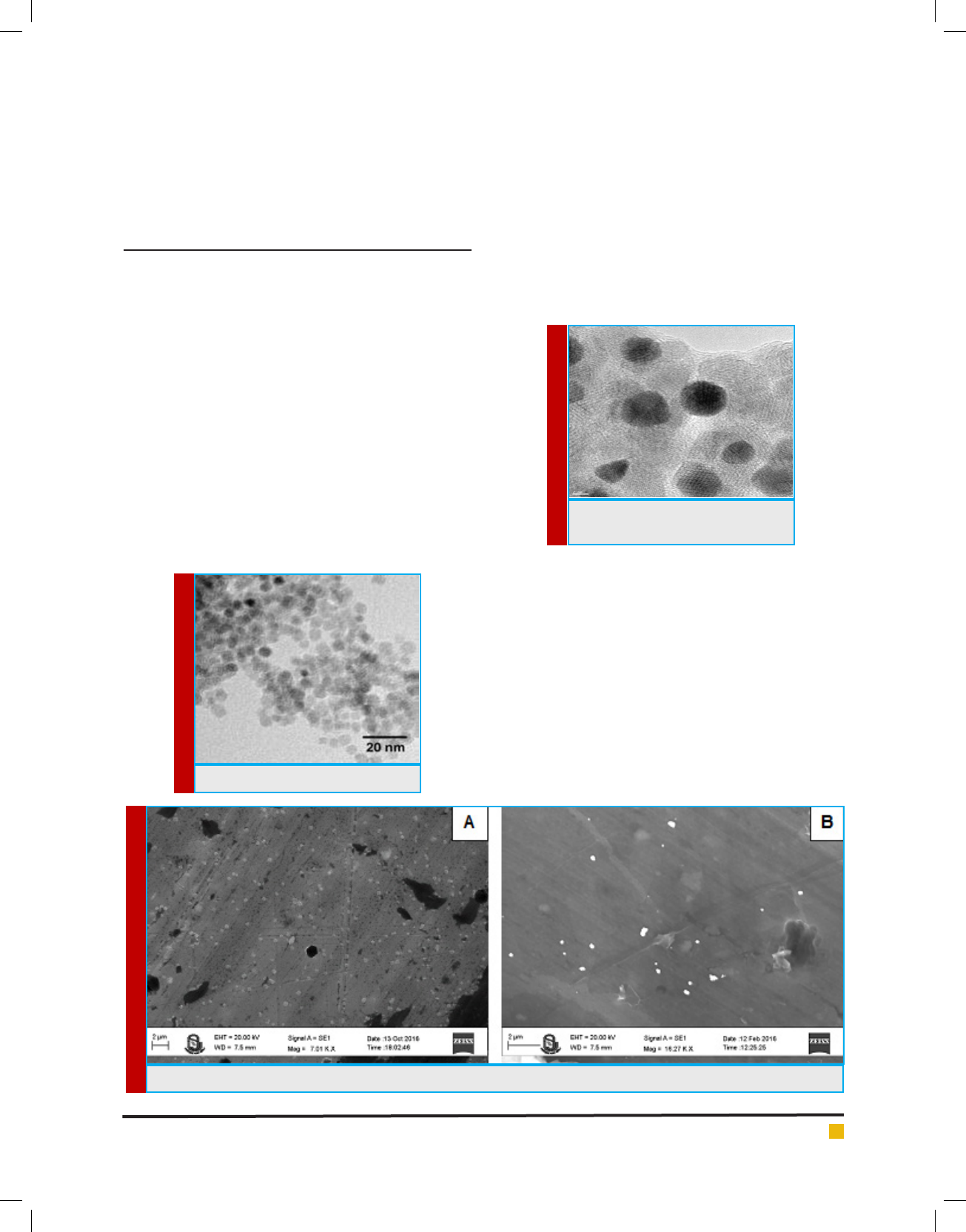
TRANSMISSION ELECTRON MICROSCOPE
The morphological characterization iron oxide nano-
particle was done by transmission electron microscopy
(TEM). A small drop of formulated iron oxide nanopar-
ticle was placed on the copper grid surface and dried it
at room temperature. TEM analysis was carried out by
JEOL-JEM2100 transmission electron microscope.
The shape of nanoparticles was spherical having aver-
age dimension of 10.8 ± 4.6nm. The TEM image of Fe
3
O
4
NPs shows in Figure 1. The morphology and magnetic
properties can be controlled by varying in pH solution,
ionic strength, temperature, reaction time, type of salts
and stirring speed. Eivari and Rahdar (2010) has reported
that Fe
3
O
4
NPs was almost spherical and their mean size
was 10 nm. Our TEM result of Fe
3
O
4
NPs morphology is
at par to this.
Figure 2 shows that the Au-Fe
3
O
4
NPs was darker than
Fe
3
O
4
NPs. TEM images shows that the average particles
size was increased from 10nm to 14nm after gold coat-
ing. The reduction of gold onto spherical surface of Fe
3
O
4
NPs and it has average size of Au-Fe
3
O
4
NPs was 14nm ±
3nm. Eivari and Rahdar (2010) has reported that the after
coating of gold onto the Fe
3
O
4
NPs it appear more darker
than Fe
3
O
4
NPs because of gold having more electron
density then iron. Our TEM result of Au-Fe
3
O
4
NPs is at
par with them.
FIGURE 1. TEM image of Fe
3
O
4
NPs
FIGURE 2. TEM images of Au-Fe
3
O
4
NPs
FIGURE 3. SEM images: (A) Fe
3
O
4
NPs (B) Au-Fe
3
O
4
NPs
SCANNING ELECTRON MICROSCOPE (SEM)
WITH ENERGY DISPERSIVE X-RAY (EDAX)
The formulated Au-Fe
3
O
4
NPs were further examine
using a Zeiss EVO-18 Scanning Electron Microscope
with Energy Dispersive X-Ray facility (SEM with EDAX),
operating at 20 kV in vacuum and at 20KX magni ca-
tion.
Con rmation of the morphology of the formulated
Fe
3
O
4
NPs and Au-Fe
3
O
4
NPs analyze by SEM with EDAX
which show in Figure 3.
After the synthesis of Fe
3
O
4
NPs it was coated by gold
with reduction of sodium borohydride in the sonication
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS FORMULATION OF DETECTION BIOCONJUGATES OF GOLD COATED IRON OXIDE NANOPARTICLE 445
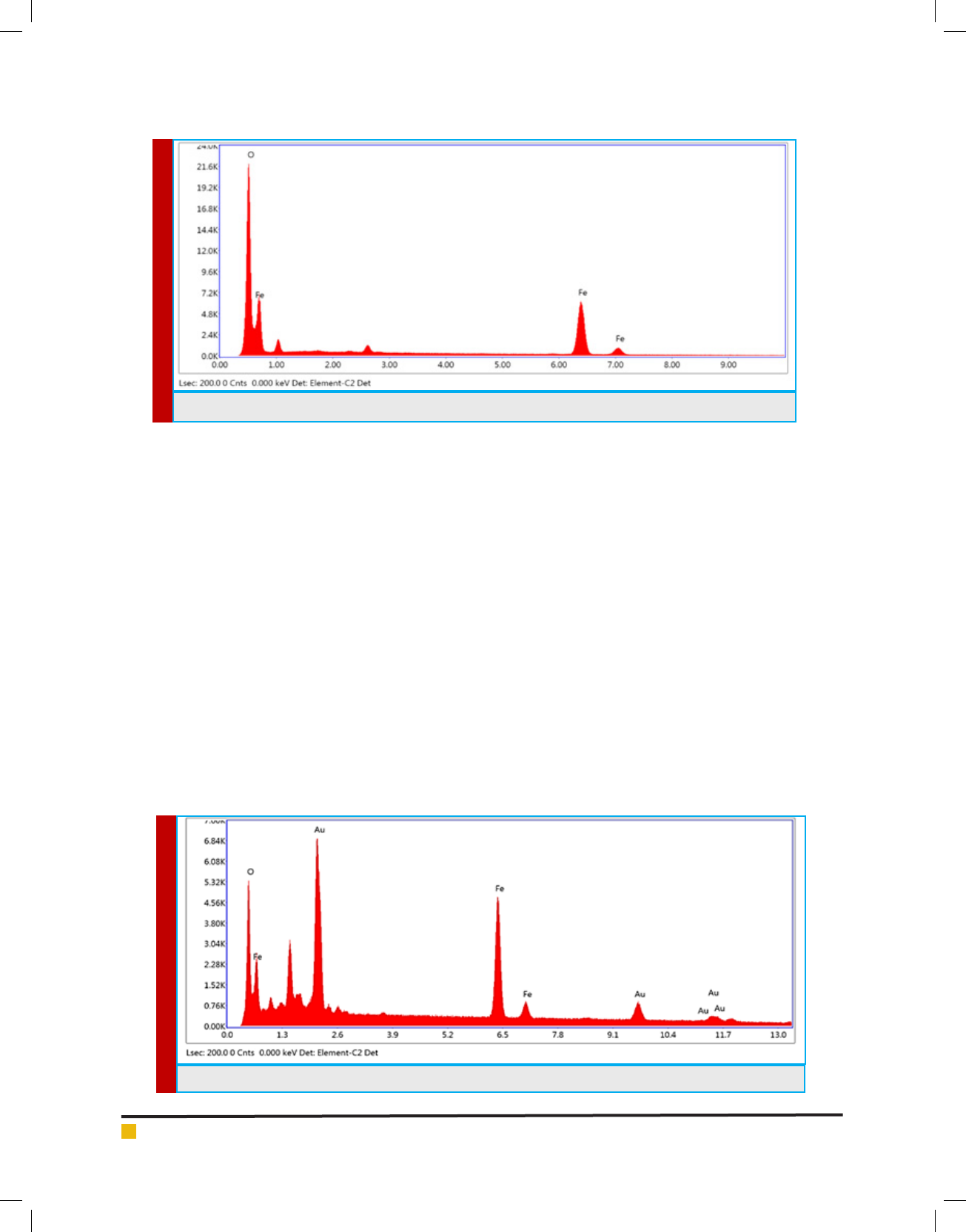
Jayesh H Kabariya and Vimal M Ramani
chamber which shows in Figure 4. The difference in the
size of Fe
3
O
4
NPs shows that the coating of gold onto
Fe
3
O
4
NPs was may be successful.
The analysis of Au-Fe
3
O
4
NPs by EDAX was done to
investigate the presence of Au and Fe in the synthe-
sized nanoparticle. EDAX spectrum of the Au-Fe
3
O
4
NPs
was shown in Figure 5, which con rms the existence of
Au and Fe in the synthesized nanoparticle. Hoskins et
al., (2012) has reported the presence of Au-Fe
3
O
4
NPs by
EDAX analysis. Our EDAX analysis result of Au-Fe
3
O
4
NPs is af rmation to this.
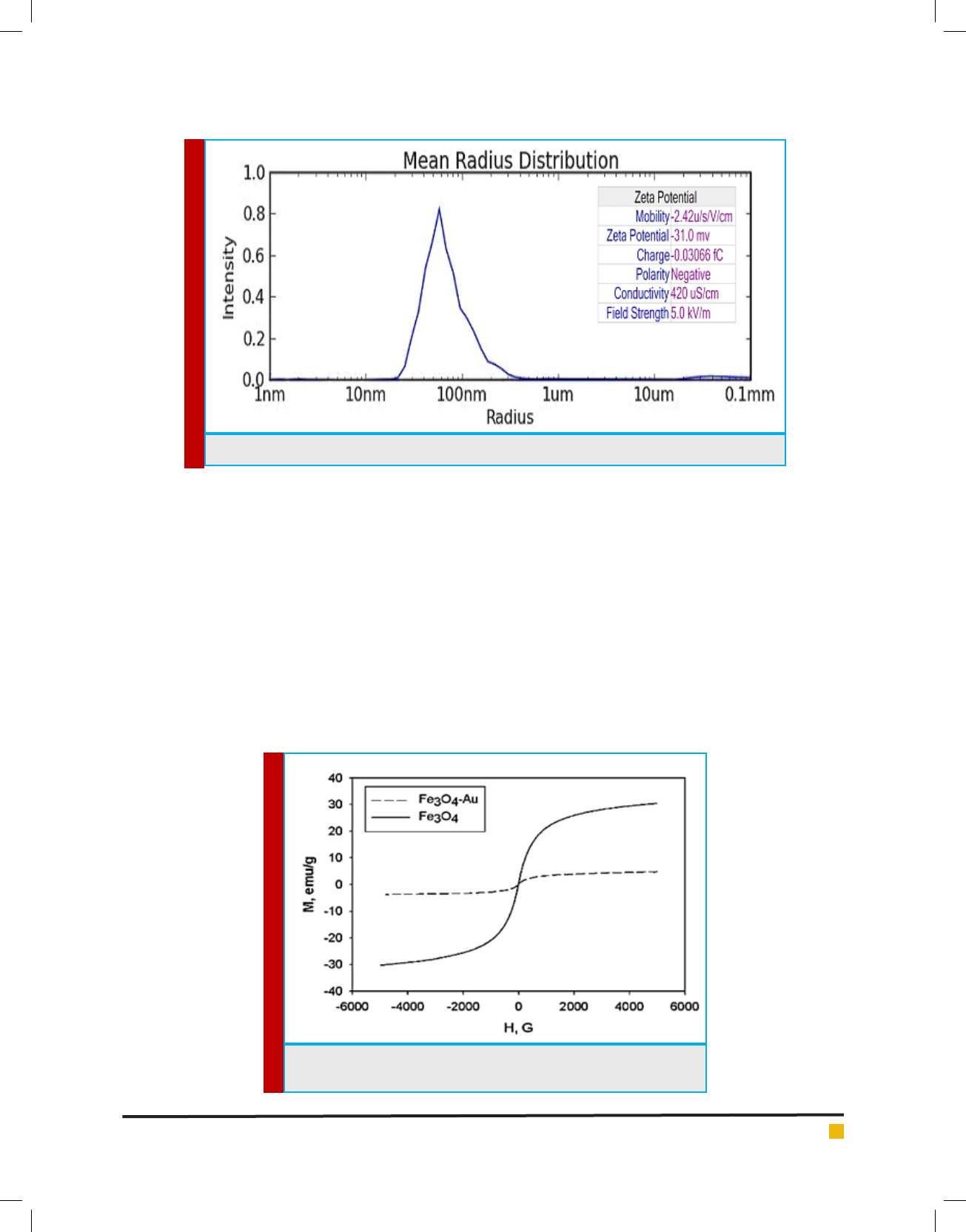
PARTICLE SIZE ANALYZER AND ZETA
POTENTIAL
In the formulation of Fe
3
O
4
NPs experiments, known
volumes (normally 500 μL) of Fe
3
O
4
NPs suspension were
taken for particle size analyzed by a Malvern Mastersizer
2000. The particle size analyzer utilizes laser technology
based on the Mie light-scattering theories. Fe
3
O
4
NPs
was characterized to examine the particle mean size and
understand the properties under different physiological
conditions and also determine the surface charges of
nanoparticle.
The average size of Fe
3
O
4
NPs was 52.04nm ± 5nm
shown in Figure 6. The Zeta Potential of synthesized
Fe
3
O
4
NPs was -31.0 mv and polarity was negative, so
it has good particle stability re ated by this result. Our
result is af rmation to study of Behera et al., (2012).
VIBRATING SAMPLE MAGNETOMETER
Iron oxide (magnetic) nanoparticle may give different
magnetic properties depending on condition of formula-
tion Gupta and Gupta (2005). At room temperature iron
oxide nanoparticle has superpara-magnetic property
where at 60 emu/g reported for saturation. The vibrating
sample magnetometer measure the iron oxide nanopar-
ticle and gold coated iron oxide nanoparticle character-
istic at 300K.
FIGURE 4. SEM-EDAX analysis of Fe
3
O
4
NPs
FIGURE 5. EDAX analysis of Au-Fe
3
O
4
NPs
446 FORMULATION OF DETECTION BIOCONJUGATES OF GOLD COATED IRON OXIDE NANOPARTICLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Jayesh H Kabariya and Vimal M Ramani
FIGURE 6. Average size of Au-Fe
3
O
4
NPs by Particle Size Analyzer and Zeta potential
FIGURE 7. Hysteresis loops of Fe
3
O
4
(solid line) and Au-Fe
3
O
4
(dotted line)
by Vibrating
Figure 7 shows hysteresis loop of uncoated & gold
coated iron oxide nanoparticle. The superpara-magnet-
ism was observed in both synthesized nanoparticles.
The magnetism form saturation to superpara-magnet-
ism curve the value of iron oxide nanoparticle and gold
coated iron oxide nanoparticle was 30 and 4.5 emu/g
respectively at 300K. Reduction in magnetism was indi-
cated that the magnetism was indicated that the forma-
tion of gold shell into iron oxide nanoparticle core.
FOURIER TRANSFORM INFRARED (FTIR)
SPECTROSCOPIC ANALYSIS
The carboxylic group attached on the surface of Au-
Fe
3
O
4
NPs by treatment of ethanolic solution having
3-mercaptopropionic acid. The activation of carboxylic
group for the bonding between amide and carboxylic
group, the activation of carboxylic group was catalyzed
by carbodimide (EDC) in presence of N-hydroxysuccin-
imide (NHS). The addition of NHS catalyzed the forma-
tion of the intermediate active esters that further react
with the amine function of the streptavidin (STV) to
yield nally the amide bond between the monoclonal
antibody a atoxin M1 (mAFM1) and the carboxyl group
on the nanoparticles.
Characteristic of C=O and N-H stretch analysis was done
with various peaks in the FTIR spectrum region shown in
Figure 8. However the sharp FTIR absorbance peaks 3426
cm
-1
speci c to amide indicate the presence of proteins.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS FORMULATION OF DETECTION BIOCONJUGATES OF GOLD COATED IRON OXIDE NANOPARTICLE 447
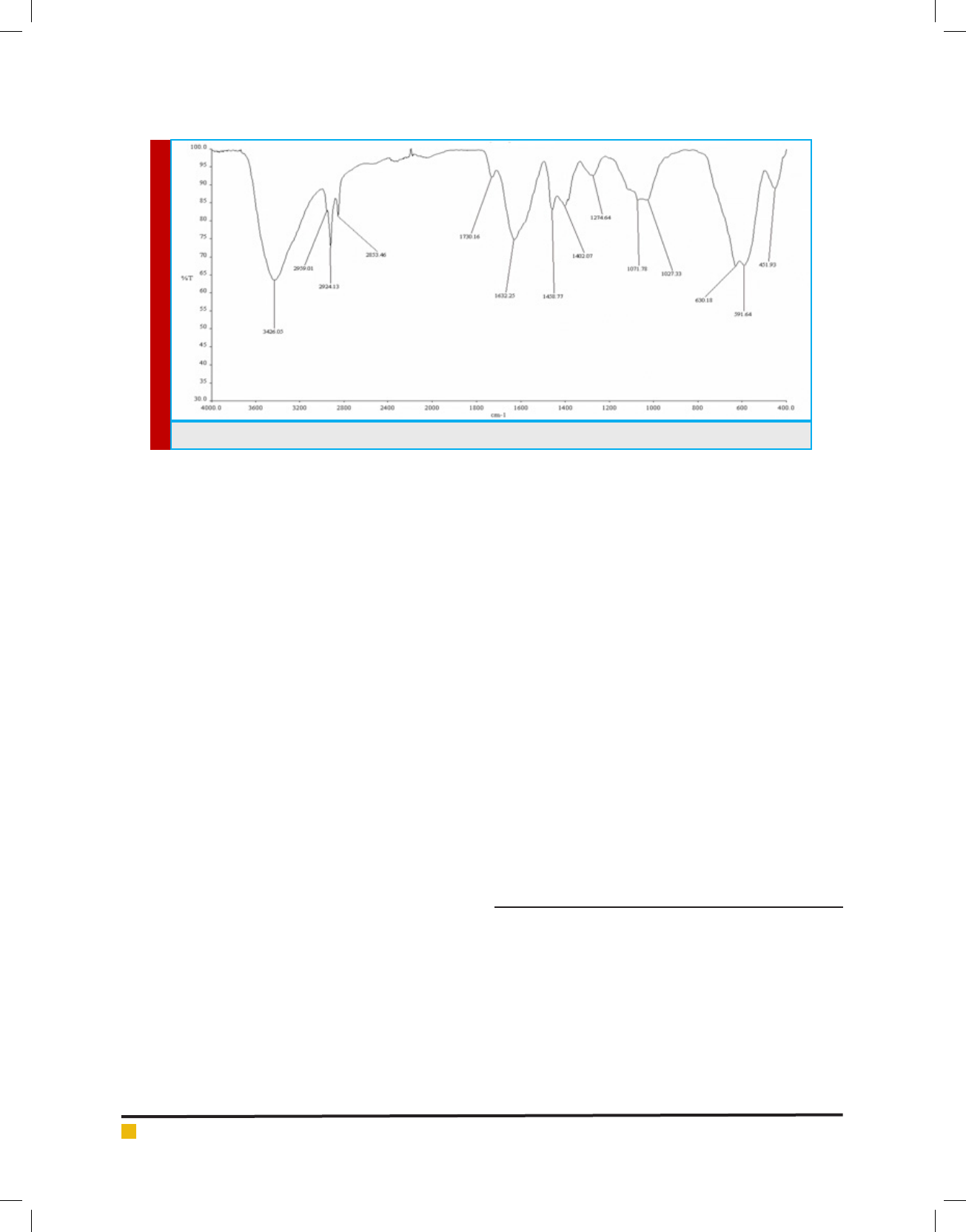
Jayesh H Kabariya and Vimal M Ramani
The FTIR spectrum in Figure 8 shows peaks at various
ranges corresponding to different stretching and bend-
ing modes of the amine and carboxylic group. Peaks at
3426 cm
−1
are assigned to N-H stretching of the amide
group. The peak at 1632 cm
−1
has been assigned to C=O
stretching of the carboxylic group. The peak at 3426
cm
−1
is due to O–H stretching. This type of study has
been reported previously to nd functional properties,
binding and to be used as detection system Adarsha
et al., (2015).
DETECTION OF BIOCONJUGATES BY
FLUORESCENCE MICROSCOPY
Fluorescence is a process where a uorophore (FITC)
absorb light (492nm) and turns in to an excitation form
with apple green color which result in emitted light
(515nm). The Au-Fe
3
O
4
NPs and its interaction with FITC
labeled STV and biotinylated monoclonal antibody of
AFM1 (bimAFM1) was measured under uorescence
microscope (Nikon Eclipse Ni) under uorescence mode at
constant ow of 50 a.u with FITC lter (EX 465-495nm,
DM 505 & BA 512-558nm). The background of image
became brighter making the particles more dif cult to
visualize so, it track with the Nikon D-element software.
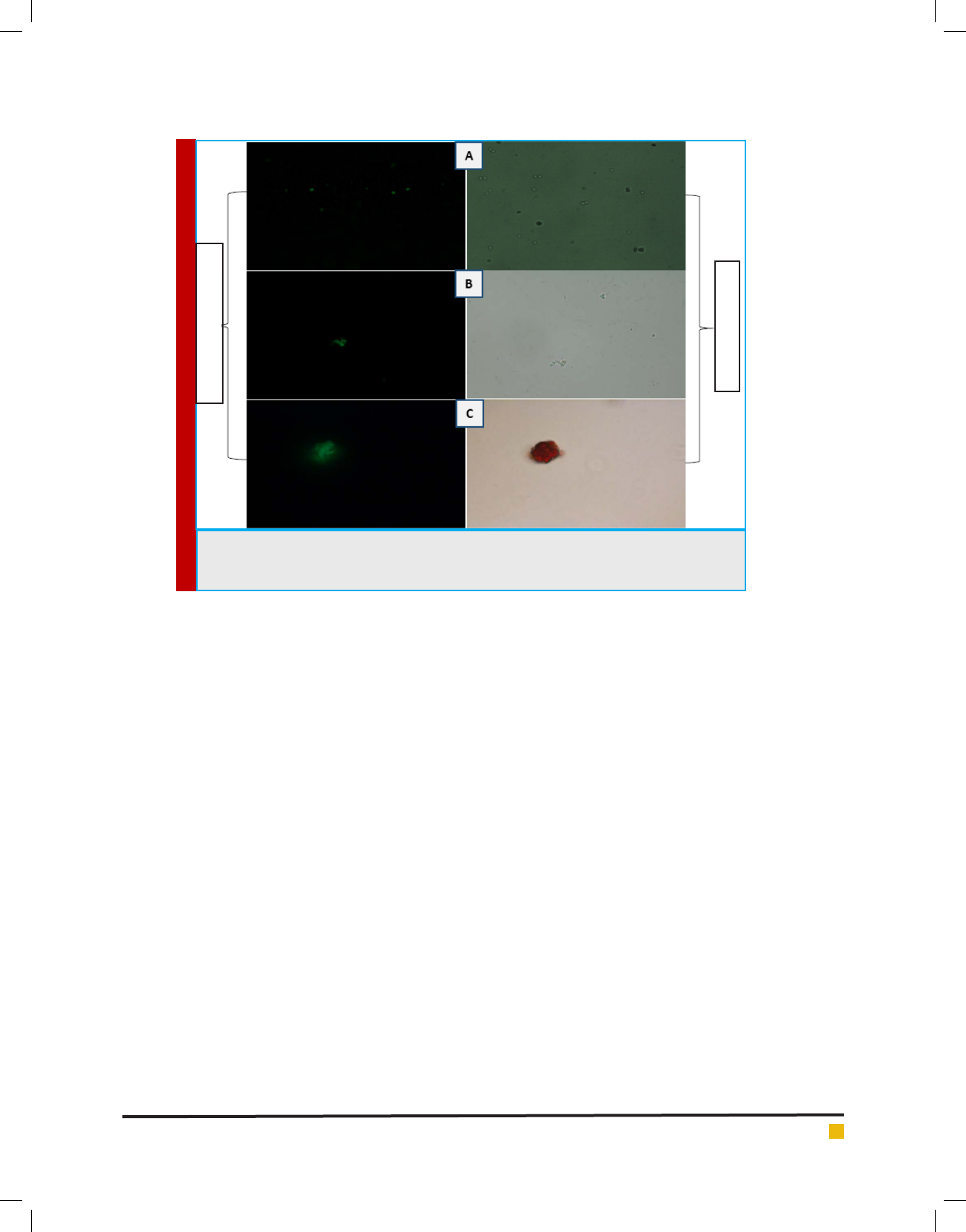
The uorescence microscopic image of Figure 9 (A)
Au-Fe
3
O
4
NPs-FITC labeled STV (B) Au-Fe
3
O
4
NPs-FITC
labeled STV-bimAFM1 antibody and (C) Au-Fe
3
O
4
NPs-
FITC labeled STV-bimAFM1 antibody-AFM1 bioconju-
gate was examined under uorescence and bright eld
shown in Figure 9. There was no any aggregation found
in case of Figure 9 (A) and (B) while in case of (C) shows
the aggregation between Au-Fe
3
O
4
NPs-FITC labeled STV-
bimAFM1 antibody and AFM1 which indicating the suc-
cessful bioconjugation. Streptavidin are used ubiquitously
FIGURE 8. FTIR spectrum of Au-Fe
3
O
4
NPs interact with STV and mAFM1
due to its remarkable speci c binding af nity with biotin
which ultimately leads to the aggregation. This aggrega-
tion could be exploited to examine at nano level due to
ultra-small structure of a atoxin M1 molecule.
The binding of bimAFM1 antibody and Au-Fe
3
O
4
NPs-FITC labeled STV was shown in uorescence micro-
scopic images. Figure 9 (A), (B) and (C) shows the green
uorescence which indicating the FITC labeled STV
binding with Au-Fe
3
O
4
NPs, bimAFM1 antibody binding
with Au-Fe
3
O
4
NPs-FITC labeled STV and aggregation
between Au-Fe
3
O
4
NPs-FITC labeled STV and bimAFM1
antibody with AFM1. By antigen–antibody reaction
anti-AFM1 and a atoxin M1 developed color by the
aggregation of gold nanoparticle conjugate Hoskins et
al., (2012). Figure 9 (C) shows the pinkish red color in
bright ied microscopy which con rm the aggregation
Au-Fe
3
O
4
NPs-FITC labeled STV-bimAFM1 antibody and
AFM1.M. Adarsha et al., (2015) has reported the detec-
tion of a atoxin B1 byFe3O4 NPs bioconjugation. Our
study is at par to this with slight difference i.e. a atoxin
M1 detection instead of a atoxin B1.
CONCLUSION
Iron oxide nanoparticles of 10±2 nm has been prepared
by the co-precipitation method and in a next step, the
nanoparticles have been coated with gold shell of gold
it con rmed by super paramagnetic properties of Au-
Fe3O4 shows a difference of the magnetization for the
coated magnetite nanoparticles in comparison with the
uncoated ones. The Fluorescence microscopic charac-
terization shows the successful bioconjugation between
Au-Fe
3
O
4
NPs-FITC labeled STV-bimAFM1 antibody and
AFM1.
448 FORMULATION OF DETECTION BIOCONJUGATES OF GOLD COATED IRON OXIDE NANOPARTICLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Jayesh H Kabariya and Vimal M Ramani
REFERENCES
Adarsha M. H., Ramani V. M. and Madhusudhan B. (2015)
Development of Fluorophore Labeled Anti-A atoxin Antibod-
ies Coated SPIONs for Detection of A atoxin B1. J. of Biona-
nosci. Vol. 9: Pages 94–101
Baniukevic J., Boyaci I. H., Bozkurt A. G., Tamer U. Ramana-
vicius A. and Ramanaviciene A. (2013) Magnetic gold nano-
particles in SERS-based sandwich immunoassay for antigen
detection by well oriented antibodies. Biosensors and Bioelec-
tronics Vol. 43: Pages 281–288
Behera S. S., Patra J. K., Pramanik K., Panda N. and Thatoi H.
(2012) Characterization and Evaluation of Antibacterial Activi-
ties of Chemically Synthesized Iron Oxide Nanoparticles. W. J.
of Nano Sci. and Eng. Vol. 2: Pages 196-200
Carrasco Y. R., Luana I., Anna G., Giulia G., Jordi M. and
Alberto R. (2018) Simultaneous Determination of AFB1 and
AFM1 in Milk Samples by Ultra High Performance Liquid
Chromatography Coupled to Quadrupole Orbitrap Mass Spec-
trometry. Beverages Vol. 4 No 43 Pages 1-9
Eivari H. A. and Rahdar. A. (2013) Some Properties of Iron
Oxide Nanoparticles Synthesized in Different Conditions.
World Applied Prog. Vol. 3 No 2: Pages 52-55
Espinosa-Calderon A., Contreras-Medina L. M., Munoz-Huerta
R. F., Millan-Almaraz J. R., Guevara-Gonzalez R. G. and Tor-
res-Pacheco., I. (2011) Methods for Detection and Quanti ca-
tion of A atoxins. intechopen. Vol. 7: Pages 109–128
Flores M.E. and Gonzalez P.E. (2017) An LC–MS/MS method
for multi-mycotoxin quanti cation in cow milk. Food Chem.
Vol. 218 Pages 378–385
Gacem M. A. and Hadj-Khelil A. O. E. (2016) Toxicology bio-
synthesis bio-control of a atoxin and new methods of detec-
tion. Asian Paci c J. Trop. Biomed. Vol. 6 No 9: Pages 808-814
Gretch D. R., Suter M. and M. F. Stinski. (1987) Anal. Biochem.
Vol. 16 No 3: Pages 263-270
Gupta A. K. and Gupta M. (2005) Synthesis and surface engi-
neering of iron oxide nanoparticles for biomedical applica-
tions. Biomaterials Vol. 26 No 18: Pages 3995–4021
Hoskins C., Min Y., Gueorguieva M., McDougall C., Volovick A.,
Prentice P., Wang Z., Melzer A., Cuschieri A. and Wang L. (2012)
Hybrid gold-iron oxide nanoparticles as a multifunctional plat-
form for biomedical application. J. of Nanobiotech. Pages 10-27
Ketney O., Santini A. and Oancea S. (2017) Recent a atoxin
survey data in milk and milk products: A review. Int. J. Dairy
Technol. Pages 320–331
Kouassi G. K., Wang P., Sreevatan S. and Irudayaraj J. (2007)
Aptamer-Mediated Magnetic and Gold-Coated Magnetic Nan-
oparticles as Detection Assay for Prion Protein Assessment.
Biotechnol. Prog. Vol. 23: Pages 1239-1244
Lyon J. L., David A., Matthew F., Stone B., Schiffer P. and
Williams M. E. (2004) Synthesis of Fe Oxide Core/Au Shell
Nanoparticles by Iterative Hydroxylamine Seeding. Nano Let-
ters Vol. 4 No 4: Pages 719–723
FIGURE 9. Fluorescence and bright eld Microscopy images. (A) Au-Fe
3
O
4
NPs-FITC labeled STV
(B) Au-Fe
3
O
4
NPs-FITC labeled STV-bimAFM1 antibody (C) Au-Fe
3
O
4
NPs-FITC labeled STV-
bimAFM1 antibody and AFM1 aggregation under uorescence and bright eld.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS FORMULATION OF DETECTION BIOCONJUGATES OF GOLD COATED IRON OXIDE NANOPARTICLE 449
Fluorescence Field
Bright Field

Jayesh H Kabariya and Vimal M Ramani
Stroka, J. and Anklam, E. (2002) New strategies for the screen-
ing and determination of a atoxins and the detection of
a atoxin-producing moulds in food and feed. TrAC Trends in
Analytical Chemistry Vol. 21 No 2: Pages 90–95
Tamer U., Cetin D., Suludere Z., Boyaci I. H., Temiz H. T.,
Yegenoglu H., Daniel P., Dinçer I. and Elerman Y. (2013) Gold-
Coated Iron Composite Nanospheres Targeted the Detection of
Escherichia coli. Int. J. Mol. Sci. Vol. 14: Pages 6223-6240
Tamer U., Gundogdu Y., Boyaci I. H. and Pekmez K. (2010)
Synthesis of magnetic core–shell Fe3O4–Au nanoparticle for
biomolecule immobilization and detection. J Nanopart Res.
Vol. 12: Pages 1187–1196
450 FORMULATION OF DETECTION BIOCONJUGATES OF GOLD COATED IRON OXIDE NANOPARTICLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS