
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(3): 416-425 (2018)
Enhanced production of alkaline protease from novel
bacterium
Bacillus cereus
GVK21 under submerged
fermentation
Keshavamurthy M
1
, Vishwanatha T
2
, Suresh Kumar M
1
and Subhaschandra M Gaddad*
1
1
Department of Post Graduate Studies and Research in Microbiology, Gulbarga University,
Kalaburagi-585106, Karnataka, India
2
Department of Microbiology, Maharani’s Science College for Women, Bengaluru - 560 001 Karnataka, India
ABSTRACT
Alkaline proteases are an important class of enzymes with potential industrial and commercial applications. In the
present study, 52 bacterial isolates from various soil samples have been evaluated for the production of extracellular
protease and selected one potent isolate based on maximum casein hydrolysis. Further, it is identi ed as Bacillus
cereus GVK21 based on biochemical characteristics and 16S rRNA gene sequence analysis (KY659318). This organism
produced 136 U/mL of protease within 48 hrs of incubation. Maximum production of protease was recorded at pH 9
and a temperature of 40°C. The present study is attempted to exploit new economical media, based on agro wastes
recipes for the increased production of alkaline proteases. B.cereus GVK21 produced high levels of protease (1762 U/
ml) on groundnut oil cake (1.5 %) as a substrate.The results of the study show that this isolate can be further exploited
for commercial production of protease.
KEY WORDS: PROTEASE;
BACILLUS CEREUS
; 16S RRNA; OIL CAKES; INDUSTRIAL PRODUCTION
416
ARTICLE INFORMATION:
*Corresponding Author: smgaddad@gmail.com
Received 12
th
July, 2018
Accepted after revision 21
st
Sep, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.3/10

Keshavamurthy et al.
INTRODUCTION
The emergence of new innovations are opening up new
avenues in the areas of industrial biotechnology for the
production of various bulk chemicals and value added
products using inexpensive substrates (Binod et al.,
2013). In the recent years, enzymes are replacing chemi-
cal catalysts in food, leather goods, pharmaceuticals
and textiles industry(Singh etal., 2016). Proteases are a
group of enzymes with wide range of applications, and
account for 40 – 60% of the total enzyme sales with two
thirds of them produced majorly from microorganisms
(Kumar etal., 2011; Deshmukh and Vidhale, 2015).
Proteases are highly complex group of hydrolytic
enzymes and occupy a pivotal status with regard to
their medicinal and industrial applications (El-Bakry
etal., 2015). Proteases are produced by a wide range of
sources(Sharma etal., 2017). The majority of commer-
cially available enzymes are obtained from microbial
origin (Raj etal., 2012). Microbial proteases play a vital
role in biotechnological processes and constitute one of
the most important groups of industrial enzymes, sell-
ing product segment in the global market accounting for
60% market share (Kumar et al., 2014).Bacillus genus
is one of the most important producers of extracellular
proteases and industrial sectors very often use Bacillus
species for the production of proteases(Contesini etal.
2018).
In the recent years, bulk chemicals and value-added
products such as organic acids, amino acids, enzymes,
ethanol, and single cell protein etc., are produced by the
utilization of agro-industrial residues as raw materials
(Pandey etal., 2000; Singhania etal., 2008). Oil cakes
or oil meals are the residues obtained after oil extraction
from the seeds and are rich in proteins, ber and energy
contents (Ramachandran etal.,2007).The increase in the
demand leads the attention of researchers to explore
novel sources for proteases, where isolation, screening
and characterization of new promising strains are a con-
tinuous process. Therefore, the present study focuses on
the selection of high yielding stable proteolytic bacte-
ria from mining soil sample which is considered as an
extreme environment region.
MATERIALS AND METHODS
SAMPLE COLLECTION AND ISOLATION
Soil samples were collected in sterile containers from
different locations within the mining area of Raichur
district (16
o
11
’
45
”
North Latitude and 76
o
38’ 31
”
East
Longitude), Karnataka, India. One gram of soil was
added into100 mL of enrichment medium [g/L: Casein –
10.0 and NaCl – 5.0 at pH 9.0] in a 250 mL of Erlenmeyer
ask and incubated at 37
o
C in a rotary shaker incuba-
tor at 140 rpm for 24 hours. A loopful of the enriched
medium was streaked on Nutrient agar (NA) plates and
incubated at 37
o
C for 24 hours. Well isolated colonies
were re-streaked on NA for con rming the purity and
transferred onto NA slants and preserved for further use.
SCREENING OF THE ISOLATES FOR
EXTRACELLULAR PROTEASE PRODUCTION
The isolates were screened for protease production on
screening medium, skimmed milk agar [g/L: skim milk -
10.0; peptone - 5.0; yeast extract - 3.0; NaCl - 5.0; agar,
20 and pH 9.0](Kumar etal., 2011). After 24 hrs growth,
the plates were observed for clear zones around the colo-
nies. Proteolytic activity was further con rmed by using
gelatin as the substrate in the medium (Pant etal.,2015).
Depending on the zone of clearance, ve isolates GVK8,
GVK21, GVK29, GVK38 and GVK40, were selected for
further experimental studies.
PROTEASE PRODUCTION UNDER SUBMERGED
FERMENTATION
Protease production by the selected isolates was carried
out by submerged fermentation. One mL of fresh bacte-
rial inoculum was added in to 250 mL Erlenmeyer ask
containing 100 mL of production medium [g/L: casein-
5.0, yeast extract - 5.0; NaCl - 5.0; MgSO
4
.
7H
2
O - 0.02
and KH
2
PO
4
- 0.05 at pH 9.0]. The asks were placed in
a rotary shaker incubator at 140 rpm at 37° C for 3-4
days. An aliquot of the culture supernatant was collected
at regular time intervals of 12 hrs and assayedfor protease
activity (Josephine etal.,2012). Based on the maximum
protease production and shorter time required, the isolate
GVK 21 is a potent strain and selected for further studies
IDENTIFICATION OF THE POTENT PROTEASE
PRODUCING ISOLATE
Morphological and biochemical characteristics of the
selected isolate (GVK21) was studied and recorded as
per Bergey’s Manual of Systematic Bacteriology (Holt
etal., 2000). The bacterial isolate was further identi ed
by 16S rDNA sequence analysis using universal primers
and genomic DNA as template. The genomic DNA of the
isolate was extracted as described by Roohi etal., (2012).
The PCR ampli ed product was sequenced at Microbial
Ecology Laboratory, National Centre for Cell Science,
Pune. Phylogenetic tree was constructed with Molecu-
lar Evolutionary Genetics Analysis (MEGA) 6.0 software
using neighbor-joining method (Tamura et al.,2011).
Duly annotated partial nucleotide sequences of the
novel bacterial strain was deposited with NCBI Genbank
(http://www.ncbi.nlm.nih.gov).
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ENHANCED PRODUCTION OF ALKALINE PROTEASE 417

Keshavamurthy et al.
SCANNING ELECTRON MICROSCOPY
Scanning Electron Microscopic (SEM) analysis was done
to observe the morphology of the isolated strain.Thin
lm of the sample was prepared on a carbon coated cop-
per grid by just dropping a small amount of the bacterial
culture on the grid. Extra solution was removed using
a blotting paper. Then the lm on the SEM grid was
allowed to air dry by putting it under a mercury lamp
for 5 min. The sample was then observed under scanning
electron microscopy TESCAN (Vega 3 LMU) at a resolu-
tion of 3 nm at various magni cations at acceleration
voltage of 20.0 KV.
PROTEASE PRODUCTION IN RELATION TO THE
GROWTH OF BACTERIA
In order to study the time course for microbial growth
and protease production, the isolate B. cereus GVK 21
was inoculated in the production medium and incubated
in rotary shaker incubator at 160 rpm at 40° C upto 72
hours. The growth of the bacterium was determined by turbi-
dometry, OD at 600 nm. After the removal of cells by centrifu-
gation, the cell free supernatant was considered as the crude
enzyme solution and protease activity was measured.
EVALUATION OF AGRO RESIDUES (OIL CAKES)
FOR ENHANCED PROTEASE PRODUCTION
Major regional Oil cakes such as neem and pongamia
were collected from local market, Bengaluru, Indiaand
were ne powdered using mixer grinderand evaluated
as substrates for the production of protease. Different
type of oil cakes such as Castor oil cake (COC), Ground-
nut oil cake (GOC), Neem oil cake (NOC) and Pongamia
oil cake (POC) were supplemented individually in to 100
mL of the optimized production mediumin 250 mL of
Erlenmeyer ask. The agro wastes were used at a con-
centration of 0.5 to 2% with an increment of 0.25 %. The
amount of protease produced was determined at every 6
h up to 72 h.
PROTEASE ASSAY
The culture was centrifuged at 10,000 rpm for 5 min
at 4° C to obtain the CFS. The protease activity of the
crude enzyme was determined by the modi ed method
of Joo et al.,(2002) brie y; 0.5 mL of CFS was added
to 0.5 mL of 1% casein (as a substrate) in 0.1 M Tris-
HCl (pH 9.0) and incubated at room temperature for 10
min. The reaction was terminated by the addition of 3
mL of 10% (w/v) trichloroacetic acid (TCA). The solution
was centrifuged at 5000 rpm for 10 min. To the 3 mL
of the clear supernatant, 5 mL 0.4 M sodium bicarbo-
nate solution and 0.5 mL of Folin Ciocalteau reagent
(FCR) were added, mixed thoroughly and incubated for
30 min at room temperature, in dark. The optical den-
sity was measured using a UV-VIS spectrophotometer
(ELICO SL-159) at 660 nm against the enzyme blank.
The amount of the released aromatic amino acids was
calculated usingtyrosine standard.
One unit of protease is de ned as the amount of the
enzyme required to release 1g of tyrosine per mL per
min under the above assay conditions. Enzyme activity
was calculated according to the formula of Pant etal.,
(2015).
RESULTS AND DISCUSSION
ISOLATION, IDENTIFICATION, SCREENING
AND CHARACTERIZATION OF PROTEASE-
PRODUCING BACTERIA
In the present study, 52 independent bacteria were iso-
lated from the soil samples collected from gold mines
and were screened for proteolytic activity on skim milk
agar plates (Figure 1). A total of 28 (53.84 %) isolates
showed proteolytic activity ranging from 11 - 37 mm
of clear zones aroundthe colonies. Five bacterial iso-
lates, GVK8, GVK21, GVK29, GVK38 and GVK40, were
selected based on the higher zone of hydrolysis on skim
milk agar plates for further study. The use of skim milk
agar medium for the ef cient screening of proteolytic
bacteria has been reported by earlier researchers (Raj
etal., 2012; Ravi etal., 2015).
The selected ve isolates were further screened for
the quantitative production of the enzyme in production
medium (Figure 2). The protease production was slower
during the rst 12 hours by all the isolates. The protease
production increased signi cantly in respect of the iso-
late GVK21 during 12 to 24 hours to reach the maximum
activity and remained at that level till 48 hours. While
with respect to the other isolates, the protease activity
increased gradually and the maximum activities were
recorded at 48 hours of incubation. This indicated that
the isolate GVK21 produces a maximum protease activity
of 136 U/mL and takes shorter time in preliminary study.
The strain GVK21 is gram positive rod, motile,
spore former and was tentatively identi ed as Bacillus
sp. based on its morphological and biochemical char-
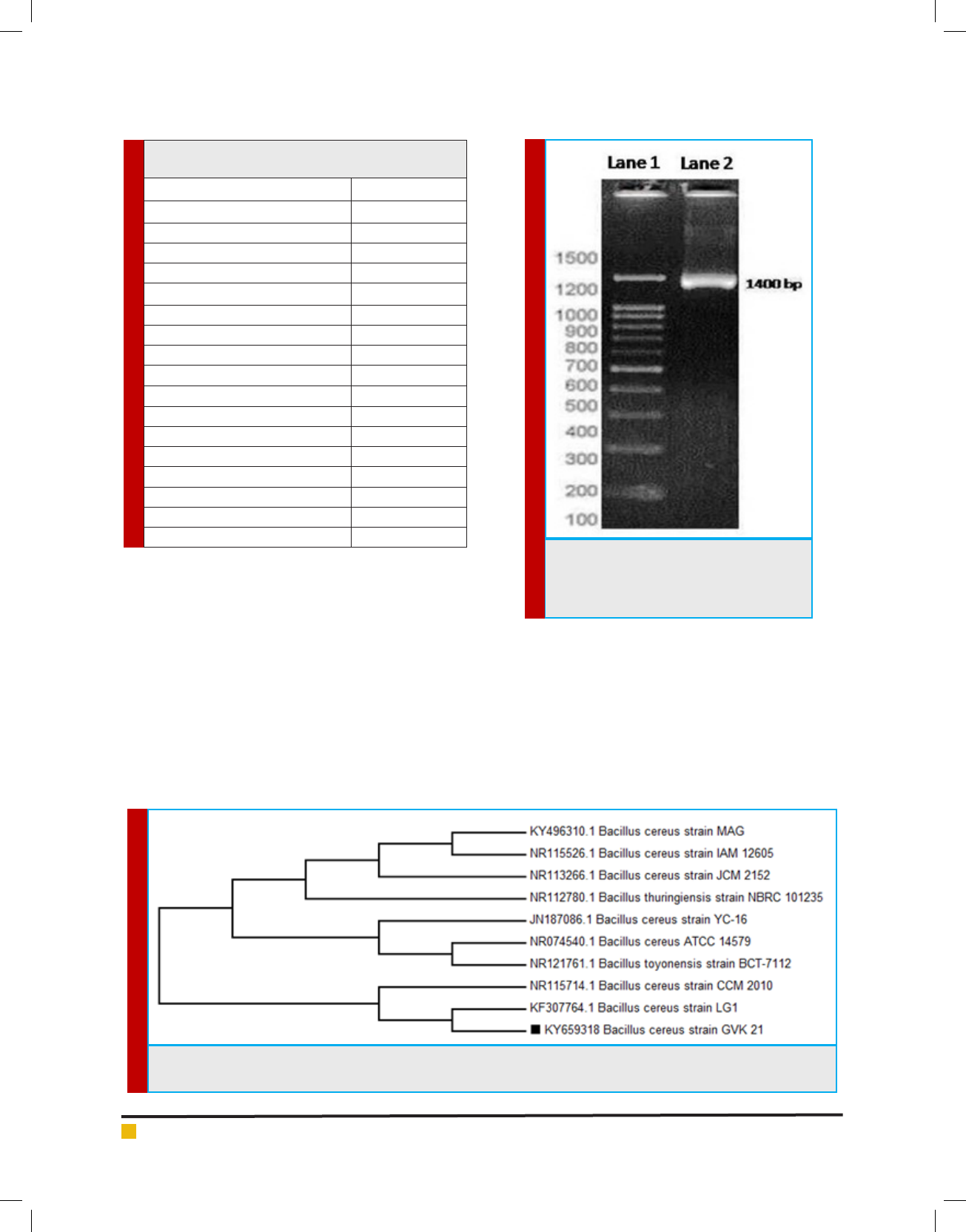
acteristics (Table 1), (Holt et al.,2000). The 16S rDNA
was ampli ed through PCR which showed1500 kb band
on 2% agarose gel (Figure 3). Subsequently, the com-
parison of the 16S rRNA gene nucleotide sequence (1387
bp) of the strain GVK21 with other 16S rRNA genes
sequences of closely related strains from NCBI database
showed that this isolate has 99 % sequence homology
with B. cereus ATCC 14579(Accession No. 074540). The
418 ENHANCED PRODUCTION OF ALKALINE PROTEASE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
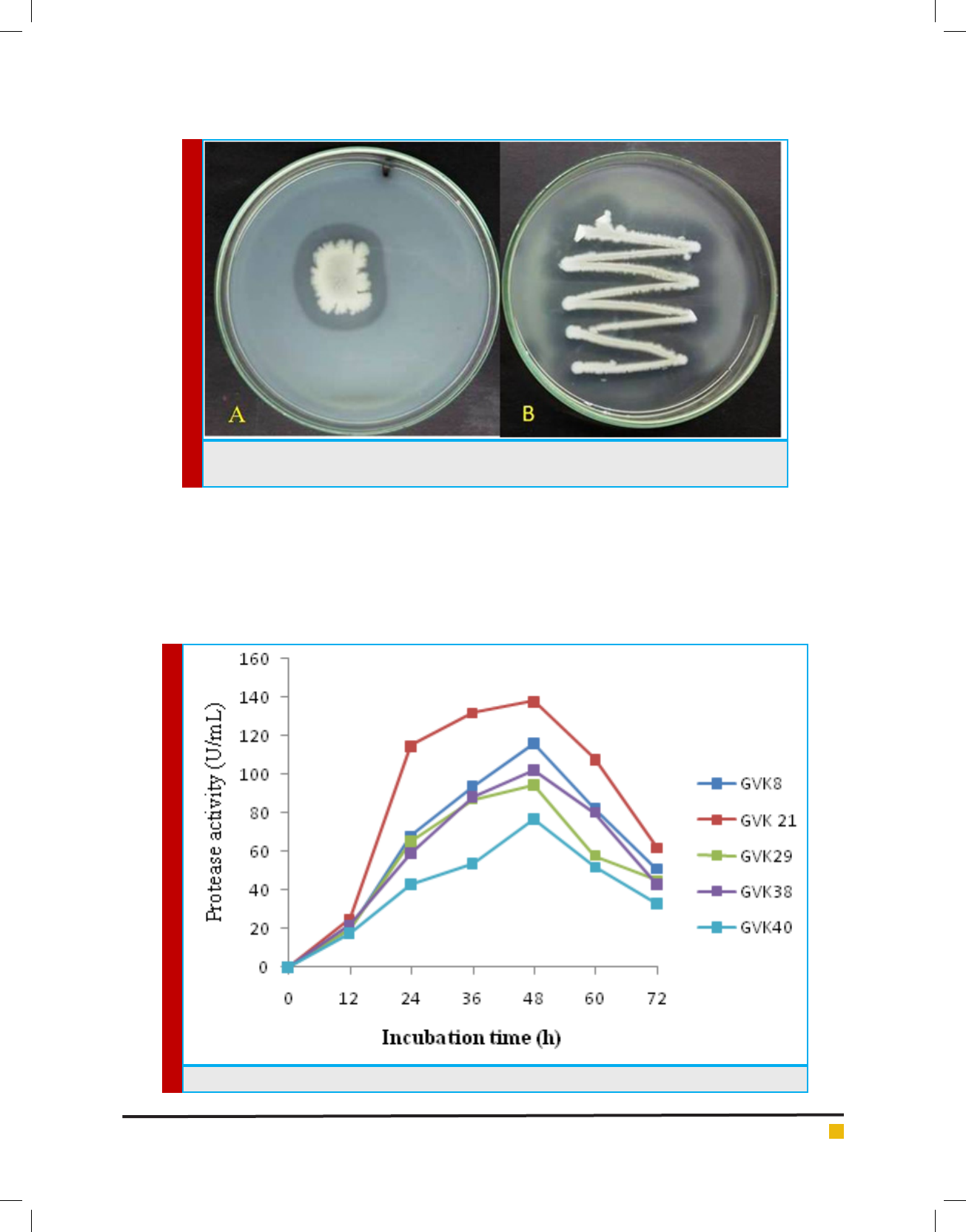
Keshavamurthy et al.
FIGURE 1. Zone of hydrolysis on A) skim milk agar B) Gelatin agar by bacterial isolate Bacillus cereus
GVK 21.
FIGURE 2. Quantitative production of protease using different bacterial isolates.
phylogenetic tree, constructed by the neighbor-joining
method indicated that the strain GVK21 is af liated
with the genus Bacillus and closely related to B. cereus
strain LG1 - Accession No. KF307764 (Figure 4). The
obtained nucleotide sequence GVK21 was submitted to
GenBank database and the accession number assigned
is KY659318 (https://www.ncbi.nlm.nih.gov/ nuccore/
KY659318). The species B. cereusATCC 14579(074540.1),
B. cereus strain JCM 2152 (113266.1), B. thuringiensis
strain NBRC 101235 (112780.1) has the closest sequence
similarity of 99%.
Bacillus is an industrially important organism and
was the rst to be used in the commercial production
of protease in 1952 (Binod et al., 2013). Several pro-
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ENHANCED PRODUCTION OF ALKALINE PROTEASE 419
Keshavamurthy et al.
teases have been produced from many Bacillus species
(Rao et. al., 1998). Bacillus species like B. subtilis (Babe
and Schmidt, 1998), B. licheniformis (Mabrouk et al.,
1999), B. sphaericus (Singh etal., 1999), B. proteolyti-
cus (Bhaskaretal., 2007), Bacillus cereus (Doddapaneni
etal., 2009; Bajaj etal., 2013) and B. megaterium (Raj-
kumaretal., 2010) have been reported for protease pro-
duction. Other than Bacillus species bacteria such as
Pseudomonas uorescens (Kalaiarasi and Sunitha, 2009),
P. aeruginosa (Raj etal., 2012), Vibrio etschnikovii (Jel-
loulietal., 2009), V. alginolyticus (Shanthakumari etal.,
2010) are also reported for protease production.
Bacillus cereus GVK 21 was subjected for secondary
screening for quantitative protease production under
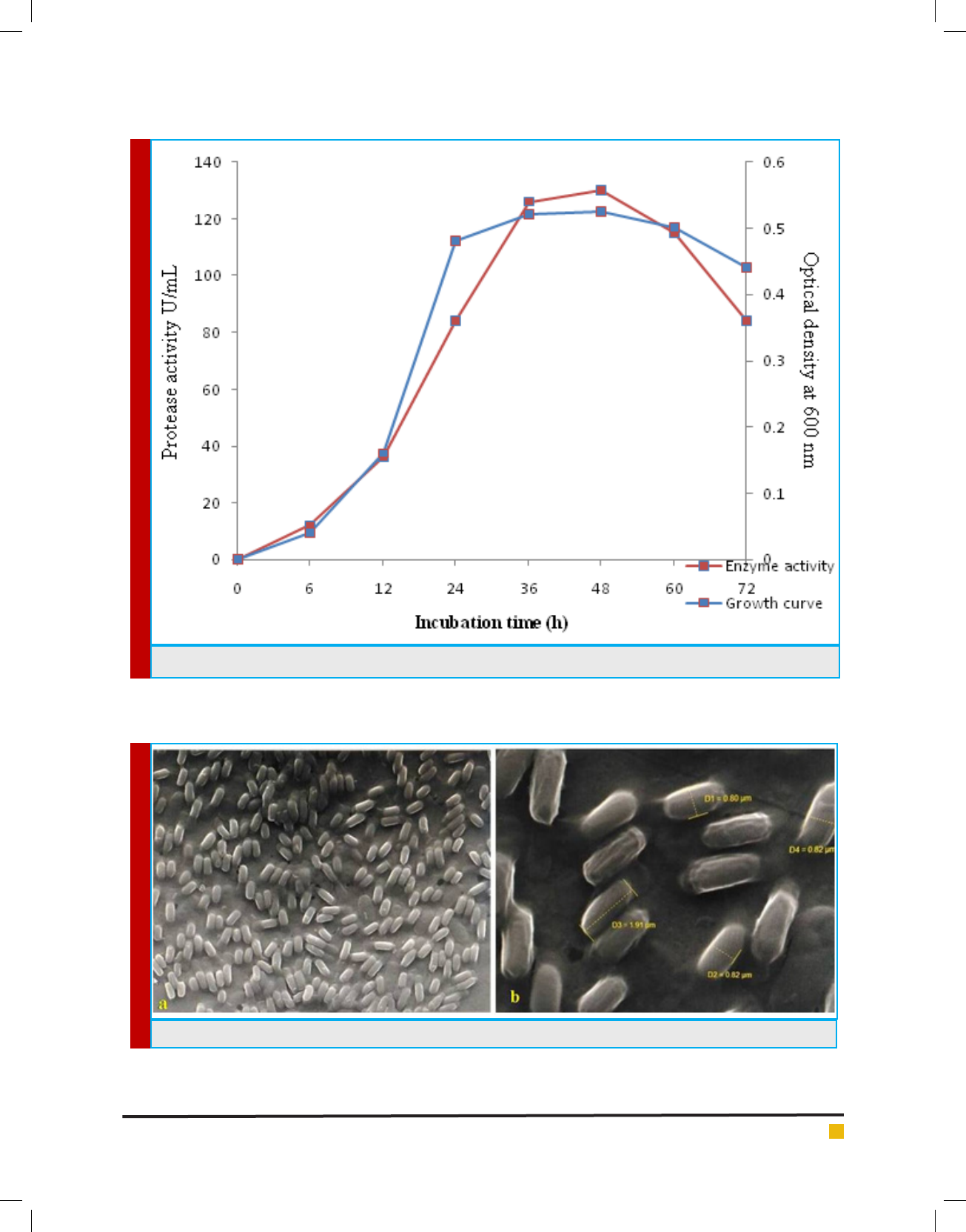
submerged condition. The protease production increased
signi cantly from 12 U/mL to 136 U/mL during6 h to
36 h of incubation and the enzyme activity was in syn-
chrony with the growth of the bacterium, wherein the
logarithmic growth was observed during 6 to 24 hours
and the maximum growth was observed at 36 hours
Table 1. Morphological and biochemical characteristics
of B. cereus strain GVK21 isolated from soil.
Morphological Characteristics Results
Gram staining Positive rods
Colour Creamish white
Motility test Motile
Spore Spore former
Physiological characteristics
Catalase Positive
Indole Negative
Methyl red Negative
Voges Proskauer Positive
Citrate utilization Positive
Oxidase reaction Positive
Casein hydrolysis Positive
Gelatin liquefaction Positive
Starch hydrolysis Positive
Nitrate reduction Positive
Growth at 4o C -
Growth at 40o C +
FIGURE 3. Agarose Gel Electrophoresis of
B.cereus DNA. Lane1- 100 bp DNA lad-
der, Lane2- 16S rDNA amplicon of B.cereus
GVK21
FIGURE 4. Phylogram obtained based on phylogenetic analysis of 16S rDNA gene sequence data showing the phyloge-
netic positions of isolate Bacillus cereus strain GVK21 and of a number of related taxa
420 ENHANCED PRODUCTION OF ALKALINE PROTEASE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Keshavamurthy et al.
FIGURE 5. Protease production with relation to growth of Bacillus cereus GVK 21.
FIGURE 6. SEM images of B.cereus GVK 21
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ENHANCED PRODUCTION OF ALKALINE PROTEASE 421

Keshavamurthy et al.
FIGURE 7. Different Oil cakes used as a substrate for protease production.
indicating that the protease production is maximum in
the late exponential and early stationary phase (Figure
5). The decline in the protease production was observed
after 48 h. Kannikar et al.,(2008) reported that Bacil-
lus sp. BA40 produced 1.158 U/mL protease activity, B.
licheniformis LBBL-11 showed 18.4 U/mL at 48 hours
(Olajuyigbe, and Ajele,2008). The results indicate that
the isolate B. cereus GVK 21 is producing 136U/mL a
comparatively higher protease activity, that too under
preliminary screening.
Chitte and Dey (2000) have also shown that the log
phase the optimum for the production ofprotease by
Streptomyces megasporus. Incubation time of 24 hours
positively in uences the production of protease from
Bacillus cereus SRM-001 (Narasimhan et al.,2015).
Incubation time of 40 h was found to enhance protease
production by Bacillus natto-NRRL-3666 (Mahajan
etal.,2010).
SEM ANALYSIS
Figure 6 shows SEM image of bacteria which con rmed
the rod shaped nature of the cells, where the cell size
(length and breadth) is found to be 1.91 µm and 0.82 µm
respectively.
ENHANCED PROTEASE PRODUCTION USING
OIL CAKES
The effect of agro-based by products as alternative
substrates for bacterial protease production under sub-
merged fermentation has been studied by several work-
ers (Praveen Kumar et al., 2008, Prasad, et al., 2014).
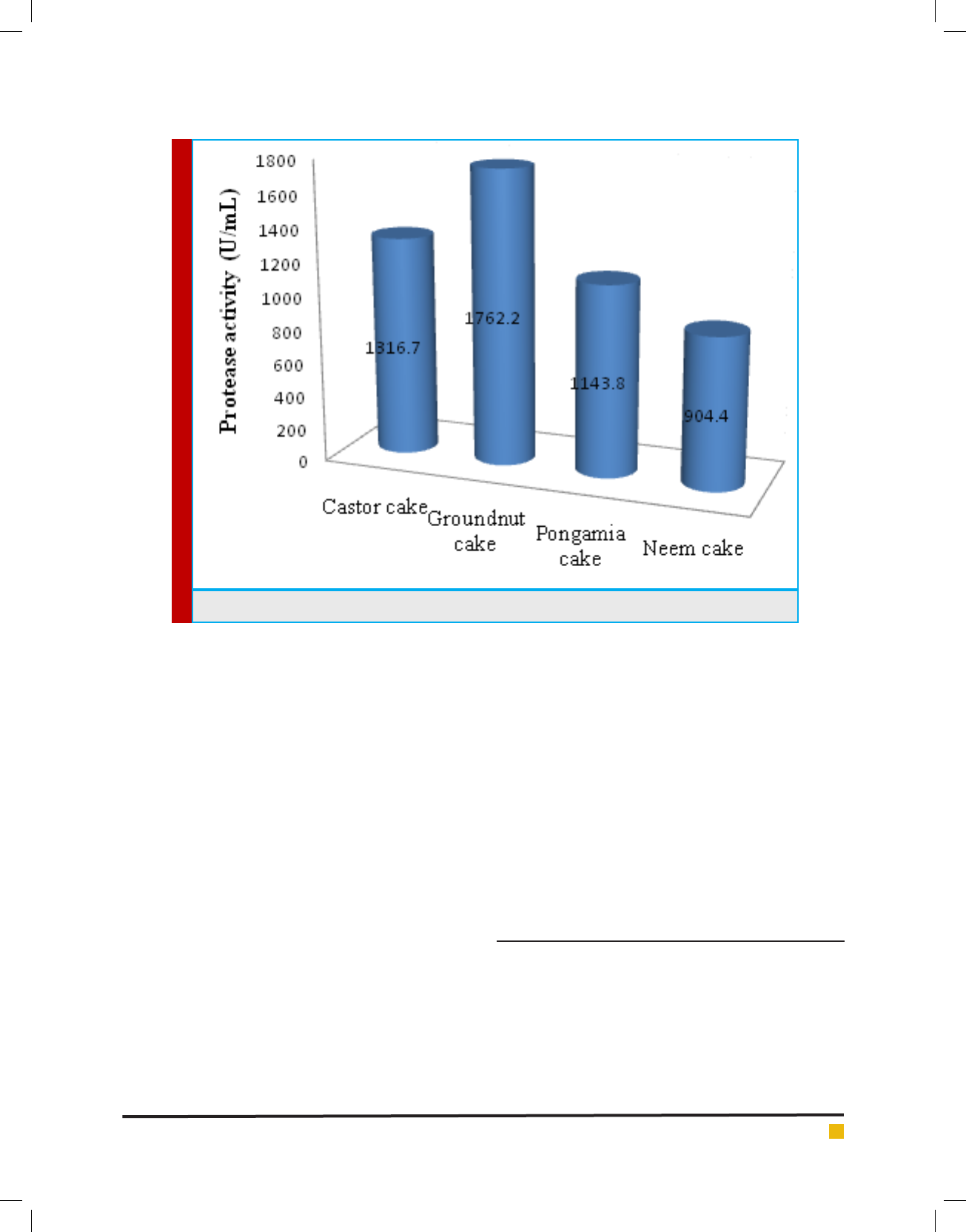
B. cereus GVK21 (KY659318) produced varying levels
of alkaline protease on various agriculture based sub-
strates. Among various oil cakes (Figure. 7) examined,
groundnut oil cake enhanced the protease production
from B. cereus GVK21 by 112 % (1762 U/ml) as com-
pared to control (665 U/ml), i.e., groundnut oil cake
supported maximum protease production. Bacillus sub-
tilis SHS-04exhibited maximum protease production
(1616.21 U/mL) by utilizing groundnut cake as substrate
(Olajuyigbe, 2013).
Other oil cakes also substantially supported protease
production, castor cake (1316 U/ml), pongamia cake
(1144 U/ ml) and neem cake (904 U/ ml) as shown in
Figure 8. Enzyme synthesis was found to be repressed
by rapidly metabolizable nitrogen sources such as amino
acids or ammonium ion concentrations in the medium
as also observed in the presence of ammonium sulphate
and potassium nitrate (Saurabh etal., 2007; Bajaj and
422 ENHANCED PRODUCTION OF ALKALINE PROTEASE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Keshavamurthy et al.
Sharma, 2011). Therefore complex nitrogen sources
are usually used for protease production. Groundnut
oil cake, a rich protein source (30–40%), which mainly
constitutes different amino acids such as arginine (11.0),
Leucine (6.1), glycine (6.0), and phenyl alanine (4.9)
and a dry matter of 92.6% comprehends slow release of
nitrogen due to its conditioned and moderately complex
nature which may favor the process organism metaboli-
cally and physiologically for ef cient production of pro-
tease (Kuo, 1967). Several reports indicate that ground-
nut cake serves as a reasonably good nitrogen source
for protease production by various microorganisms
(Kuberan etal., 2010; Kranthi etal., 2013, Olajuyigbe,
2013).
The substrate in the growth medium constitutes a
major cost determining factor for the commercial pro-
duction of industrial enzymes. The high cost of protease
production is another major hindrance for wide range
of industrial and medicinal applications of this enzyme
(Jayasree etal., 2009). Utilization of agro industrial resi-
dues as carbon and nitrogen sources for the bulk pro-
duction of industrial enzymes may play a signi cant
role not only in reducing the production cost and also
contribute towards growing environmental concerns by
addressing the agro industrial waste disposal and man-
agement problems because of tremendous quantities
of agricultural residues generated through agricultural
practices and industrial processes (Bajaj and Wani, 2011;
Singh and Bajaj 2015). Earlier workers also reported that
defatted oil meals like soybean meal (Saurabh et al.,
2007), cotton seed cake (Bajaj etal., 2013) and ground-
nut meals (Olajuyigbe, 2013) are the cost-effective alter-
natives for industrial production processes. Hence utili-
zation of a low-priced nitrogen source is an important
criterion for economic production of industrial enzymes.
Requirement of speci c nitrogen source differs from one
organism to other even among the same species isolated
from different sources (Bajaj and Sharma, 2011).
CONCLUSION
Bacillus cereus GVK 21 (KY659318) isolated from the soil
samples from a mining area exhibited higher proteolytic
activity of 136 U/mL which signi cantly increased to
1762 U/mL on ground nut cake, comparatively higher
than already reported and thus can be exploited as a
potential source for large scale production of protease
enzyme to cope up the needs of industrial applications
and the demand of the global market.
FIGURE 8. Enhanced Protease production using different oil cakes.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ENHANCED PRODUCTION OF ALKALINE PROTEASE 423

Keshavamurthy et al.
ACKNOWLEDGMENTS
Authors wish to acknowledge Dr. Yogesh Souche, Scien-
tist, National Centre for Cell Science, Govt of India, Pune
for sequencing and identi cation of Bacteria and B.M.S
College of Engineering, Bengaluru, Karnataka, India for
SEM analysis. Authors are thankful to the Maharani’s
Science College for Women, Bengaluru, Karnataka, India
for providing facilities to carry out this work.
CONFLICT OF INTEREST
Authors declare that they have no con ict of interest in
the publication.
REFERENCES
Babe, L., and Schmidt, B., 1998. Puri cation and biochemi-
cal analysis of WprA, a 52-kDa serine protease secreted by B.
subtilis as an active complex with its 23-kDa propeptide.Bio-
chimicaet Biophysica Acta (BBA)-Protein Structure and Molec-
ular Enzymology,1386(1),pp.211-219.
Bajaj, B.K. and Sharma, P., 2011. An alkali-thermotolerant
extracellular protease from a newly isolated Streptomyces sp.
DP2.New biotechnology,28(6), pp.725-732.
Bajaj, B.K. and Wani, M.A., 2011. Enhanced phytase production
from Nocardia sp. MB 36 using agroresidues as substrates:
Potential application for animal feed production.Engineering
in Life Sciences,11(6), pp.620-628.
Bajaj, B.K., Sharma, N. and Singh, S., 2013. Enhanced produc-
tion of brinolytic protease from Bacillus cereus NS-2 using
cotton seed cake as nitrogen source.Biocatalysis and Agricul-
tural Biotechnology,2(3), pp.204-209.
Bhaskar, N., Sudeepa, E.S., Rashmi, H.N. and Selvi, A.T., 2007.
Partial puri cation and characterization of protease of Bacil-
lus proteolyticus CFR3001 isolated from sh processing waste
and its antibacterial activities.Bioresource Technology,98(14),
pp.2758-2764.
Binod, P., Palkhiwala, P., Gaikaiwari, R., Nampoothiri, K.M.,
Duggal, A., Dey, K. and Pandey, A., 2013. Industrial Enzymes-
Present status and future perspectives for India.
Chitte, R.R. and Dey, S., 2000. Potent brinolytic enzyme from
a thermophilic Streptomyces megasporusstrain SD5.Letters in
applied microbiology,31(6), pp.405-410.
Contesini, F.J., Melo, R.R.D. and Sato, H.H., 2018. An overview
of Bacillus proteases: from production to application.Critical
reviews in biotechnology,38(3), pp.321-334.
Doddapaneni, K.K., Tatineni, R., Vellanki, R.N., Rachcha,
S., Anabrolu, N., Narakuti, V. and Mangamoori, L.N., 2009.
Puri cation and characterization of a solvent and detergent-
stable novel protease from Bacillus cereus. Microbiological
Research,164(4), pp.383-390.
El-Bakry, M., Abraham, J., Cerda, A., Barrena, R., Ponsá, S.,
Gea, T. and Sánchez, A., 2015. From wastes to high value
added products: novel aspects of SSF in the production of
enzymes.Critical Reviews in Environmental Science and Tech-
nology,45(18), pp.1999-2042.
Holt, J.G., Krieg, N.R., Sneath, P., Staley, J.T. and Williams, S.T.,
Bergey’s Manual of Determinative Bacteriology. 2000.Lippin-
cot Williams & Wilkins. pag, pp.571-572.
Jayasree, D., Kumari, T.D.S., Kishor, P.B.K., Lakshmi, M.V. and
Narasu, M.L., 2009. Optimization of production protocol of
alkaline protease by.Streptomyces pulvereceus; Inter JRI Sci-
ence and Technology,1(2).
Jellouli, K., Bougatef, A., Manni, L., Agrebi, R., Siala, R.,
Younes, I. and Nasri, M., 2009. Molecular and biochemi-
cal characterization of an extracellular serine-protease from
Vibrio metschnikovii J1.Journal of industrial microbiology &
biotechnology,36(7), pp.939-948.
Joo, H.S., Kumar, C.G., Park, G.C., Kim, K.T., Paik, S.R. and
Chang, C.S., 2002. Optimization of the production of an extra-
cellular alkaline protease from Bacillus horikoshii. Process
Biochemistry,38(2), pp.155-159.
Josephine, F.S., Ramya, V.S., Devi, N., Ganapa, S.B. and Vishwa-
natha, T., 2017. Isolation, production and characterization of
protease from Bacillus sp isolated from soil sample. Journal
of Microbiology and Biotechnology Research, 2(1), pp.163-
168.
Kalaiarasi, K. and Sunitha, P.U., 2009. Optimization of alkaline
protease production from Pseudomonas uorescens isolated
from meat waste contaminated soil. African Journal of Bio-
technology,8(24).
Kranthi, V.S., Rao, D.M. and Jaganmohan, P., 2012. Protease
production by Rhizopus stolonifer through solid state fermen-
tation.Cent. Euro. J. Exp. Bio,1, pp.113-117.
Kumar, E.V., Srijana, M., Kumar, K.K., Harikrishna, N. and
Reddy, G., 2011. A novel serine alkaline protease from Bacillus
altitudinis GVC11 and its application as a dehairing agent.Bio-
process and Biosystems engineering,34(4), pp.403-409.
Kumar, D., Kumar, V., Verma, A.K. and Dubey, A., 2013. Kinetic
characterization and immobilization of partially puri ed
extracellular alkaline protease from rhizospheric soil bacte-
rium Bacillus subtilis strain EN4.J. Pure Appl. Microbiol,7(1),
pp.727-732.
Kumar, P.P., Mathivanan, V., Karunakaran, M., Renganathan,
S. and Sreenivasan, R.S., 2008. Studies on the effects of pH
and incubation period on protease production by Bacillus spp.
using groundnut cake and wheat bran.Indian Journal of Sci-
ence and Technology,1(4), pp.1-4.
Mabrouk, S.S., Hashem, A.M., El-Shayeb, N.M.A., Ismail, A.M.
and Abdel-Fattah, A.F., 1999. Optimization of alkaline protease
productivity by Bacillus licheniformis ATCC 21415. Biore-
source Technology,69(2), pp.155-159.
Maghsoodi, V., Kazemi, A., Nahid, P., Yaghmaei, S. and
Sabzevari, M.A., 2013. Alkaline protease production by immo-
bilized cells using B. licheniformis. Scientia Iranica, 20(3),
pp.607-610.
Mahajan, P.M., Gokhale, S.V. and Lele, S.S., 2010. Production
of nattokinase using Bacillus natto NRRL 3666: media opti-
424 ENHANCED PRODUCTION OF ALKALINE PROTEASE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Keshavamurthy et al.
mization, scale up, and kinetic modeling. Food Science and
Biotechnology,19(6), pp.1593-1603.
Narasimhan, M.K., Chandrasekaran, M. and Rajesh, M., 2015.
Fibrinolytic enzyme production by newly isolated Bacillus
cereus SRM-001 with enhanced in-vitro blood clot lysis poten-
tial.The Journal of general and applied microbiology,61(5),
pp.157-164.
Olajuyigbe, F.M. and Ajele, J.O., 2008. Some properties of
extracellular protease from Bacillus licheniformis LBBL-11 iso-
lated from iru, a traditionally fermented African locust bean
condiment. African Journal of Biochemistry Research, 2(10),
pp.206-210.
Olajuyigbe, F.M., 2013. Optimized production and properties of
thermostable alkaline protease from Bacillus subtilis SHS-04
grown on groundnut (Arachis hypogaea) meal. Advances in
Enzyme Research,1(04), p.112.
Pandey, A., Soccol, C.R., Nigam, P., Soccol, V.T., Vandenberghe,
L.P. and Mohan, R., 2000. Biotechnological potential of agro-
industrial residues. II: cassava bagasse. Bioresource technol-
ogy,74(1), pp.81-87.
Pant, G., Prakash, A., Pavani, J.V.P., Bera, S., Deviram, G.V.N.S.,
Kumar, A., Panchpuri, M. and Prasuna, R.G., 2015. Production,
optimization and partial puri cation of protease from Bacillus
subtilis.Journal of Taibah University for Science,9(1), pp.50-55.
Prasad, R., Abraham, T.K. and Nair, A.J., 2014. Scale up of
production in a bioreactor of a halotolerant protease from
moderately halophilic Bacillus sp. isolated from soil.Brazilian
Archives of Biology and Technology,57(3), pp.448-455.
Raj, A., Khess, N., Pujari, N., Bhattacharya, S., Das, A. and
Rajan, S.S., 2012. Enhancement of protease production by
Pseudomonas aeruginosa isolated from dairy ef uent sludge
and determination of its brinolytic potential. Asian Paci c
Journal of Tropical Biomedicine,2(3), pp.S1845-S1851.
Rajkumar, R., Jayappriyan, K.R., Kannan, P.R. and Rengasamy,
R., 2010. Optimization of Culture Conditions for the Production
of Protease from Bacillus megaterium.Journal of Ecobiotech-
nology.
Rao, M.B., Tanksale, A.M., Ghatge, M.S. and Deshpande, V.V.,
1998. Molecular and biotechnological aspects of microbial
proteases.Microbiology and molecular biology reviews,62(3),
pp.597-635.
Ramachandran, S., Singh, S.K., Larroche, C., Soccol, C.R. and
Pandey, A., 2007. Oil cakes and their biotechnological appli-
cations–A review. Bioresource technology, 98(10), pp.2000-
2009.
Ravi, M., Rayudu, K., Gaddad, S.M. and Jayaraj, Y.M., 2015.
Studies on the potent protease producing bacteria from soil
samples.Int. J. Curr. Microbiol. App. Sci,4(1), pp.983-988.
Rupali R. Deshmukh and Vidhale N N.,2015. Effect of pH on
the production of protease by Fusarium oxysporum using
agroindustrial waste. Biosci. Biotech. Res. Comm. 8(1): 78-83
(2015)
Santong, K., Chunglok, W., Lertcanawanichakul, M. and Ban-
grak, P., 2011. Screening and isolation of Bacillus sp. produc-
ing thermotolerant protease from raw milk.Walailak Journal
of Science and Technology (WJST),5(2), pp.151-160.
Saurabh, S., Jasmine, I., Pritesh, G. and Kumar, S.R., 2007.
Enhanced productivity of serine alkaline protease by Bacillus
sp. using soybean as substrate.Malaysian Journal of Microbi-
ology,3(1), pp.1-6.
Shanthakumari, A.R., Nagalakshmi, R. and Ramesh, S., 2010.
Scaleup and media optimization of protease by Vibrio algino-
lyticus.Journal of Ecobiotechnology.
Sharma, K.M., Kumar, R., Panwar, S. and Kumar, A., 2017.
Microbial alkaline proteases: Optimization of production
parameters and their properties.Journal of Genetic Engineer-
ing and Biotechnology,15(1), pp.115-126.
Singh, J., Vohra, R.M. and Sahoo, D.K., 1999. Alkaline protease
from a new obligate alkalophilic isolate of Bacillus sphaeri-
cus.Biotechnology letters,21(10), pp.921-924.
Singh, S. and Bajaj, B.K., 2015. Medium optimization for
enhanced production of protease with industrially desirable
attributes from Bacillus subtilis K-1. Chemical Engineering
Communications,202(8), pp.1051-1060.
Singh, R., Kumar, M., Mittal, A. and Mehta, P.K., 2016. Micro-
bial enzymes: industrial progress in 21st century. 3 Bio-
tech,6(2), p.174.
Singhania, R.R., Soccol, C.R. and Pandey, A., 2008. Application
of tropical agro-industrial residues as substrate for solid-state
fermentation processes. InCurrent developments in solid-state
fermentation(pp. 412-442). Springer, New York, NY.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M.
and Kumar, S., 2011. MEGA5: molecular evolutionary genet-
ics analysis using maximum likelihood, evolutionary distance,
and maximum parsimony methods. Molecular biology and
evolution,28(10), pp.2731-2739.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ENHANCED PRODUCTION OF ALKALINE PROTEASE 425