Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(3): 409-415 (2018)
On the prevalance of selected serotypes of
enterobacteriaceae pathogens isolated from polluted
ecosystem
G. Archana
1
and Judia Harriet Sumathy V
2
1
Research Scholar, PG Department of Bi otechnology, Women’s Christian College, Chennai, India
2
Associate Professor, PG Department of Biotechnology, Women’s Christian College, Chennai, India
ABSTRACT
Salmonella is a genus of rod shaped (bacillus) Gram negative bacteria of the family Enterobacteriaceae. Salmo-
nella species are non-spore-forming, predominantly motile with cell diameters between 0.7 and 1.5 μm, lengths
from 2 to 5μm, and peritrichous agella(all around the cell body). They arechemotrophs, obtaining their energy
fromoxidation and reduction reactionsusing organic sources. They are alsofacultative aerobes, capable of generat-
ingATPwith oxygen (“aerobically”) when it is available, or whenoxygenis not available, using other electron accep-
tors or fermentation (“anaerobically”). Salmonellaspecies areintracellular pathogens; certain serotypes cause illness.
Non-typhoidal serotypes can be transferred from animal-to-human and from human-to-human. They usually invade
only the gastrointestinal tract and causeSalmonellosis, the symptoms of which can be resolved withoutantibiotics.
Typhoidal serotypes can only be transferred from human-to-human, and can cause food-borne infection,typhoid
fever, and paratyphoid fever. Typhoid fever is caused bySalmonellainvading the bloodstream (the typhoidal form),
or in addition spreads throughout the body, invades organs, and secretesendotoxins(the septic form). This can lead
to life-threateninghypovolemic shockandseptic shock, and requiresintensive careincluding antibiotics. The present
study is aimed at studying the Serotype of this Enterobacteriaceae pathogen isolated from sewage and drinking water
environments.
KEY WORDS: ENTEROBACTERIACEAE, SALMONELLA, TYPHOIDAL SEROTYPE, SEWAGE AND DRINKING WATER ENVIRONMENTS
409
ARTICLE INFORMATION:
*Corresponding Author: judiawcc@gmail.com,
archanamsc.bt@gmail.com
Received 27
th
June, 2018
Accepted after revision 19
th
Sep, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.3/9

410 A STUDY ON THE PREVALANCE OF SELECTED SEROTYPE OF ENTEROBACTERIACEAE PATHOGEN ISOLATED BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
G. Archana and Judia Harriet Sumathy V
INTRODUCTION
Salmonella species are facultative intracellular patho-
gens.A facultative organism uses oxygen to makeATP;
when it is not available, it “exercises its option”—the lit-
eral meaning of the term—and makes ATP byfermenta-
tion, or by substituting one or more of four less ef cient
electron acceptors as oxygen at the end of the elec-
tron transport chain: sulfate, nitrate, sulfur, or fuma-
rate Cabada et al., 1975). Most infections are due to
ingestion of food contaminated by animal feces, or by
human feces. Salmonellaserotypes can be divided into
two main groups—typhoidal and non-typhoidal (CDC,
2005).
Non-typhoidal serotypes are more common, and usu-
ally cause self-limiting gastrointestinal disease. They
can infect a range of animals, and arezoonotic, mean-
ing they can be transferred between humans and other
animals (Cooke, and Wain, 2005). Typhoid fever caused
bySalmonellaserotypes are strictly adapted to humans
or higher primates—these include Salmonella Typhi,
Paratyphi A, Paratyphi B, and Paratyphi C (D’Aoust,
1989). In the systemic form of the disease, Salmonellae
pass through the lymphatic system of the intestine into
the blood of the patients (typhoid form) and are carried
to various organs (liver, spleen, kidneys) to form second-
ary foci (septic form). Endotoxins rst act on the vascu-
lar and nervous apparatus, resulting in increased perme-
ability and decreased tone of the vessels, upset of thermal
regulation, and vomiting and diarrhoea (Hudault et al.,
2001). In severe forms of the disease, enough liquid and
electrolytes are lost to upset the water-salt metabolism,
decrease the circulating blood volume and arterial pres-
sure, and cause hypovolemic shock.Septic shock may
also develop. Shock of mixed character (with signs of
both hypovolemic and septic shock) is more common
in severe Salmonellosis. Oliguria and azotemia may
develop in severe cases as a result of renal involvement
due tohypoxiaandtoxemia.
Mechanisms of infection differ between typhoidal
and nontyphoidal serotypes, owing to their different
targets in the body and the different symptoms that they
cause. Both groups must enter by crossing the barrier
created by the intestinal cell wall, but once they have
passed this barrier, they use different strategies to cause
infection. Nontyphoidal serotypes preferentially enterM
cellson the intestinal wall by bacterial-mediatedendo-
cytosis, a process associated with intestinal in amma-
tion and diarrhoea. They are also able to disrupttight
junctionsbetween the cells of the intestinal wall, impair-
ing the cells’ ability to stop the ow ofions, water, and
immune cells into and out of the intestine. The combi-
nation of the in ammation caused by bacterial-medi-
ated endocytosis and the disruption of tight junctions is
thought to contribute signi cantly to the induction of
diarrhoea (Murray, 1991).
Salmonellae are also able to breach the intestinal
barrier via phagocytosis and traf cking by CD18-pos-
itive immune cells, which may be a mechanism key to
typhoidalSalmonella infection. This is thought to be a
more stealthy way of passing the intestinal barrier, and
may, therefore, contribute to the fact that lower numbers
of typhoidalSalmonellaare required for infection than
nontyphoidalSalmonella (Olsen et al., 2001). Typhoidal
serotypes can use this to achieve dissemination through-
out the body via themononuclear phagocyte system, a
network of connective tissue that contains immune cells,
and surrounds tissue associated with the immune system
throughout the body (Parras et al., 1984).
Salmonellosis is also known to be able to causeback
painorspondylosis. It can manifest as ve clinical pat-
terns: gastrointestinal tract infection, enteric fever, bac-
teremia, local infection, and the chronic reservoir state.
The initial symptoms are nonspeci c fever, weakness,
and myalgia among others. In the bacteremia state, it
can spread to any parts of the body and this induces
localized infection or it forms abscesses (Popoff, 2001).
The forms of localizedSalmonellainfections are arthritis,
urinary tract infection, infection of the central nervous
system, bone infection, soft tissue infection, etc.Infec-
tion may remain as the latent form for a long time, and
when the function ofreticular endothelial cellsis dete-
riorated, it may become activated and consequently, it
may secondarily induce spreading infection in the bone
several months or several years after acute salmonellosis
(Silverman, 1979).
MATERIALS AND METHODS
By plate count method 1 ml of the sample was prepared
and transferred to 9 ml of saline and was maintained
as master dilution. From this (10ˉ
¹
to 10ˉ
6
) dilutions
were prepared and 1 ml of sample was poured to cool
sterilized agar count plate and incubated at 37°C for 24
hours. Colony was counted by colony counter. Mor-
phological study was achieved by microscopic observa-
tion of Grams staining, Motility test, Catalase test and
Oxidase test. A small portion of suspected colony was
streaked on medias such as Nutrient Agar, MacConkey
Agar and Eosin Methylene Blue Agar. Biochemical tests
were performed using Standard Protocol. Following this
serological typing was done. Depression plates were
taken and were marked as A, B and C. In A depression
plate it was marked as negative control in which phe-
nolized saline suspension was added. In B depression
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS A STUDY ON THE PREVALANCE OF SELECTED SEROTYPE OF ENTEROBACTERIACEAE PATHOGEN ISOLATED 411
G. Archana and Judia Harriet Sumathy V
Table 1. Biochemical Test for Salmonella
paratyphi A
S. No. Biochemical Tests Results
1. Catalase Positive
2. Oxidase Negative
3.
TSI Test
Butt
Slant
Gas
H2S
Acid
Acid
Negative
Negative
4. Indole Negative
5. Methyl Red Positive
7. Voges Proskauer Positive
8. Citrate Positive
Table 2. Antibiotic Sensitivity Test for Klebsiella pneumoniae
S. No Name of the Antibiotics Zone of inhibition in mm Interpretation
1. Amikacin ( AI ) 13 mm Resistant
2. Chloramphenicol ( C ) 21 mm Sensitive
3. Co – trimoxazole ( CT ) 13 mm Resistant
4. Tetracycline ( T ) 20 mm Sensitive
5. Gentamycin ( G ) 14 mm Intermediate
6. Ceftriaxone ( CTR ) 22 mm Sensitive
7. Cephotaxime ( CTX ) No zone Resistant
8. Nor oxacin ( NX ) 18 mm Sensitive
9. Meropenem ( MR ) No zone Resistant
10. Imipenem ( I ) No zone Resistant
Zone of inhibition
Below 10 mm – least active
Between 11-25 mm – active
Above 26 mm – very active
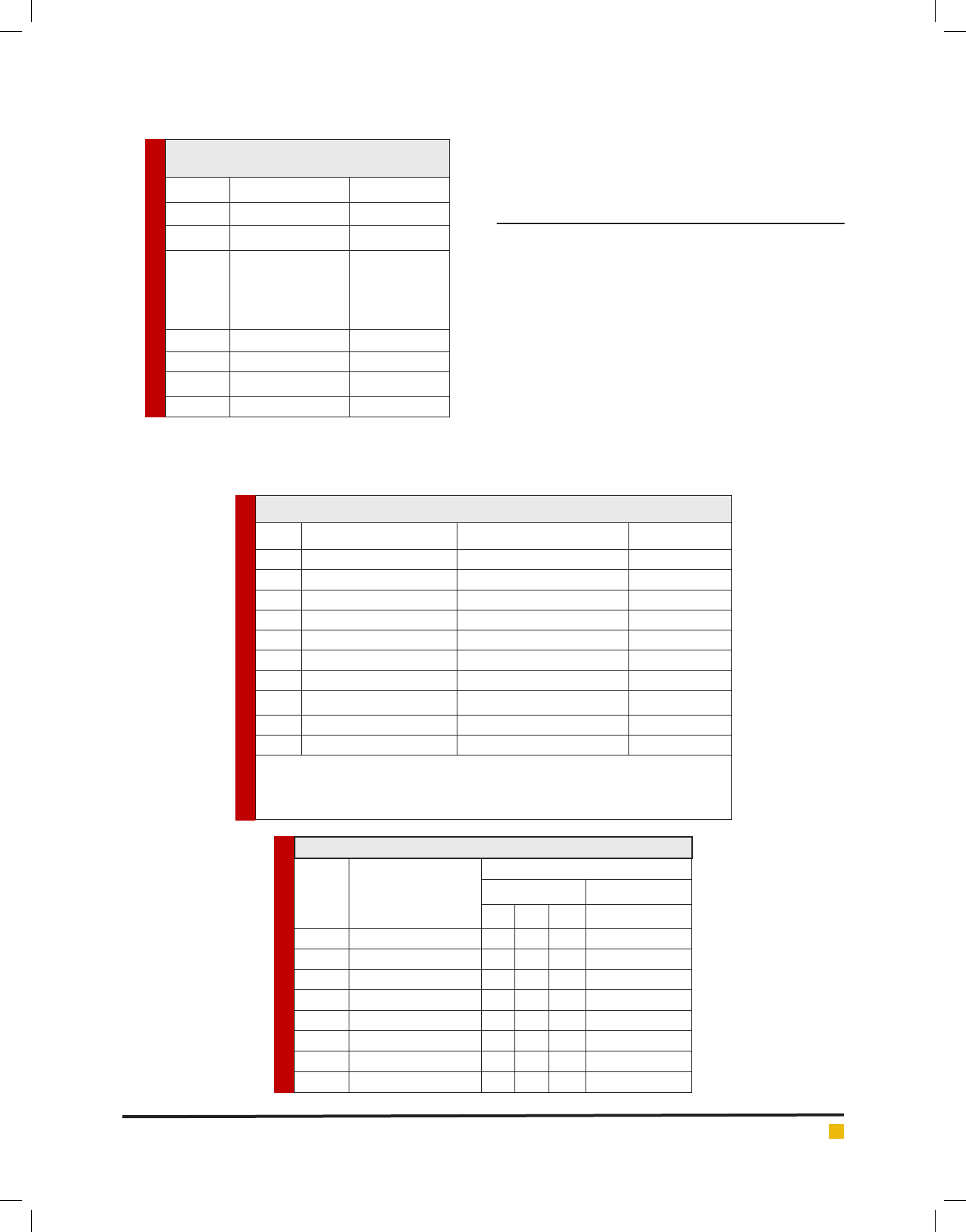
Table 3. Serotyping of Salmonella paratyphi A from Sewage Sample
S. No. Name of the Isolate
Antiserum
O Antigen H Antigen
1 2 12 Phase 1 – a
1. S. paratyphi A – 1 -+- -
2. S. paratyphi A – 2 --+ -
3. S. paratyphi A – 3 -+- -
4. S. paratyphi A – 4 --- +
5. S. paratyphi A – 5 +- - -
6. S. paratyphi A – 6 --+ -
7. S. paratyphi A – 7 --- +
8. S. paratyphi A – 8 --- +
tive control which contain phenolized saline suspension
of known organism and antiserum.
RESULTS AND DISCUSSION
The total number of positive and negative samples
obtained from sewage and drinking water for Salmo-
nella paratyphi A was found to be 13. In identi cation
of bacterial isolate of morphological characteristics by
Grams staining and motility for Salmonella paratyphi
A, it wasfound to be Gram negative small rods and
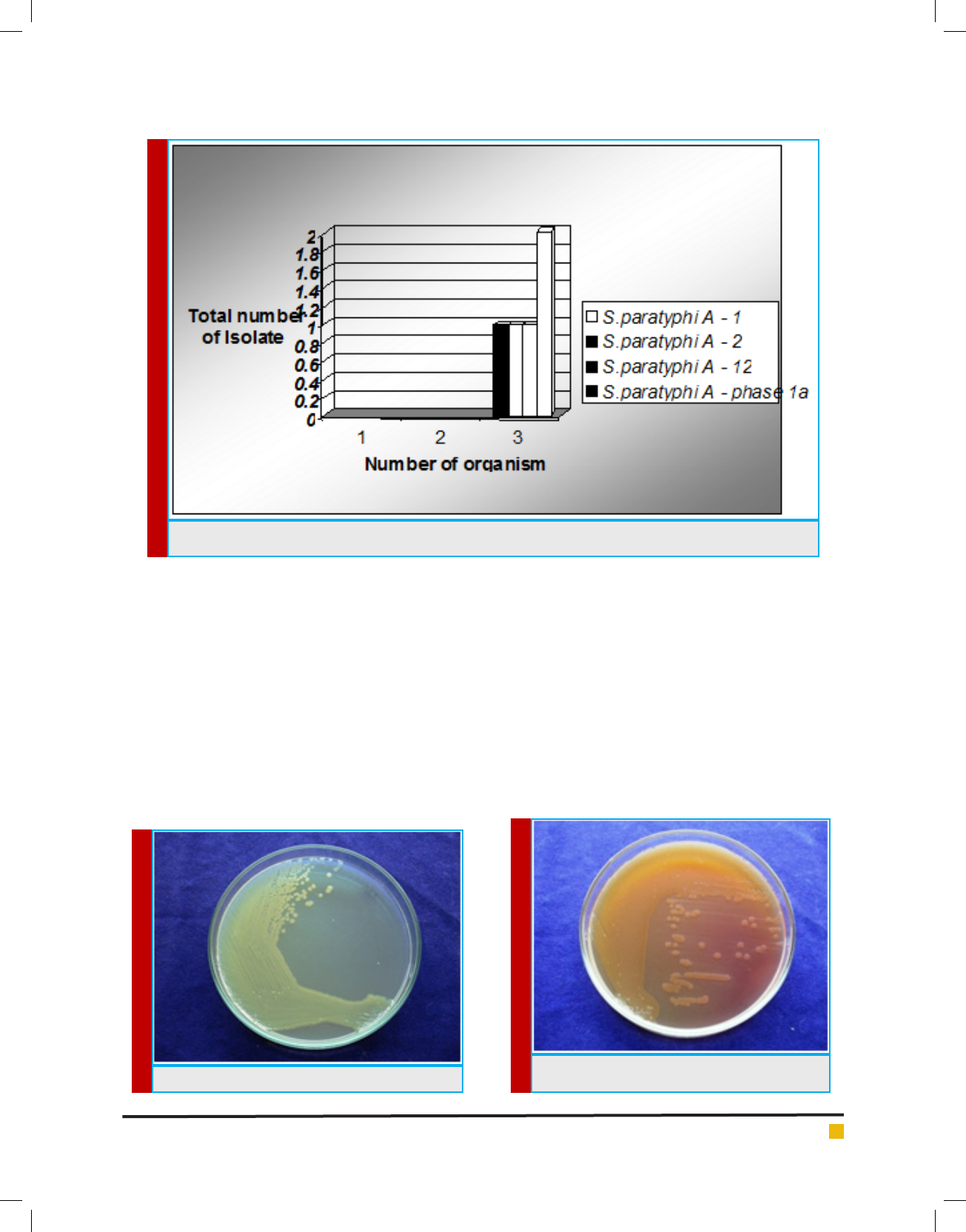
motile. Cultural characteristics of Salmonella paratyphi
Aon Nutrient Agar formed large transparent colonies,
on Mac Conkey Agar lactose fermenting colonies and on
Blood Agar moist colonies. Biochemical Test, Antibiotic
Sensitivity Test and Serotype study results indicate the
prevalence of Salmonella paratyphi A in the sewage and
drinking water samples collected for the present study
(Tables 1 – 4 and Figures 1 – 8).
plate it was marked as test in which phenolized saline
suspension and antiserum of respective organism was
added and in C depression plate it was marked as posi-
G. Archana and Judia Harriet Sumathy V
412 A STUDY ON THE PREVALANCE OF SELECTED SEROTYPE OF ENTEROBACTERIACEAE PATHOGEN ISOLATED BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
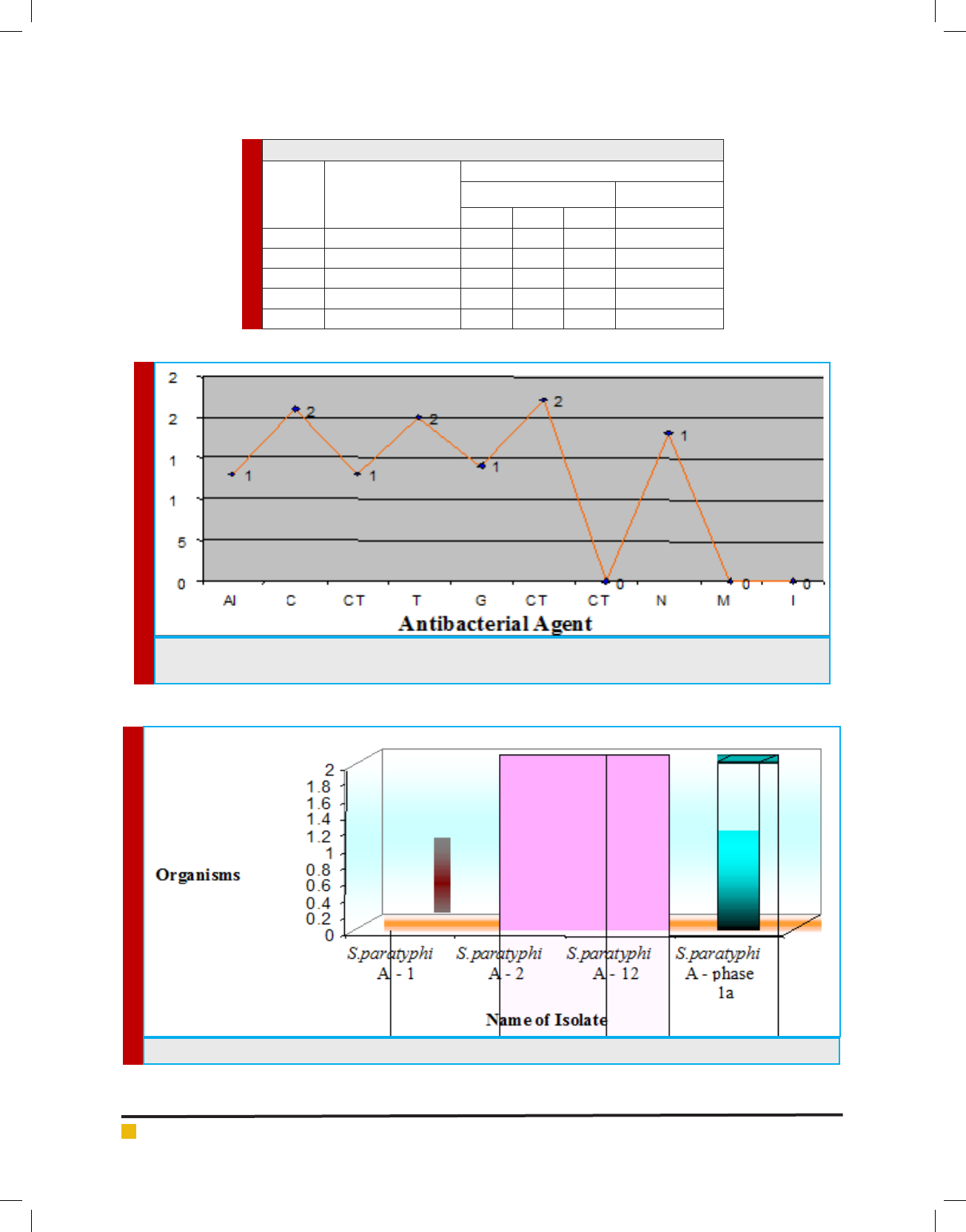
Table 4. Serotypying of Salmonella paratyphi Afrom Drinking Water Sample
S. No. Name of the Isolate
Antiserum
O Antigen H Antigen
1 2 12 Phase 1 - a
1. S. paratyphi A – 1 -+--
2. S. paratyphi A – 2 +- - -
3. S. paratyphi A – 3 ---+
4. S. paratyphi A – 4 --+-
5. S. paratyphi A – 5 ---+
FIGURE 1. Antibacterial Activity of Salmonella paratyphi A. AI - Amikacin, C - Chloramphenicol, CT - Co-trimoxazole,
T - Tetracycline, G - Gentamycin, CTR - Ceftriaxome, NX - Nor oxacin, MR - Meropenem, I – Imipenem
FIGURE 2. Serotypying of Salmonella paratyphi A from Sewage Sample
G. Archana and Judia Harriet Sumathy V
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS A STUDY ON THE PREVALANCE OF SELECTED SEROTYPE OF ENTEROBACTERIACEAE PATHOGEN ISOLATED 413
FIGURE 3. Serotypying of Salmonella paratyphi A from Water Sample
FIGURE 4. S.paratypi A on Nutrient Agar
FIGURE 5. S.paratypi A on Nutrient Agar
Salmonella infection (salmonellosis) is a common
bacterial disease that affects the intestinal tract. Sal-
monella bacteria typically live in animal and human
intestines and are shed through feces. Humans become
infected most frequently through contaminated water or
food. Typically, people with salmonella infection have no
symptoms. Others develop diarrhea, fever and abdomi-
nal cramps within eight to 72 hours. Most healthy peo-
ple recover within a few days without speci c treatment.
In some cases, the diarrhea associated with salmonella
infection can be so dehydrating as to require prompt
medical attention. Life-threatening complications also
may develop if the infection spreads beyond your
intestines. The risk of acquiring salmonella infection is
higher if one travels to countries with poor sanitation.
Thus the present study was aimed at studying this Enter-
obacteriacea pathogen which revealed high prevalence
pattern in the water samples collected from polluted
environments.
G. Archana and Judia Harriet Sumathy V
414 A STUDY ON THE PREVALANCE OF SELECTED SEROTYPE OF ENTEROBACTERIACEAE PATHOGEN ISOLATED BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
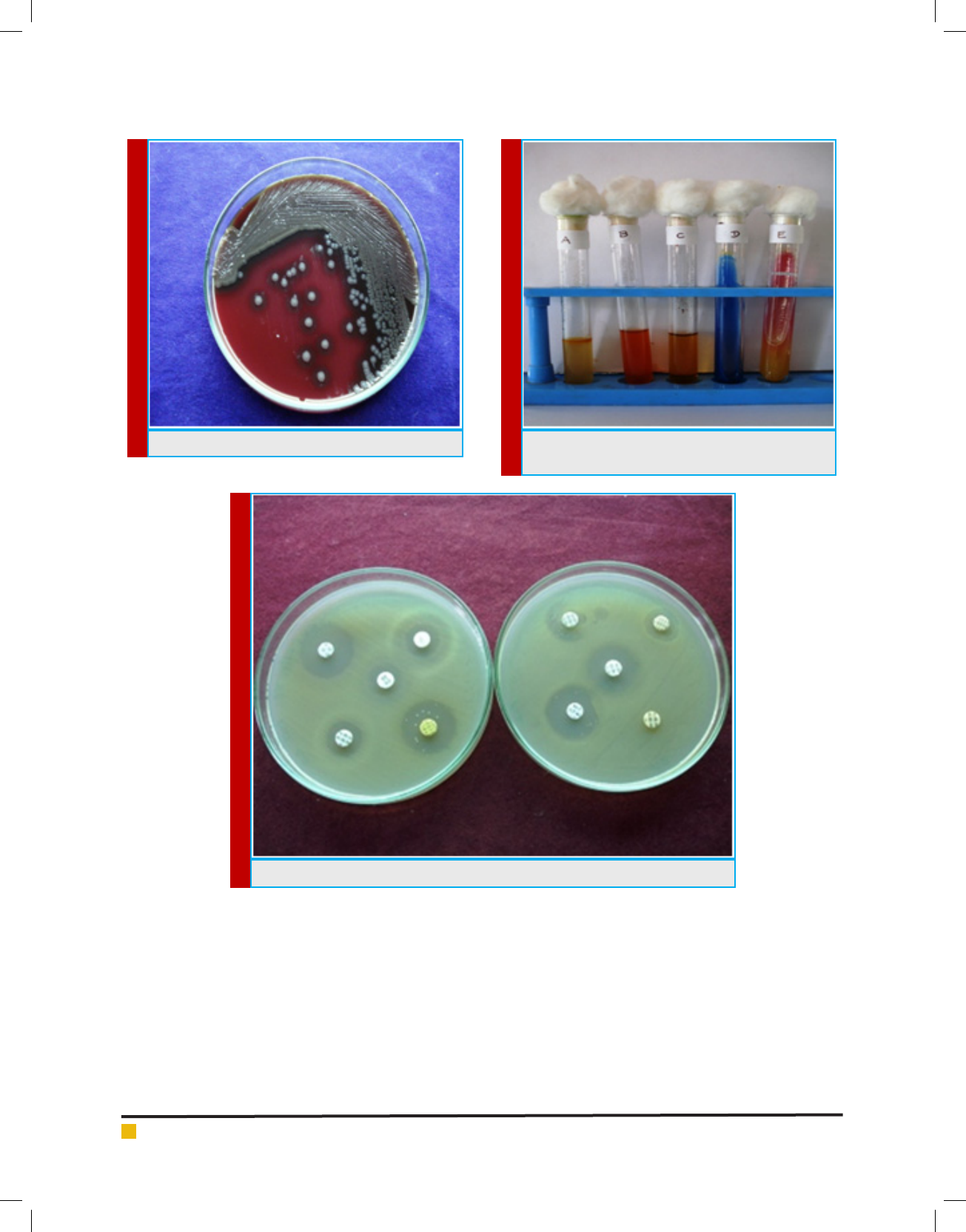
FIGURE 6. S.paratypi A on Blood Agar
FIGURE 7. IMViC test for S. paratyphi A. A = Indole B
= Methyl Red C = Voges Proskaur. D = Citrate E = TSI
FIGURE 8. Antibiotic sensitivity test for Salmonella paratyphi A
REFERENCES
Cabadaj, R., Pipova, M. and Turek, P. (1975). Poultry, eggs, and
their products as sources of human Salmonellosis in Slovakia.
In Proceedings of the World Veterinary Congress. Japan, p.
168.
CDC. (2005). Salmonella Surveillance Annual Summary US
Department of Health and Human Services, Centers for Disease
Control and Prevention, Atlanta, Georgia.
Cooke, F. and Wain, J. (2005). Antibiotic resistance in Salmo-
nella infections, in Maskell D, Mastroeni P (Eds), Salmonella
Infections. Clinical, immunological and molecular aspects.
Cambridge University Press, Cambridge, pp. 25-56.
D’Aoust, J.Y. (1989). Salmonella in Foodborne Bacterial Patho-
gensed. Doyle, M.P. pp. 327-445. New York, Marcel Dekker.
Hudault, S., Guignot, J. and Servin, A.L. (2001). Escherichia coli
strains colonizing the gastrointestinal tract protect germfree
mice against Salmonella typhimurium infection. Gut 49:47-55.
Murray, C.J. (1991). Salmonellae in the environment. Revue
Scienti que et Technique. Of ce International des Epizooties
10, 765-785.

G. Archana and Judia Harriet Sumathy V
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS A STUDY ON THE PREVALANCE OF SELECTED SEROTYPE OF ENTEROBACTERIACEAE PATHOGEN ISOLATED 415
Olsen, S.J., Bishop, R., Brenner, F.W., Roels, T.H., Bean, N. and
Tauxe, R.V. (2001). The changing epidemiology of Salmonella
trends in serotypes isolated from humans in the United States,
1987–1997. J. Infectious Disease; 183, 753-761.
Parras Jimenez, L., Espigares Garcia, M. and Rodriguez-Con-
treras, R. (1984). Resistencia de origen plasmidico a los b-lacta
micos en el genero Salmonella. Laboratorio 78, 31-41.
Popoff, M.Y. (2001). Antigenic Formulas of the Salmonella
Serovars, 8th ed. W.H.O. Collaborating Centre for Refer-
ence and Research on Salmonella, Institute Pasteur, Paris,
France.
10. Silverman, M. (1979). Phase variation in Salmonella genetic
analysis of a recombinational switch. Proc. National Academy
Science USA 76, 391-395.