
Toxicological
Communication
Biosci. Biotech. Res. Comm. 11(3): 434-441 (2018)
Histopathological and ultrastructural changes in the
gill and liver of fresh water sh
Channa punctatus
exposed to sodium arsenite
Titikksha Das
1
* and Mamata Goswami
2
1
Department of Zoology, Gauhati University, Guwahati, 781014, Assam
2
Department of Zoology, Cotton College, Guwahati, 781001, Assam
ABSTRACT
Arsenic, is one of the most important and concerned global environmental toxicants. Correlations have been found
between chronic arsenic poisoning and many severe health effects including cancers, hypertension and ischemic
heart disease etc. However, the proper understanding of the role of arsenic in the cause of these diseases is still
limited. In this work, we studied the toxicity effect of sodium arsenite in the gill and liver tissues of fresh water sh
Channa punctatus and for the rst time observed the histopathological as well as surface ultrastructural changes on
it. The liver and gill tissues of Channa punctatus were exposed to sub-lethal (12 ppm: parts per million) concentration
of sodium arsenite (NaAsO2) for 96 hours. The histopathological effects of sodium arsenite on the liver and gill tissues
were studied by light microscopy. The surface ultrastructural changes on the same tissues were investigated by scan-
ning electron microscopy (SEM). The results were compared with the normal structure of liver and the gill tissue of a
control group of Channa punctatus. Gill tissues exposed to arsenic showed hyperplasia, desquamation, and necrosis
of epithelium, epithelial lifting, oedema, lamellar fusion, collapsed secondary lamellae, curling of secondary lamellae
and aneurism in the secondary lamellae. Hepatic lesions in the form of cloudy swelling of hepatocytes, congestion,
vacoular degeneration, karyolysis, dilation of sinusoids and nuclear hypertrophy were observed in the liver tissue of
the exposed group. Thus it has been shown that sodium arsenite can produce signi cant damage in the ultrastructure
of liver and gill tissues. Also the histological and ultrastructural changes on the liver and the gill tissue indicate that
arsenic is biologically reactive and gives rise to acute poisoning.
KEY WORDS:
CHANNA PUNCTATUS
, GILL, HISTOPATHOLOGY, LIVER, SODIUM ARSENITE
434
ARTICLE INFORMATION:
*Corresponding Author: titikkshadas.89@gmail.com
Received 12
th
July, 2018
Accepted after revision 27
th
Sep, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.3/12

Titikksha Das and Mamata Goswami
INTRODUCTION
Contamination of water by arsenic compounds and its
toxicological effect on aquatic organism is a major world-
wide problem. Geogenic processes and anthropogenic dis-
turbances are the two main causes of dispersal of arsenic
in aquatic environment (Bears et. al., 2006; Gonazalez et.
al., 2006). Several countries including Argentina, Bangla-
desh, Chile, China, India, Japan, Mexico, Mongolia, Nepal,
Poland, Taiwan, Vietnam, and some part of United States
have been reported with high concentration of arsenic in
groundwater (Anowar et. al., 2002; Mitra et. al., 2002;
Smith et. al., 2001; Chowdhury et. al., 2000). A correlation
has been found between chronic arsenic poisoning and
many health effects including cancers, melanosis, hyper-
keratosis, restrictive lung disease, peripheral vascular dis-
ease, gangrene in leg, skin, lung, bladder, liver, diabetes
mellitus, hypertension and ischemic heart disease (Ana-
war et. al., 2002). It is evident that arsenic exposure has
multiple effects at the molecular level for instance liver
chromosomal DNA fragmentation, expression of certain
proteins, differential expression of genes involved in cell
cycle regulation, signal transduction, stress response,
apoptosis, cytokine production, growth-factor and hor-
mone-receptor production (Hossain et. al., 2003; Tabellini
et. al., 2005; Ahmed et. al., 2008; Sangeeta et. al., 2012
Paruruckumani et al., 2015).
Both in laboratory and eld studies histopathologi-
cal investigations have been long recognised as reli-
able biomarkers of stress in sh and in the evaluation
of the health of sh exposed to contaminants. The gills,
liver and kidney are the common primary target organs
for many chemicals primarily because of their vital
role within the body (Chowdhury et. al., 2000; Hossain
et. al., 2000, Paruruckumani et al., 2015).
In this work, we studied the toxicity effect of sodium
arsenite in the gill and liver tissues of fresh water sh
Channa punctatus and for the rst time observed the his-
topathological as well as surface ultrastructural changes
on it. We also estimated a critical value of concentration
of sodium arsenite above which shes are likely to be
killed. A commonly useful measure of toxicity LC50 is
used for this purpose. The goal of this study was, rstly,
to observe any histological changes, arsenic could bring
to the vital organs of living animal and secondly, to sub-
stantiate the role of arsenic as a toxic environmental
agent which can cause many severe health effects.
MATERIALS AND METHODS
For the present study healthy and disease free shes
Channa punctatus (weight 22-50 gm) were collected from
local markets in Guwahati. After disinfection with a dip of
2% potassium permanganate (KMnO
4
) solution the shes
were acclimatised in aquaria for two weeks before initia-
tion of experiment. The water provided in the aquaria was
from the tap water in the laboratory and was changed
on the following day. The shes were fed everyday with
sh food available in the market. Proper aeration was
done during these periods. Sodium Arsenite (NaAsO
2
),
molecular weight-129.91 Merck, India (Ltd.) was procured
for performing the experiment. A stock solution was pre-
pared with water from which the test concentration was
prepared by dilution. The control group of shes were
kept in similar conditions without adding sodium arsen-
ite. Fishes were exposed to 5 different concentration of
Sodium Arsenite of 5, 15, 25, 35 and 45 ppm. The toxicity
bioassay was performed in semi-static system in triplicate
with 10 specimens exposed for each concentration in each
set in accordance with the standard methods of acute tox-
icity bioassay procedures (APHA, 2005).
Fishes were transferred to each aquarium and exposed
to ve different concentrations such as 5, 15, 25, 35 and
45 ppm of sodium arsenite. In all cases, control groups
of shes were maintained. Each experimental trial was
carried out for a period of 96 hours. The mortality rate of
the sh was recorded at logarithmic time intervals that
is, after 6, 12, 24, 48, 72 and 96 hours of exposure. The
test media was renewed daily during the experimental
period. The data obtained in course of the investigation
were analysed statistically to see whether there is any
in uence of different treatment concentrations on the
mortality of the sh. Fishes were exposed to sub lethal
concentration i.e. 12ppm of sodium arsenite along with
a control group for 96 hours. At the end of the exposure
period, shes were randomly selected for histopatho-
logical examinations. Gill, liver, tissues were isolated
from normal and experimental sh. Physiological saline
solution (0.75% NaCl) was used to rinse and clean the
tissue. They were xed in aqueous Bouins solution for
24 hours, processed through graded series of alcohols,
cleared in xylene and embedded in paraf n wax. Sec-
tions were cut at 4 micron thickness and stained with
Hematoxylin and eosin stain. Histopathological lesions
were examined and photographed with the help of com-
puter attached Bright Field Microscope (Leica DM 3000).
Gills and liver tissues of both the control and treated
groups were rapidly removed and processed routinely
for scanning electron microscopic studies. Gills and liver
tissues were cut into small pieces of 1 mm thickness and
xed in 2.5 % glutaraldehyde prepared in cacodylate
(sodium phosphate) buffer adjusted to pH 7.4 for 24
hours and afterward washed in phosphate buffer for 15
min. After dehydration in ascending series of acetone,
samples were immersed in Tetra Methyl Silane for 10
minutes at 4 degree centrigrate. Then they were brought
to room temperature to dry. The specimens were mounted
on Aluminium Stubs coated with gold and observed
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOPATHOLOGICAL AND ULTRASTRUCTURAL CHANGES IN THE GILL AND LIVER 435
Titikksha Das and Mamata Goswami
through scanning electron microscope in Sophisticated
Analytical Instrument Facility (SAIF), North-Eastern Hill
University (NEHU), Shillong – 793022.
RESULTS AND DISCUSSION
The mortality rate of Channa punctatus to different con-
centration of sodium arsenite can be seen in Figure 1. In
the present study, it was observed that 45 ppm sodium
arsenite in water induced death of all the exposed shes
within 96 hours. The 96 hours LC50 of sodium arsenite
for Channa punctatus was found to be 25 ppm. Fishes
treated with a concentration of 5, 10 and 12 ppm sur-
vived for more than 90 days with zero mortality rates.
The sub lethal concentration of sodium arsenite for the
exposed group of sh was 12 ppm. The control group of
sh were in good condition without any morphological
changes. But the sodium arsenite treated sh showed
rapid movement of ns and operculum. They produced
a lot of slime around their body. Their overall activities
decreased with time.
In the liver tissue of control channa punctatus, there
was normal structure and systematic arrangement of
hepatocytes. Hepatic cells were roundish, polygonal
containing clear spherical nucleus which can be seen in
the Figure 2. The normal histological arrangement was
FIGURE 1. Graphical representation of 96 hours LC50 of Sodium
arsenite treated Channa Punctatus. It shows mortality rate of
Channa Punctatus to different concentration of sodium arsenite,
(ppm: parts per million).
FIGURE 2 Optical Micrograph of liver tissue of control group of Channa punc-
tatus. (N-Nucleus, HC-Hepatic cell, GC-Granular cytoplasm).
436 HISTOPATHOLOGICAL AND ULTRASTRUCTURAL CHANGES IN THE GILL AND LIVER BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Titikksha Das and Mamata Goswami
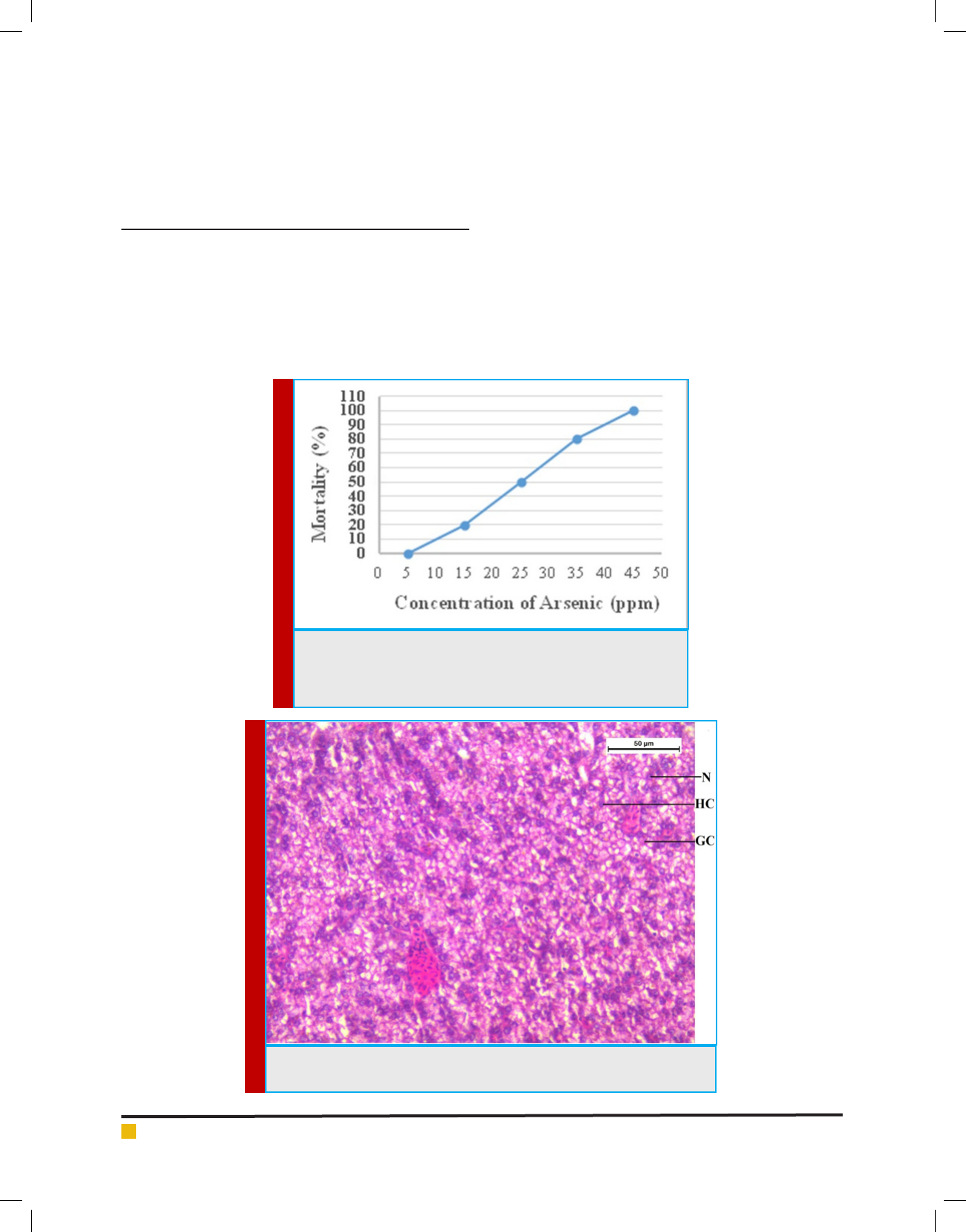
FIGURE 3. Optical Micrograph of liver tissue of sodium arsenite treated Channa
punctatus. (VF-Vacuole formation, NH-Nuclear Hypertrophy, CD-Cytoplasmic
Deformation).
FIGURE 4. Scanning Electron Micrograph of Liver tissue in the control-
group of Channa punctatus.
not found in the liver tissue of sodium arsenite treated
channa punctatus. A micrograph of liver tissue of sodium
arsenite treated channa punctatus is shown in the Figure
3. The micrograph shows a lot of rupture of blood ves-
sels, necrotic tissue with marked loss of hepatocytes and
extensive area of vacuolation in the liver tissue. Figure 3
also reveals large lipid droplets and abundant glycogen
in most of the area of hepatocytes of liver tissue.
A Scanning Electron Micrograph of liver tissue
in control channa punctatus is shown in the Figure 4
which represents normal ultrastructural morphology of
hepatocytes. Serous membranes with some connective
tissue are seen in the surface of the liver tissue. Hepatic
cells are seen with clear spherical nucleus. Liver is the
primary organ for detoxi cation of foreign compounds
(Gernhofer et al., 2011) and one of the most affected
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOPATHOLOGICAL AND ULTRASTRUCTURAL CHANGES IN THE GILL AND LIVER 437
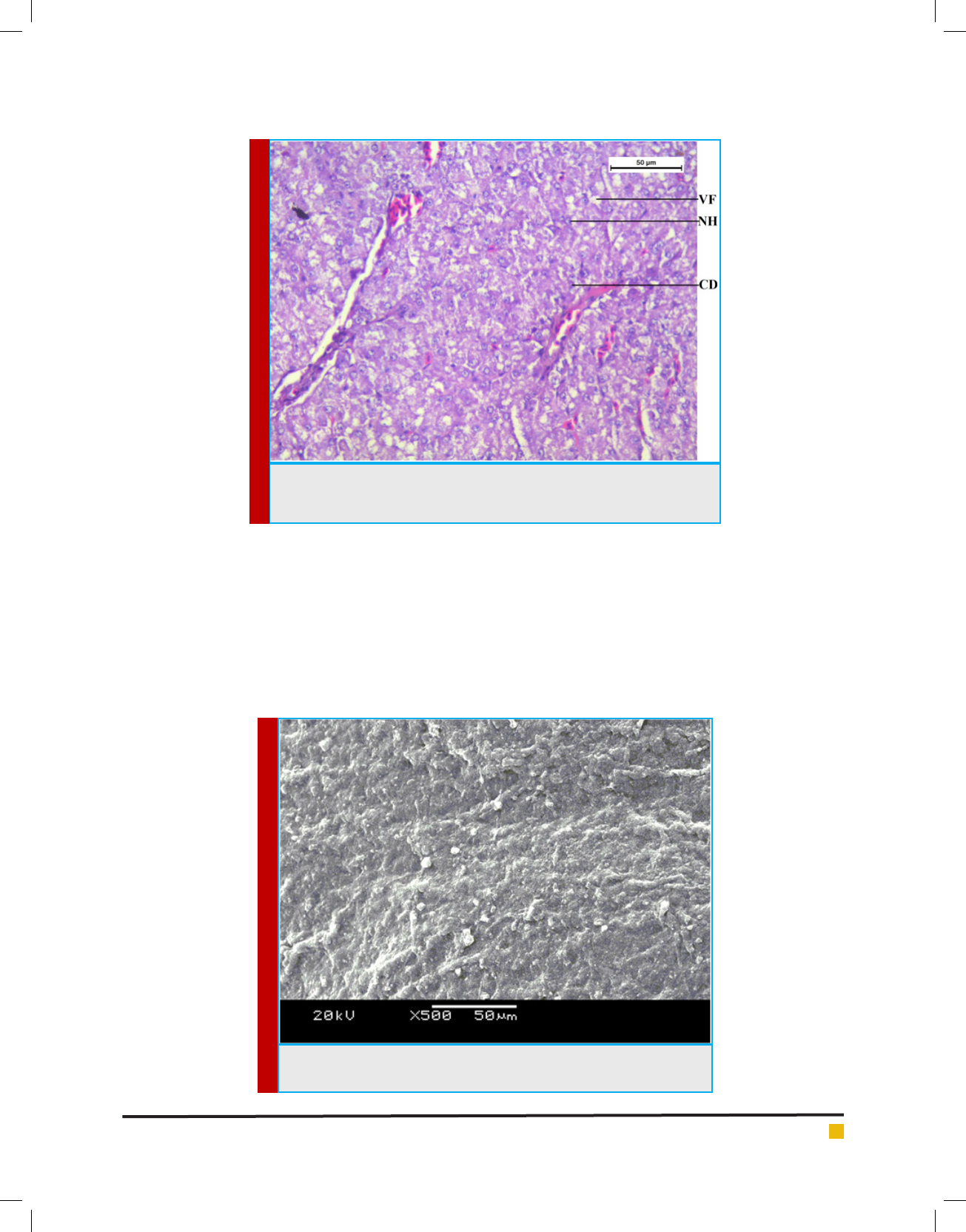
Titikksha Das and Mamata Goswami
FIGURE 5. Scanning Electron Micrograph of Liver tissue in the group of
Channa punctatus treated with sodium arsenite (CSH- Cloudy Swell-
ing of Hepatocytes, VD-Vacuolar Degeneration, N-Necrosis).
FIGURE 6. Optical Micrograph of Gill tissue of control group of Channa
punctatus. (PGL-Primary Gill Lamellae, CA-Central axis, SGL-Secondary Gill
Lamellae, GZ-Growth Zone).
organs by contaminants in water (Camargo, Martinez,
2007). In our study it has been found that sodium arsen-
ite caused several damages in the liver tissue which
includes destruction of normal arrangement of the cells,
vacuolar degeneration of cytoplasm, necrosis and cloudy
swelling of hepatocytes. These changes are represented
in the Figure 5. In earlier studies (Ahmed et al., 2008;
Sangeeta et al., 2012) on sodium arsenite treated Channa
punctatus showed concentration dependent reduced
cell viability and chromosomal DNA fragmentation of
liver cells. Finding of Ahmed et al., 2008, revealed that
lower concentration of sodium arsenite induced apop-
totic death of cells while higher concentration induced
necrotic cell death.
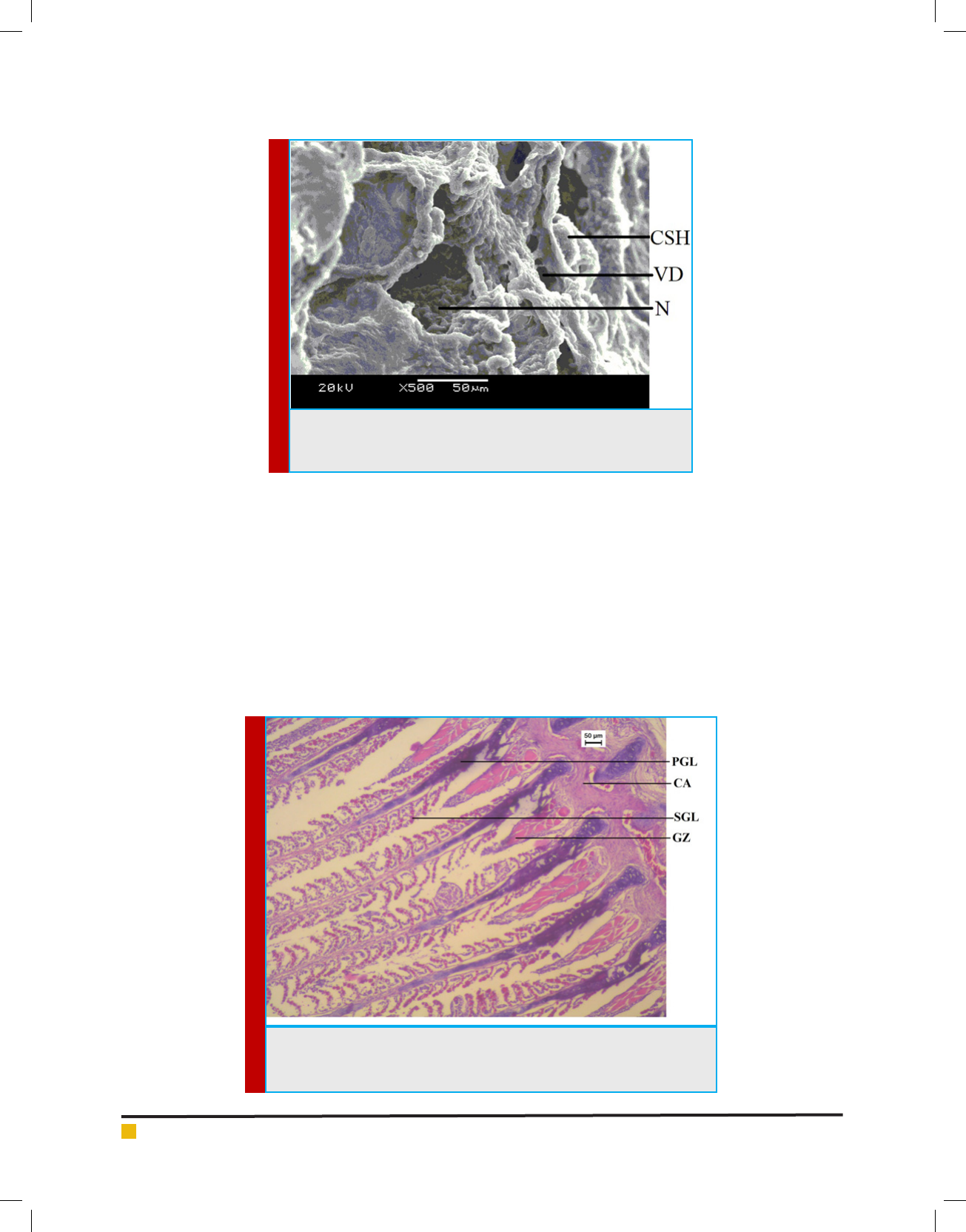
In Channa punctatus there are four pairs of semicir-
cular gill arches. Each gill arch has a row of microscopic
primary gill lamellae on which secondary gill lamellae
are arranged bilaterally. In the control group normal
structure of gill lamellae were observed (Figure 6). The
histology of the treated sub lethal exposure revealed loss
of structural integrity of lamellae. It also shows destruc-
tion of cartilaginous gill bar, degenerated primary and
secondary gill lamellae, lamellar fusion and capillary
lumen and that can be clearly seen from Figure 7. Anal-
438 HISTOPATHOLOGICAL AND ULTRASTRUCTURAL CHANGES IN THE GILL AND LIVER BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
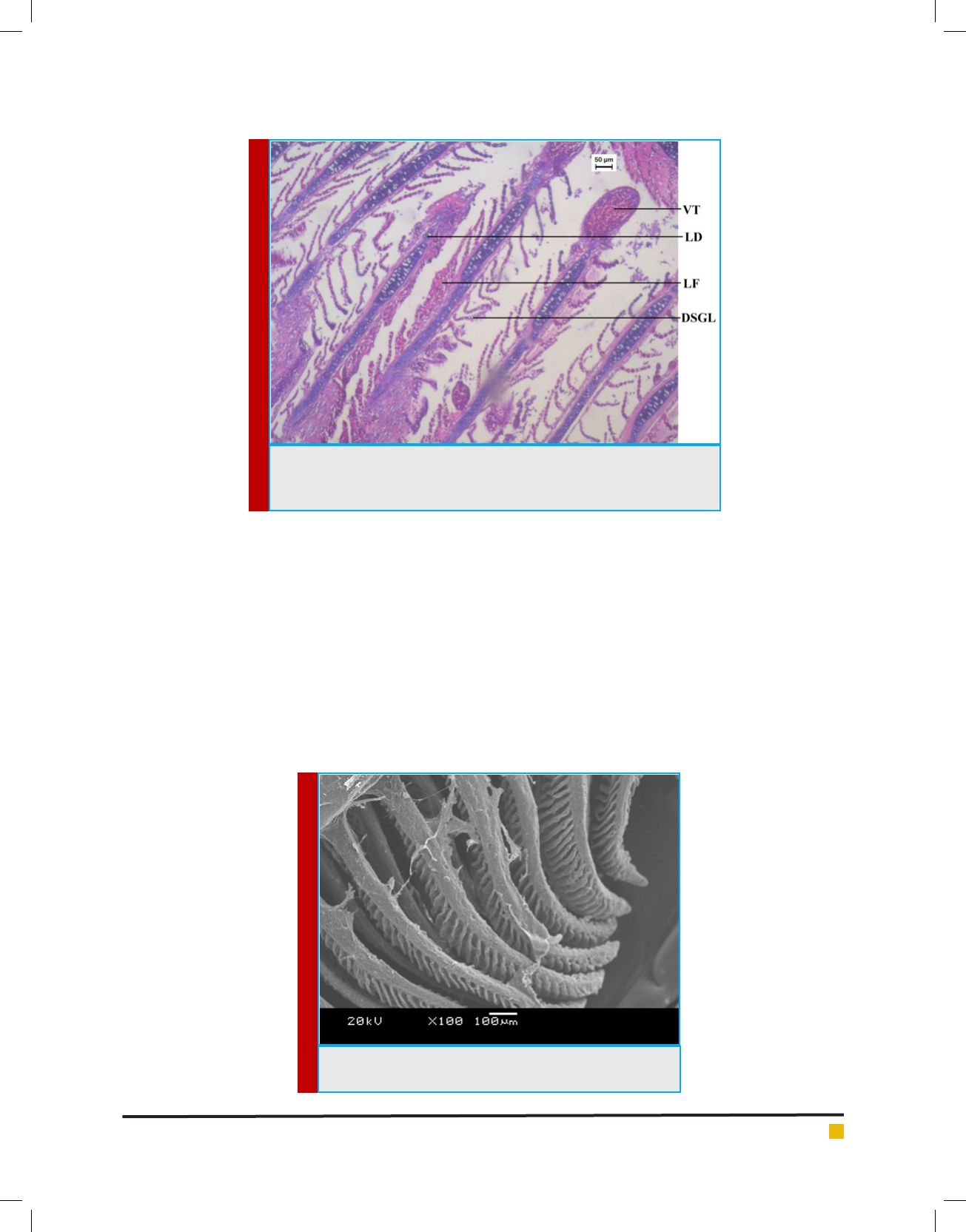
Titikksha Das and Mamata Goswami
FIGURE 7. Optical Micrograph of Gill tissue of Channa punctatus treated with
Sodium arsenite. (VT-Vacuolization at tip region, LD- Lamellar Disorganisa-
tion, LF-Lamellar Fusion, DSGL-Distorted Secondary Gill Lamellae).
FIGURE 8. Scanning Electron Micrograph of Gill tissue in the
controlgroup of Channa punctatus.
ogous structural changes could be seen from the gill
tissue of Channa punctatus exposed to arsenic trioxide
(Agnihotri et al., 2010). Their ndings revealed degener-
ative changes in cartilaginous bar and increased mucous
secretion between the spaces of primary gill lamella
while capillary lumen developed enlarged spaces in gills
of Channa punctatus exposed to arsenic trioxide. The
secondary gill lamellae of arsenic trioxide treated sh
showed destruction of epithelial cells, vacuolization in
the tip of the primary gill ray, gill hyperplasia and lamel-
lar fusion (Agnihotri et al., 2010). Pathological lesions in
the gill tissue induced by sodium arsenite were similar
to cadmium induced gill tissue of Labeo rohita (Muthu-
kumaravel et al., 2013). Copper induced gill tissues of
Oreochrombis mossambicus showed marked alternations
which were studied by Radhika and Krishnamoorthy
(Radhika et al., 2010).
Figure 8 shows a normal architecture of gills in the
control group of sh. Normal structure of primary gill
lamella, secondary gill lamella and micro ridges on the
normal gill epithelium were observed. In the gill tis-
sue of sodium arsenite treated sh fusion of secondary
lamella, necrosis and deformation of the gill tissue were
observed and that can be seen in the Figure 9. The SEM
micrograph of gill in sodium arsenite treated Channa
Punctatus (Figure 9) also reveals swelling and curling
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOPATHOLOGICAL AND ULTRASTRUCTURAL CHANGES IN THE GILL AND LIVER 439

Titikksha Das and Mamata Goswami
of secondary lamellae, complete fusion of secondary
lamellae and surface wrinkling in numerous areas of the
gill tissue. These observations are in accordance to those
reported in Surface ultrastructural changes in the gill
and liver tissue of Asian sea bass Lates calcarifes (Bloch)
exposed to copper (Paruruckumani et al., 2015)
CONCLUSION
This work presents a unique evidence of arsenic toxic-
ity in shes and how its sub lethal concentration causes
ultrastructural damages on gill and liver tissue. We also
have seen high sensitivity and behavioural changes in
the treated sh. The data obtained from the concen-
tration dependent study of sodium arsenite to Channa
punctatus can be used to set a standard for human expo-
sure to arsenic. Further studies on the nature of arsenic
induced damages observed on the cellular structure of
the concerned tissue could provide some insight into the
mechanism of arsenic poisoning on human being.
REFERENCES
Anwar, H. M., Akai, J., Mostofa, K. M., Saifullah, S., & Tareq, S.
M. (2002). Arsenic poisoning in ground water health risk and
geochemical sources in Bangladesh. Environ In, 36, 962-968.
Ahmed, K., Akhand, A. A., Hasan, M., Aslam, M., & Hasan, A.
(2008). Toxicity of arsenic (Sodium Arsenite) to fresh water
spotted snakehead Channa punctatus (Bloch) on cellular death
and DNA content. American-Eurasian J Agric & Environ Sci,
4, 18-22.
Agnihotri, U. S., Bahadure, R. B., & Akarte, S. R. (2010). Gill
lamellar changes in fresh water sh Channa punctatus due
FIGURE 9 Scanning Electron Micrograph of Gill tissue in the group of
Channa punctatus treated with sodium arsenite (CSL-Curling of Sec-
ondary Lamellae, SSL-Swelling of Secondary Lamellae, FSL-Fusion of
Secondary Lamellae).
to in uence of arsenic trioxide. Biosci Biotech Res Comm, 3,
61-65.
Bears, H., Richards, J. G., & Schulte, P. M. (2006). Arsenic
exposure alters hepatic arsenic species composition and stress
mediated gene expression in the common Killi sh (Fundulus
heteroclitus). Aqua Toxico, 77, 257-266.
Chowdhury, U. K., Biswas, B. K., Chowdhury, T. R., Samanta,
G., Mandal, B. K., & Basu, G. C. (2000). Ground water arsenic
contamination in Bangladesh and West Bengal, India. Environ
Health Perspect, 108, 393-397.
Camargo, M. M., & Martinez, C. (2006). Biological and physio-
logical biomarkers in Prochilodus lineatus submitted to in situ
tests in an urban stream in southern Brazil. Environ Toxicol
Pharmacol, 21, 61-69.
Gernhofer, M., Pawet, M., Schramm, M., Muller E., & Trieb-
skorn, R. (2011). Ultrastructural biomarkers as tools to charac-
terize the health status of sh in contaminated streams. Aqua
Ecosyst Stress Recov, 8, 241-260.
Gonazalez, H. O., Roling, J. A., Baldwin W. S., & Bain, L. J.
(2006). Physiological changes and differential gene expression
in mummichog (Fundulus heteroclitus) exposed to arsenic.
Aqua Toxico, 77, 43-52.
Hossain, K., Akhand, A. A., Kato, M., Du, J., Takeda, K., Wu,
J., Takeuchi, K., Liu, W., Suzuki, H., & Nakashima, I. (2000).
Arsenic induces apoptosis of murine T lymphocytes through
membrane raft-linked signaling for activation of c-Jun amino-
terminal kinase. J Immunol,165, 4290-4297.
Hossain, K., Akhand, A. A., Kawamoto, Y., Du, J., Takeda, K.,
Wu, J., Youshihara, M., Tsuboi, H., Takeuchi, K., Kato, M.,
Suzuki, H., & Nakashima, I. (2003). Caspase activation is accel-
erated by the inhibition of arsenic-induced, membrane raft-
dependant Akt activation. Free Radic Biol Med, 34, 598-606.
Mitra, A. K., Bose, B. K., Kabir, H., Das, B. K., & Hussain,
M. (2002). Arsenic-related health problems among hospital
440 HISTOPATHOLOGICAL AND ULTRASTRUCTURAL CHANGES IN THE GILL AND LIVER BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Titikksha Das and Mamata Goswami
patients in southern Bangladesh. J Health Popul Nutr, 20, 198-
204.
Muthukumaravel, K., Prithiviraj, N., Ramesh, M., Sekar, V., &
Sheik Mohamed Salahuen, B. (2013). Light and scanning elec-
tron microscopic evaluation and effect of Cadmium on the gill
of the freshwater sh, Labeo rohita. Int J Pharmace Biol Arch,
4, 999-1006.
Paruruckumani, P. S., Maharajan, A., Ganapiriya, V., Naraya-
naswamy, Y., & Raja Jeyasekar, R. (2015). Surface ultrastruc-
tural changes in the gill and liver tissue of Asian sea bass Lates
calcarifes (Bloch) exposed to copper. Biol Trace Elem Res, 168,
5000-5007.
Radhika, R., & Krishnamoorthy, R. (2010). Effect of copper sul-
phate on histological changes in the fresh water sh Oreo-
chromic Massambicus. J Ecotoxicol Environ Monit, 20, 431-
435.
Smith, A. H., Lingas, F. O., & Rahman, M., (2001). Contami-
nation of drinking-water by arsenic in Bangladesh: A public
health emergency. Bull World Health Organ, 78, 1023-1103.
Sangeeta, D., Unni, B., Bhattacharjee, M., Wann, S. B., & Gan-
gadhar Rao, P. (2012). Toxicological effects of arsenic exposure
in a freshwater teleost sh Channa punctatus, African J Bio-
tech, 11, 4447-4454.
Tabellini, G., Tazzari, P. L., Bortul, R., Evanquelisiti, C., Billi,
A. M., Grafone, T., Baccarani, M., & Martelli, A. M. (2005).
Phosphoinositide 3-Kinase/Akt inhibition increases arsenic
trioxide-induced apoptosis of acute promyelocytic and T-cell
leukaemias. J Haematol, 130, 716-725.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS HISTOPATHOLOGICAL AND ULTRASTRUCTURAL CHANGES IN THE GILL AND LIVER 441