Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(3): 376-386 (2018)
Antifungal peptides: Biosynthesis, production and
applications
Narjis Fathima Mirza
1
, Snehasri Motamarry
1
, Preetha Bhadra
2
and Bishwambhar Mishra
2
*
1
Department of Biotechnology, Sreenidhi Institute of Science and Technology, Ghatkesar, Hyderabad–501301,
India
2
Department of Biotechnology, Centurion University of Technology and Management, Bhubaneswar–752050
India
ABSTRACT
Fungal infections in animal, plants and fungal contamination of food for humans and livestock result in substantial
worldwide economic losses. In the last few years, fungal infection has increased strikingly by a rise in the number
of deaths of acquired immunode ciency syndrome (AIDS) cancer patients, transplant patients owing to fungal infec-
tions. The growth rate of fungi is very slow as compared to bacteria and very dif cult to identify. Approximately 100
peptides have been investigated to date for their antifungal properties, which can be of great importance to overcome
the human diseases. Insects secrete such compounds, which can be peptides, as a part of their immune defense reac-
tions. Antifungal peptides are excellent models for drug discovery exhibiting unique characteristics such as high
speci city, broad spectrum, low level of resistance reaching and unique mode of action. The aim of this review is to
provide information on research on these important peptides.
KEY WORDS: ANTIFUNGAL; PEPTIDES; MODE OF ACTION; FUNGAL INFECTION; FUNGI CIDAL
376
ARTICLE INFORMATION:
*Corresponding Author: mishra.bishwambhar@gmail.com
Received 11
th
July, 2018
Accepted after revision 27
th
Sep, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.3/5
INTRODUCTION
Many research advances have been made in medicine at
present. Be it in the treatment of HIV-AIDS, cancer, or
organ transplantation, the success rates have increased
drastically over past 50 years. Even though success rates
have been increased, many patients are left with compro-
mised immune systems (Wisplinghoff etal., 2004). The
Patients, receiving chemotherapy, organ transplantation,
use of prosthetic Devices and vascular catheters, dialysis
etc., are easily susceptible to manybacterial, viral and
fungal infections (Spellberg etal., 2008). Even though
fungal species are serious pathogens, they get lesser
attention when compared to bacterial and viral infec-

Narjis Fathima Mirza etal.
tions as, the frequency of occurrence of fungal infec-
tions has been comparatively less to bacterial and viral
infections (Georgopapadakou etal., 1996; Wisplinghoff
et al., 2004; Porto et al., 2012). Human fungal infec-
tions, caused by Aspergillus fumigatus, Cryptococcusne-
oformans, Candida albicans, are increasing in a number
o mmune-compromised patients (Blanco et al., 2008).
Fungal pathogens such as Candida species and Aspergil-
lus species are more common and account up to 19%
of cases (Schelenz etal., 2009). C. albicans is known as
major fungal pathogen and is 4
th
most common cause of
nosocomial infections (Banerjee etal., 1991; Beck-Sague
etal., 1993; Wisplinghoff etal., 2004; Xiao etal., 2013;
Chen etal., 2016; Ageitos etal., 2017; Bondaryk etal.,
2017).
Only a limited number of antifungal drugs are avail-
able such as echinocandins, polyenes etc., (Gupte etal.,
2002). Amphotericin B, which was discovered in 1956,
is still used for treatment many fungal infections. Just
like bacterial resistance, fungal pathogens have also
developed resistance in past 20 years. (Gold etal., 2002;
Georgopapadakou etal., 1996). The fact that fungal and
bacterial infections are different and bacterial infec-
tions are treated more easily is because, fungal cells are
eukaryotic and bacterial cells are prokaryotic. The main
concern in treating fungal infections is that any chemi-
cal substance that is successful in damaging the eukary-
otic cell wall of fungi may also cause possible damage
to human cells, unlike antibiotics, which won’t have
any effect on humans. Any chemical substance that is
toxic to fungus may also be toxic to humans (Moham-
mad et al., 2015). Therefore, there is need to discover
new biochemical targets in fungi. Antifungal peptides
are treatment alternatives, derived from natural sources
and are effective against fungal infections, thus, safe for
immune compromised patients (Gold etal., 2002; Ravi
etal., 2011; Thakur etal., 2012; Jia etal., 2016; Wang
etal., 2016; Veltri etal., 2017).
Antifungal peptides from natural sources are much
cheaper than commercial antifungal drugs and are
also better alternative to combat resistance. Antifungal
peptides are cationic biomolecules with weight around
1.3 kDa to 30 kDa (Mohammad etal., 2015). Antifun-
gal peptides are classi ed into two types based on their
mode of action. First group are, lytic peptides, (Rees
etal., 1997; Shai etal., 1995). These peptides are amphi-
pathic in nature (contain a positive and a neutral charge)
and disrupt the membrane structure by xing onto its
surface (Leuschner etal., 2004; Shai etal., 1995). The
second group of peptides act by inhibiting the synthesis
of cell wall or essential cell wall components such as
glucan, chitin (Fernández etal., 2004; Lata etal., 2010;
Joseph et al., 2012; Liu et al., 2016; Bondaryk et al.,
2017).
SOURCES OF ANTIFUNGAL PEPTIDES
Bacterial Peptides Iturins
Iturin was one of rst antifungal peptides, ever iso-
lated. It is produced by different strains of Bacillus sub-
tilis (Georgopapadakou et al., 1996). They are cyclic
lipopeptides and act by disrupting the cell membrane
of fungi, hence leaking its vital ions (XinZhao et al.,
2013; Lemaitre etal., 1997). Iturin A, of iturin family,
was observed to inhibit A. avus and F. moniliforme
growth and had Minimal inhibitory concentration (MIC)
of 22.0 μg/ml against Saccharomyces cerevisiae. It was
found to be effective against dermatomycoses. (De Lucca
etal., 1999). But iturin A was also observed to be hemo-
lytic. Bacillomycin F, another family member of iturin,
is known to inhibit strains such as Byssochlamys fulva,
A.niger, C.albicans, and F.oxysporumand had MIC of
40.0μg/ml for A.niger (De Lucca etal., 1999). Bacillo-
mycin D produced by Bacillus amyloliquefaciens was
found to be effective against a plant pathogenic fungi
Fusarium graminearum and Candida species. MIC of
(12.5-25) μg/ml was observed against various Candida
species (Tabbene etal., 2015; Qin Gu etal., 2017).
Syringomycins: Syringomycins are produced by Pseu-
domonas syringae are small cyclic lipodepsipeptides with
ergosterol as a binding site in yeast. The most prevalent of
Syringomycinsis syringomycin-E (SE) which was found to
be lethal to many strains such as A. avus, A. fumigatus,
A.niger, F. moniliforme and F. oxysporum showing LD95
of 1.9 μg/ml. it showed MIC of (0.8–12.5) μg/ml against
C. neoformans (De Lucca et al., 1999). Syringotoxin B,
syringostantin A which were lipodepsinonapeptides were
found to be effective against Candida, Cryptococcus, and
Aspergillus species. Syringostantin A had MIC of 5.0μg/
ml against A. fumigatus. Syringotoxin B had MIC of
3.2μg/ml against C. albicans (Sorensen etal., 1996; Zhao
etal., 2013; Chereddy etal., 2014; Deslouches etal., 2015;
Gao etal., 2016; Kubicek-Sutherland etal., 2017).
Pseudomycins: Pseudomycins, another family, structur-
ally related to syringomycins also have antifungal activ-
ity against wide ranges of species. Existing as pseudomy-
cins (A, B, and C), these have shown antifungal activity
against Ceratocystis ulmi, C. Albicans, Rynchosporium
secalis,Rhizoctonia solani,Sclerotiniasclerotiorum Ver-
ticillium albo-atrum, Verticillium dahliae, Thielavio-
pis basicola, F. oxysporum, F. culmorum. The MIC of
pseudomycin A, against C. neoformans was 1.56 μg/ml
whereas 3.12 μg/ml was observed against C. albicans
(De Lucca etal., 1999).
Plant Peptides: Large number of antifungal peptides are
identi ed from plant sources, but only few were tested
and found to be effective.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS 377
Narjis Fathima Mirza etal.
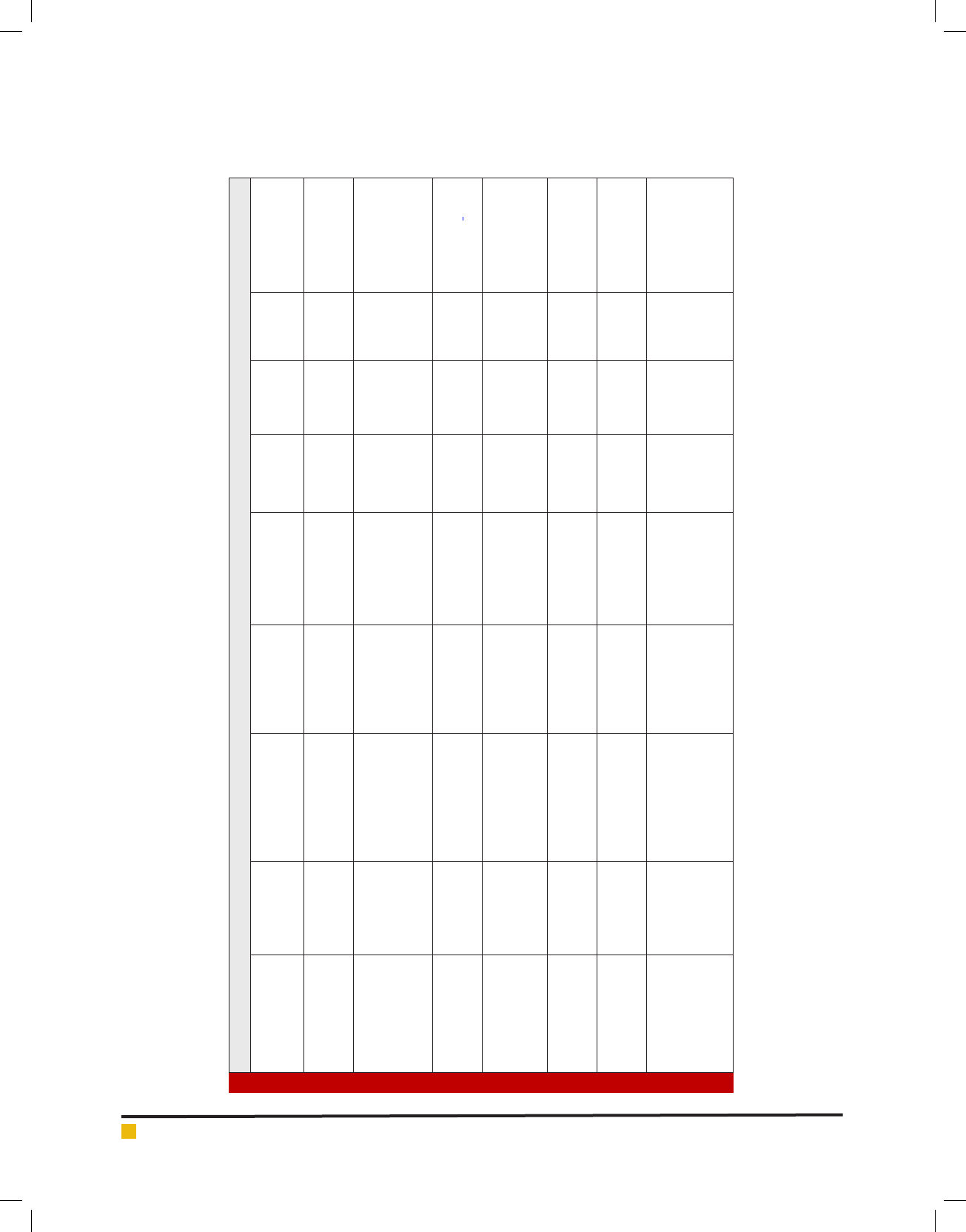
Table 1. Antifungal peptides from bacterial sources
Peptide name Family/group Structure source
Fungal species
effected
Typical
target
organism
Mode of
action
In vitro
MIC (μg/
ml)
Reference
Bacillomycin F Iturins lipopeptide B. subtilis.
Byssochlamys fulva,
A. niger, C.albicans,
and F.oxysporum
A. niger lysis 40
(De Lucca etal.,
1999; Bionda etal.,
2016)
iturin A Iturins lipopeptide
Bacillus
amyloliquefaciens
A. avus, F.
moniliforme, S.
cerevisiae
S. cerevisiae lysis 22.0
(Georgopapadakou
etal., 1996; De
Lucca etal., 1999;
Brandenburg etal.,
2015)
bacillomycin D Iturins lipopeptide
Bacillus
amyloliquefaciens
F. graminearum and
Candida species.
Candida
species
lysis 12.50-25.0
(Tabbene etal.,
2015; Qin Gu etal.,
2017,)
syringomycin-E (SE)
Syringomycins
lipodepsipeptide
Pseudomonas
syringae
A. avus, A.
fumigatus, A.niger,
F. moniliforme and
F. oxysporum
C.
neoformans
lysis 0.8–12.5
(De Lucca etal.,
1999; Falciani etal.,
2014)
syringostantin A
Syringomycins
lipodepsinonapeptides
Pseudomonas
syringae
Candida,
Cryptococcus, and
Aspergillus species
A. fumigatus lysis 5.0
(Sorensen etal.,
1996; Falciani etal.,
2014)
Syringotoxin B
Syringomycins
Lipodepsinonapeptide
Pseudomonas
syringae
Candida,
Cryptococcus, and
Aspergillus species.
C. albicans lysis 3.2
(Sorensen etal.,
1996; Lyu etal.,
2016)
pseudomycin A Pseudomycins lipodepsinonapeptides
Pseudomonas
syringae
C. albicans, F.
oxysporum , F.
culmorum, C.
neoformans
C. albicans lysis 3.12
(De Lucca etal.,
1999; Brunetti etal.,
2016)
378 ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Narjis Fathima Mirza etal.
Table 2. Antifungal peptides from plant sources
Peptide name Family/group
No. of
amino acids
source
Target
organism
In vitro MIC
(μg/ml)
Reference
Ib-AMP3 Plant defensins 20
Impatiens
balsamina
F. moniliforme 50.0
(De Lucca etal., 1999;
Asano etal., 2013)
Frangufoline Cyclopeptides *534
Rhamnus
frangula
A. niger 5.0
(Gournelis etal., 1997;
De Lucca 2000; Tan
etal., 2006; Choe
etal., 2015)
Rugosanine A
Cyclopeptides
*585 Ziziphus rugosa A. niger 5.0
(Gournelis etal.,
1997; De Lucca 2000;
Tan etal., 2006; Cole
etal., 2016)
Nummularine
Cyclopeptides
*587
Ziziphus
nummularia
A. niger 5.0
(Gournelis etal., 1997;
De Lucca 2000; Tan
etal., 2006; Dobson
etal., 2014)
ACE-AMP1
Lipid transfer
proteins
93 Allium cepa L F. oxysporum 10.0
(De Lucca 2000; Dutta
etal., 2015)
Table 3. Antifungal peptides from fungal sources
Peptide name Structure source
Typical target
organism
Mode of action
In vitro MIC
(μg/ml)
Reference
Caspofungin lipopeptide G.lozoyensis Candida spp glucan synthesis 8 - 64
(Bartizaletal.,
1997;Groll etal.,
1999; Kuhn
etal., 2002;
Deresinski etal.,
2003; Porto
etal., 2012)
Anidulafungin
(LY303366)
Lipopeptide A. nidulans Candida spp glucan synthesis 0.5 - 4.0
(Lucca etal.,
1999; Denning
etal., 1997;
Ghannoum etal.,
2005; De Lei
etal., 2013)
Cilofungin (LY121019) Lipopeptide A. nidulans C. albicans Glucan synthesis 0.62
(De Lucca 2000;
Joseph etal.,
2012)
Echinocandin B Lipopeptide A. nidulans C. albicans Glucan synthesis 0.625
(De Lucca 2000;
Veltri etal.,
2017)
Aculeacin Lipopeptide
A.
aculeatus C. albicans
Glucan synthesis 0.2
(De Lucca etal.,
1999; Chen
etal., 2016)
Trichopolyn
Amino-
lipopeptide
Trichoderma
polysporum C. albicans Unknown
0.8 (De Lucca 2000;
Liu etal., 2016)
Leucinostatin
Amino-
lipopeptide
Penicillium
lilacinum
C. neoformans
Unknown
0.5
(De Lucca 2000;
Zhao etal.,
2013 )
Plant defensins
Plant defensins are eight disul de-linked cysteines with
a single helix and triple-stranded b-sheet (Bruix etal.,
1995). Ib-AMP
3
, isolated from Impatiens balsamina, was
observed to be lethal against germinated conidia of A.
avus by 42%, where as it was non-lethal against non-
germinated conidia.It had MIC of 50.0μg/ml against
F. moniliforme (De Lucca et al., 1999; Asano et al.,
2013).
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS 379
Narjis Fathima Mirza etal.
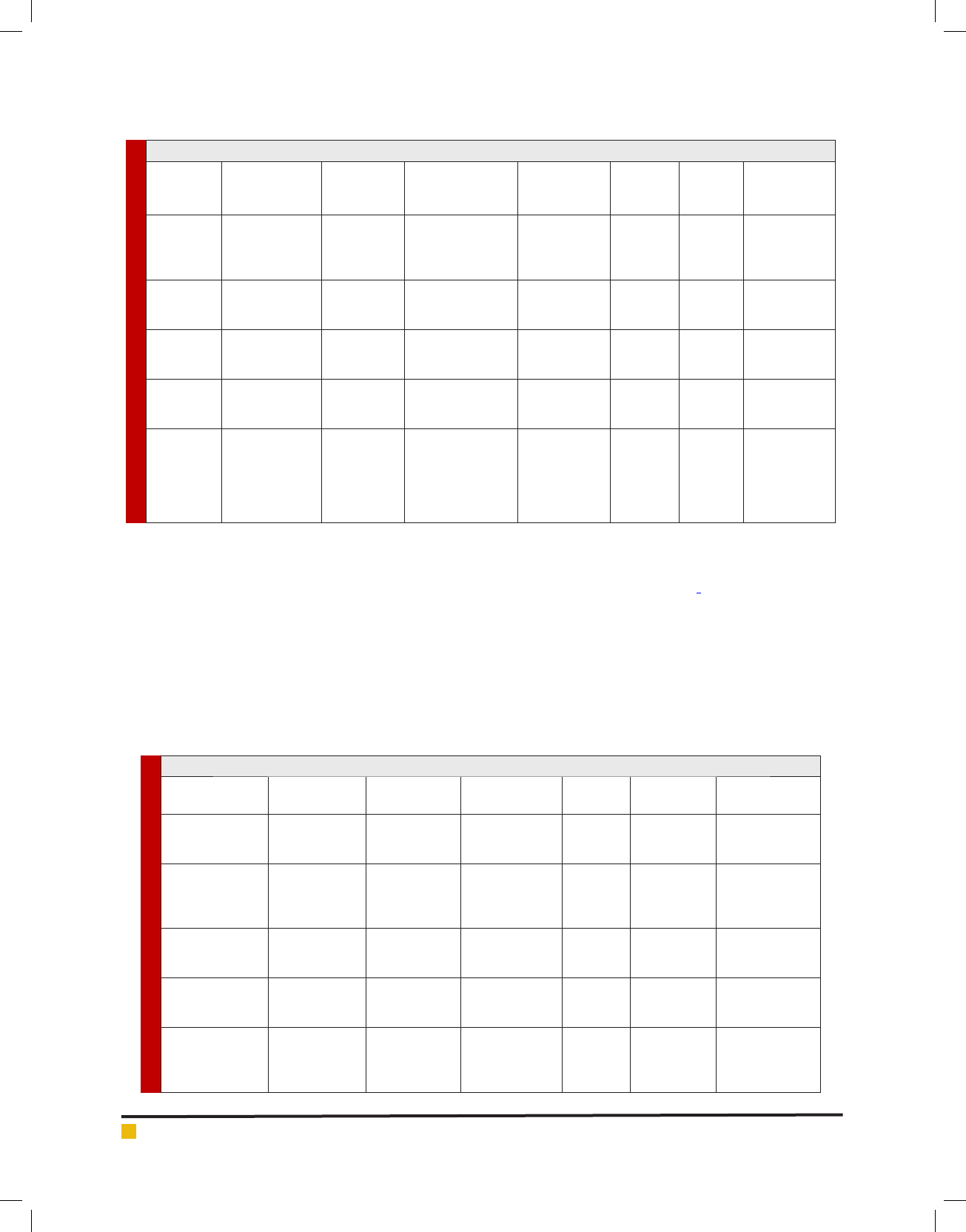
Table 5. Antifungal peptides from amphibian sources
Peptide name
No. of amino
acids
source
Typical Target
organism
Mode of
action
In vitro MIC
(μg/ml)
Reference
Magainin 2
23 Xenopus laevis C. albicans
Lysis 80.0
(Zasloff etal.,
2002; Bondaryk
etal., 2017)
Dermaseptin b 27
Phyllomedusa
sauvagii
C.
neoformans
Lysis
60.0
(Landon
etal., 1997;
Brandenburg
etal., 2015)
Dermaseptin s 34 P. sauvagii C. neoformans Lysis
5.0
(Landon etal.,
1997; Brunetti
etal., 2016)
Skin-PYY (SPYY)
36 P. bicolor A. fumigatus
Membrane
permiation
80.0
(Vouldoukis etal.,
1996; Brunetti
etal., 2016)
Brevinin-2R 24 Rana ridibunda C. albicans — 3.0
(Conlon
etal., 2003;
Anunthawan
etal., 2015 )
Cyclopeptides: Cyclopeptides from different species of
Rhamnaceae family were observed to have antifungal
activities. Frangufoline, from barks of Rhamnus fran-
gula were observed to have anti-bacterial and anti-
fungal properties. It showed MIC of 5.0 μg/ml for A.
niger. Nummularine (B, K, R, and S), from stem barks
of Ziziphus nummularia, Rugosanine (A and B) from
stem barks of Ziziphus rugosa and abyssenine-C from
stem barks of Ziziphus abyssinica, were all observed to
have antifungal properties against A. niger with MIC of
5 μg/ml. However, they were observed to be well effec-
tive against A. niger but not against C. albicans and
their mechanism of action was also unknown (Gournelis
etal., 1997; De Lucca 2000; Tan etal., 2006).
Lipid transfer proteins and other peptides: ACE-
AMP1 is a lipid transfer protein, produced by seeds of
Allium cepa which was observed to be effective against
F. oxysporum with MIC of 10.0 μg/ml (Cammue etal.,
1995; De Lucca 2000). Apart from the above antifun-
gal peptides, some other peptides include, Chitinases
and glucanases, which hydrolyze chitin, glucan, and
Table 4. Antifungal peptides from insect sources
Peptide
name
Family/group
No. of
amino acids
source
Typical Target
organism
Mode of
action
In vitro
MIC (μg/
ml)
Reference
Cecropin A
Cecropins
37 Hyalopora cecropia F. oxysporum, lysis 12.4
(De Lucca
etal.,1998;
Joseph etal.,
2012)
Cecropin B
Cecropins
35 Hyalopora cecropia
A. fumigatus
lysis 9.5
(Nappi etal.,
2001; Xiao
etal., 2013)
Drosomycin
Cysteine-rich
peptides
44
Drosophila
melanogaster and
Podisus maculiveris
F.oxysporum lysis 5.9
(De Lucca,
2000; Veltri
etal., 2017)
Thanatin
Cysteine-rich
peptides
21
Podisus
maculiveris F. oxysporum Unknown
5.0
(Bulet etal.,
2005; Wang
etal., 2015)
Heliomicins
Insect Defensins 44
Heliothis
virescens
C. neoformans
Unknown
12.0
Nappi etal.,
2001; De Lucca
2000; Zhao
etal., 2013;
Ageitos etal.,
2017)
380 ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Narjis Fathima Mirza etal.
the essential cell wall components of fungi. Prematins,
members of PR-5 protein family, act by permeabilizing
fungal membranes. Similarly, Thionins inhibit by per-
meabilizing fungal membranes and were found to be
effective against F. graminearum and F. sporotrichioides
(Velazhahan etal., 2001; Asano etal., 2013).
Fungal Peptides: Antifungal peptides from fungi
are more active than those compared to bacteria and
plants. Echinocandins are lipopeptides which inhibit
1,3--glucan synthase (Gregory etal., 2007). Glucan is
the major component of cell wall of fungi and inhibi-
tion of glucan may result in osmotic instability and in
cell lysis. (Lee etal., 1995; Gregory etal., 2007; Osorio
etal., 2015; Liu etal., 2016). The MIC90 value of echi-
nocandins was found to be ≤2 μg/mL against Candida
spp (Zaas etal., 2005). A-192411.29 had anti- fungicidal
activity against C. albicans, C. tropicalis and C. glabrata
(Vazquez et al., 2005; Kaconis etal., 2011; Chu et al.,
2013). But, the echinocandins do not show any antifun-
gal activity against Cryptococcus spp, Trichosporon spp,
Fusarium spp, zygomycetes (Zaas etal., 2005; Kazemza-
deh-Narbat etal., 2010). They also, do not affect human
cells, as human cells do not contain 1,3--D-glucan.
However, echinocandins are labeled category C and
are toxic to embryos (Gregory etal., 2007; Lakshmaiah
Narayana etal., 2014).
Micafungin from Coleophoma empedra, caspofungin
from Glarea lozoyensis and anidulafungin from A.
nidulans of echinocandin family have been approved
so far (Murdoch etal., 2004; Montgomery etal., 2013).
Of these, anidulafungin displays least MIC values fol-
lowed by micafungin and caspofungin being most. This
was observed against Candida spp. (Zaas et al., 2005;
Mojsoska etal., 2015). Caspofungin, also known as (MK-
0991) is a second generation pneumocandin from Glarea
lozoyensis (Abruzzo et al., 1997; Groll et al., 1999;
López-Garcia etal. 2005; Popovic etal., 2012 ). It was
fungicidal against C.albicans and C. parapsilosis (Bar-
tizal et al., 1997; Kuhn et al., 2002; Deresinski et al.,
2003; Ordonez etal., 2014 ). It was observed be effec-
tive against hyphal tips A. fumigatus although not com-
pletely lethal (Krishnan etal., 2005). It was also lethal
against several molds such as Alternaria sp., Curvularia
sp., Acremonium sp., Bipolaris sp., and Trichodermasp
(Kahn et al., 2006). Micafungin also known as FK463
had antifungal activity against disseminated candidi-
asis and aspergillosis (Petraitisetal., 2000; Lakshmaiah
Narayana etal., 2015).
The optimal concentration of FR463 at single infu-
sion was observed to be 2.5-25 mg (Azuma etal., 1998;
Pettengell etal., 1999; Kasetty etal., 2015; Kang etal.,
2017). Anidulafungin (V-echinocandin), previously
known as LY303366 is a semisynthetic echinocandin
currently used as antifungal drug (Krause etal., 2004;
Harder et al., 2013; Kang et al., 2017).It is a lipopep-
tide produced by A. nidulans, (Lei etal., 2013) and acts
by inhibiting glucan synthase (Denning et al., 1997;
Anunthawan etal., 2015). It was observed to be effec-
tive against Candidemia and other Candida infections
and esophageal candidiasis. MIC of (0.5 to 4.0) μg/ml
was observed in Candida spp. However, Anidulafungin
displays low MICs against strains of C. parapsilosis and
is not effective inactive against C. neoformans and Blas-
tomyces dermatitidis (De Lucca etal., 1999; Ghannoum
etal., 2005; Ben Lagha etal., 2017).
Echinocandin B from A. nidulans and A. rugulosus
was effective against C. albicans with MIC of 0.625 μg/
ml. Cilofungin (LY121019), isolated from Aspergillus
spp. had MIC of 0.62 μg/ml. Amino-lipopeptides such as
Trichopolyns from Trichoderma polysporum have MIC
of (0.78 - 6.25) μg/ml for C. albicans. Other families of
potent antifungal peptides include the leucinostatins
and helioferins families also consist of antifungal pop-
erties, but, where toxic, hemolytic to mammalian cells in
vitro (De Lucca 2000; Lei etal., 2013; Osorio etal., 2015;
Chen etal., 2016; Ageitos etal., 2017).
Insect Peptides: Cecropins
Cecropins (A and B) are linear lytic peptides, made up
of an 11- amino acid sequence, produced in hemolymph
giant silk moth, Hyalopora cecropia. Cecropin B was
observed lethal against F. oxysporum (approximately
95%), A. fumigatus 9.5 μg/ml (De Lucca et al., 1998;
Nappi etal., 2001). cecropin A was observed to be more
fungicidal at neutral pH and was more affective against
Fusarium moniliforme and Fusarium oxysporum with
total killing of 12.4 μg/ml (De Lucca etal.,1998).
Drosomycin: Drosomycin is a Cysteine-rich peptide
containing 44 amino acid with a twisted three-stranded
sheet structure steadied by disul de bonds. It is isolated
from Drosophila melanogaster and Podisus maculiveris
and was found to be effective against F.oxysporum with
MIC value of 5.9 μg/ml (De Lucca, 2000 ).
Glycin-rich peptides
Antifungal peptides, such as holotricin-3, and tenecin-3
are glycine-rich peptides isolated from insects (Nappi
etal., 2001). Tenecin-3 was studied to be effective against
C. albicans (Ganz, 2003). Holotricin-3, was isolated from
larval hemolymph of Holotrichia diomphalia, and was
observed to inhibit C. albicans growth (Lee etal., 1995).
Thanatin:Thanatin is another non-hemolytic Cysteine-
rich peptide containing 21 amino acid residues and
is smaller compared to drosomycin. It was affective
against many strains such as Trichoderma viride, Alter-
naria brassicola, Neurospora crassa, Botrytis cinerea,
and Fusarium culmorum, A. fumigatusT. mentagro-
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS 381
Narjis Fathima Mirza etal.
phytes and F. oxysporum (Fehlbaum etal., 1996; Bulet
etal., 2005). MIC of 5.0 μg/ml was observed against F.
oxysporum. However Thanatin was not effective against
yeast (Mandard etal., 1998).
Heliomicin: Heliomicin from Heliothis virescens
(tobacco budworm), was observed to have antifungal
activity against C. neoformans, with MIC of 12.0 μg/ml
(De Lucca 2000; Nappi etal., 2001).
Amphibian Peptides: Magainins: Magainins was the
rst among the antifungal peptides from amphib-
ian sources. They are amphiphilic, non-hemolytic and
are produced by Xenopus laevis (African clawed frog).
Magainin 2 inhibited C. albicans growth and had MIC of
80.0 μg/ml (De Lucca etal., 1999; Zasloff etal., 2002).
Dermaseptins: Dermaseptins are linear, lytic,peptides
produced by Phyllomedusa sauvagii (South American
arboreal frog). Dermaseptin was lethal towards for A.
avus, A. fumigatus, and F. oxysporum, with LD50
values observed as 3 μM, 0.5 μM, and 0.8 μM, respec-
tively (Landon etal., 1997). Dermaseptin b was effective
against yeasts and some lamentous fungi such as C.
neoformansand had MIC value of 60.0μg/ml. Dermasep-
tin s had MIC of 5.0μg/mlfor C. neoformans. (De Lucca
etal., 1999).
Skin-PYY (SPYY): Skin-PYY (SPYY), is an antifungal
compound produced by Phyllomedusa bicolor (South
American tree frog). It was observed to inhibit C. neo-
formans, C. albicans, and A. fumigatus and had MIC val-
ues of 20 μg/ml, 15 μg/ml, and 80 μg/ml, respectively
(Vouldoukis etal., 1996).
Brevinin: Brevinin-2R isolated from skin of Rana
ridibunda (red frog). It is non-hemolytic, 24 amino acid
peptide with -helical conformation. It was observed to
have MIC of 3.0 μg/ml against C. albicans (Conlon etal.,
2003).
FUTURE PROSPECTS
Emerging fungal resistance to conventional therapies
necessitates the development of novel antifungal strate-
gies. In this context, Anti-fungal peptides draw the atten-
tion as alternative potential antifungal agents (Brunetti
etal., 2016). These peptides are relatively safe, tolerated
and highly effective. As per the information available in
the literatures, only few antifungal peptides are used in
antifungal therapy (Brandenburg etal., 2015). There are
various problems addressed which is limiting the uses
of these peptides, such as low bioavailability, hemolytic
activity, instability, high cost of production, possible
aggregation, loss of activity in high salt concentrations,
poor ability to cross physiological barriers (Chen etal.,
2016; Ageitos etal., 2017).
Due to these effects, the therapeutic use of antifungal
peptides is signi cantly decreased now a day. However,
the utilization of these peptides could be enhanced by
chemical optimization and new delivery strategies. With
the advancement of new research strategies, the wide
variety of natural antimicrobial peptides should be char-
acterized both structurally and functionally for making
them extremely promising source of ideas in design the
novel antifungal peptides. In particular, application of
dendrimers as scaffolds for assembling well de ned
macromolecular polyvalent molecules or synthesis de
novo of per se active linear and branched peptide mim-
ics makes them extremely promising for use as new gen-
eration antifungal peptides.As found in several studies,
the modes of antifungal action must be well understood
(Deslouches et al., 2015; Gao et al., 2016; Kubicek-
Sutherland etal., 2017). Hopefully, all these efforts will
result in the development of a novel class of antifungal
agents to their full potential.
CONCLUSION
Antifungal peptides are excellent models for drug dis-
covery exhibiting unique characteristics such as low
level of resistance reaching the absent, high speci city,
broad spectrum, and unique mode of action. Despite the
distinctiveness, only few examples of antifungal pep-
tides have successfully reached the market.
ACKNOWLEDGEMENTS
All the authors want to acknowledge Sreenidhi Institute
of Science and Technology, Hyderabad and Centurion
University, Bhubaneswar for providing digital library to
explore the information to execute this work.
REFERENCES
Ageit os, J. M., Sánchez-Pérez, A., Calo-Mata, P., and Villa, T.
G. (2017). Antimicrobial peptides (AMPs): ancient compounds
that represent novel weapons in the ght against bacteria. Bio-
chem. Pharmacol. 133, 117–138. doi: 10.1016/j.bcp.2016.09.018
Anunthawan, T., De La Fuente-Nunez, C., Hancock, R. E., and
Klaynongsruang, S. (2015). Cationic amphipathic peptides KT2
and RT2 are taken up into bacterial cells and kill planktonic
and bio lm bacteria. Biochim. Biophys. Acta 1848, 1352–1358.
doi: 10.1016/j.bbamem.2015.02.021
Asano T, Miwa A, Maeda K, Kimura M, Nishiuchi T (2013). The
Secreted Antifungal Protein Thionin 2.4 in Arabidopsis thali-
ana suppresses the Toxicity of a Fungal Fruit Body Lectin from
Fusarium graminearum. PLoS Pathog 9(8): e1003581. https://
doi.org/10.1371/journal.ppat.1003581
Bartizal K, Gill CJ, Abruzzo GK, etal.. (1997). In vitro preclinical
evaluation studies with the echinocandin antifungal MK-0991
(L-743,872) Antimicrob Agents Chemother.; 41:2326–32.
382 ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Narjis Fathima Mirza etal.
Ben Lagha, A., Haas, B., Gottschalk, M., and Grenier, D. (2017).
Antimicrobial potential of bacteriocins in poultry and swine
production. Vet. Res. 48:22. doi: 10.1186/s13567-017-0425-6
Bionda, N., Fleeman, R. M., De La Fuente-Núñez, C., Rodriguez,
M. C., Reffuveille, F., Shaw, L. N., etal.. (2016). Identi cation
of novel cyclic lipopeptides from a positional scanning com-
binatorial library with enhanced antibacterial and antibio lm
activities. Eur. J. Med. Chem. 108, 354–363. doi: 10.1016/j.
ejmech.2015.11.032
Blanco, J. L., and M. E. Garcia (2008). Immune response to fun-
gal infections. Vet. Immunol. Immunopathol. 125:47–70. doi:
10.1016/j.vetimm.2008.04.020
Bondaryk, M., Staniszewska, M., Zielinska, P., and Urbanczyk-
Lipkowska, Z. (2017). Natural antimicrobial peptides as inspi-
ration for design of a new generation antifungal compounds.
J. Fungi 3:46. doi: 10.3390/jof3030046
Brandenburg, K., and Schürholz, T. (2015). Lack of new anti-
infective agents: passing into the pre-antibiotic age? World J.
Biol. Chem. 6, 71–77. doi: 10.4331/wjbc.v6.i3.71
Bruix, M., C. Gonzales, J. Santoro, F. Soriano, A. Rocher, E.
Mendez, and M. Rico (1995). 1HNMR studies on the structure
of a new thionin from barely endosperm. Biopolymers 36:751–
763. https://doi.org/10.1002/bip.360360608.
Brunetti, J., Falciani, C., Roscia, G., Pollini, S., Bindi, S., Scali,
S., et al.. (2016). In vitro and in vivo ef cacy, toxicity, bio-
distribution and resistance selection of a novel antibacterial
drug candidate. Sci. Rep. 6:26077. doi: 10.1038/srep26077
Bulet P, Stocklin R (2005). Insect antimicrobial peptides: struc-
tures, properties and gene regulation. Protein and Peptide Let-
ters; 12:3-11. DOI: 10.2174/0929866053406011.
Cammue, B. P. A., K. Thevissen, M. Hendricks, K. Eggermont, I.
J. Goderis, P. Proost, J. Van Damme, R. W. Osborn, F. Guerbette,
J.-C. Kader, and W. F. Broekaert. (1995). A potent antimicrobial
protein from onion seeds showing sequence homology to plant
lipid transfer protein. Plant Pathol; 109:445–455. https://doi.
org/10.1104/pp.109.2.445.
Chen, W., Ding, H. & Feng, P (2016) iACP: a sequence-based tool
for identifying anticancer peptides. Oncotarget. 7, 16895–16909.
Chereddy, K. K., Her, C. H., Comune, M., Moia, C., Lopes, A.,
Porporato, P. E., etal.. (2014). PLGA nanoparticles loaded with
host defense peptide LL37 promote wound healing. J. Control.
Release 194, 138–147. doi: 10.1016/j.jconrel.2014.08.016
Choe, H., Narayanan, A. S., Gandhi, D. A., Weinberg, A., Mar-
cus, R. E., Lee, Z., et al.. (2015). Immunomodulatory peptide
IDR-1018 decreases implant infection and preserves osseointe-
gration. Clin. Orthop. Relat. Res. 473, 2898–2907. doi: 10.1007/
s11999-015-4301-2
Chu, H. L., Yu, H. Y., Yip, B. S., Chih, Y. H., Liang, C. W., Cheng,
H. T., etal.. (2013). Boosting salt resistance of short antimicro-
bial peptides. Antimicrob. Agents Chemother. 57, 4050–4052.
doi: 10.1128/AAC.00252-13
Cole, J. N., and Nizet, V. (2016). Bacterial evasion of host anti-
microbial peptide defenses. Microbiol. Spectr. 4:VMBF-0006-
2015. doi: 10.1128/microbiolspec.VMBF-0006-2015
Conlon JM, Sonnevend A, Patel M, Davidson C, Nielsen PF,
Pál T, Rollins-Smith LA. (2003). Isolation of peptides of the
brevinin-1 family with potent candidacidal activity from the
skin secretions of the frog Rana boylii. J Pept Res. 2003 Nov;
62(5):207-13. DOI: 10.1034/j.1399-3011.2003.00090.x
De Lucca A, Bland J, Jacks T, Grimm C, Cleveland T, Walsh T.
(1997). Fungicidal activity of cecropin A. Antimicrob Agents
Chemother; 41: 481-483.
De Lucca A, Bland J, Jacks T, Grimm C, Walsh T. (1998). Fun-
gicidal and binding properties of the natural peptides cecro-
pin B and dermaseptin. Med Mycol;36(5):291-8. https://doi.
org/10.1080/02681219880000461.
De Lucca A, Bland J, Vigo C, Jacks T, Peter J, Walsh T. (2000).
D-cecropin B: proteolytic resistance, lethality for pathogenic
fungi and binding properties. Medical Mycology; 38:301-308.
https://doi.org/10.1080/714030954.
De Lucca A, Jacks T, Takemoto J, Vinyard B, Peter J, Navarro
E, et al.. (1999). Fungal lethality, binding, and cytotoxicity
of syringomycin-E. Antimicrobial agents and chemotherapy;
43:371-3.
De Lucca A. (2000). Antifungal peptides: potential candi-
dates for the treatment of fungal infections. Expert opin-
ion on investigational drugs; 9(2):273-299. http://dx.doi.
org/10.1517/13543784.9.2.273
De Lucca, Thomas J. Walsh. (1999). Antifungal Peptides: Novel
Therapeutic Compounds against Emerging Pathogens. Antimi-
crob Agents Chemother. 1999 Jan; 43(1): 1–11.
Denning DW. (1997). Echinocandins and pneumocandins - a
new antifungal class with a novel mode of action. J Antimi-
crob Chemother. 40 (5): 611–614. https://doi.org/10.1093/
jac/40.5.611.
Deresinski SC; Stevens DA. (2003). Caspofungin. Clin Infect
Dis. 36 (11): 1445–1457. Doi:10.1086/375080.
Deslouches, B., Steckbeck, J. D., Craigo, J. K., Doi, Y., Burns,
J. L., and Montelaro, R. C. (2015). Engineered cationic antimi-
crobial peptides to overcome multidrug resistance by ESKAPE
pathogens. Antimicrob. Agents Chemother. 59, 1329–1333.
doi: 10.1128/AAC.03937-14
Dobson, A. J., Purves, J., and Rolff, J. (2014). Increased sur-
vival of experimentally evolved antimicrobial peptide-resist-
ant Staphylococcus aureus in an animal host. Evol. Appl. 7,
905–912. doi: 10.1111/eva.12184
Dutta, P., and Das, S. (2015). Mammalian antimicrobial pep-
tides: promising therapeutic targets against infection and
chronic in ammation. Curr. Top. Med. Chem. 16, 99–129. doi:
10.2174/1568026615666150703121819
Falciani, C., Lozzi, L., Scali, S., Brunetti, J., Bracci, L., and Pini,
A. (2014). Site-speci c pegylation of an antimicrobial pep-
tide increases resistance to Pseudomonas aeruginosa elastase.
Amino Acids 46, 1403–1407. doi: 10.1007/s00726-014-
1686-2
Fehlbaum P, Bulet P, Chernych S, etal. (1996). Structure-activ-
ity analysis of thanatin, a 21-residue inducible insect defense
peptide with sequence homology to frog skin antimicrobial
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS 383

Narjis Fathima Mirza etal.
peptides. Proc Natl Acad Sci USA; 93: 1221-1225. https://doi.
org/10.1073/pnas.93.3.1221.
Fernández‐Carneado J, Kogan MJ, Pujals S, Giralt E. (2004).
Amphipathic peptides and drug delivery. Peptide Science;
76:196-203. https://doi.org/10.1002/bip.10585.
Ganz T. (2003) Defensins: antimicrobial peptides of innate
immunity. Nature Reviews Immunology; 3:710-20. https://doi.
org/10.1038/nri1180.
Gao, Y., Wu, D., Xi, X., Wu, Y., Ma, C., Zhou, M., etal.. (2016).
Identi cation and characterisation of the antimicrobial pep-
tide, phylloseptin-PT, from the skin secretion of Phyllomedusa
tarsius, and comparison of activity with designed, cationicity-
enhanced analogues and diastereomers. Molecules 21:E1667.
doi: 10.3390/molecules21121667
Georgopapadakou N, Walsh T. (1996). Antifungal agents:
chemotherapeutic targets and immunologic strategies. Antimi-
crob Agents Chemother; 40:279- 291.
Ghannoum MA, Chen A, Buhari M, etal.. (2005). Multi-echino-
candin resistant Candida parapsilosis: an emerging pathogen
[abstract M-722a]. Abstracts of the 45th Interscience Confer-
ence on Antimicrobial Agents and Chemotherapy; December
16–19, 2005; Washington, DC: American Society for Micro-
biology.
Gold, W., H.A.Stout, J.F.Pagano, and R.Donovick. (2002).
Amphotericins A and B, antifungal antibiotics produced by a
Streptomycete. I. Invitrostudies, p.579–586.Antibiot.
Gournelis, D. C., G. G. Laskaris, and R. Verpoorte. (1997).
Cyclopeptide alkaloids. Nat. Prod. Rep. 14:75–82. https://doi.
org/10.1039/np9971400075.
Gregory Eschenauer,Daryl D DePestel,and Peggy L Carver.
(2007). Comparison of echinocandin antifungals. Ther Clin
Risk Manag. 2007 Mar; 3(1): 71–97. https://doi.org/10.2147/
tcrm.2007.3.1.71.
Groll A, Walsh T. (1999). Preliminary evaluation of the anti-
fungal echinocandin MK-0991. Current Opinions in Anti-
infective Investigational Drugs; 1:334-335.
Gupte, M., P. Kulkarni, and B. N. Ganguli. (2002). Antifungal
antibiotics. Appl. Microbiol. Biotechnol. 58:46–57. https://doi.
org/10.1007/s002530100822.
Harder, J., Tsuruta, D., Murakami, M., and Kurokawa, I. (2013).
What is the role of antimicrobial peptides (AMP) in acne vul-
garis? Exp. Dermatol. 22, 386–391. doi: 10.1111/exd.12159
Jia, J., Liu, Z. & Xiao, X. pSuc-Lys: Predict lysine succinyla-
tion sites in proteins with PseAAC and ensemble random forest
approach. J. Theor. Biol. 394, 223–230 (2016).
Joseph, S., Karnik, S., Nilawe, P., Jayaraman, V. K. & Idicula-
Thomas, S(2012). ClassAMP: a prediction tool for classi cation
of antimicrobial peptides. IEEE/ACM Trans. Comput. Biol. Bio-
inform. 9(5), 1535–1538
Kaconis, Y., Kowalski, I., Howe, J., Brauser, A., Richter, W.,
RazquinOlazaran, I., et al.(2011). Biophysical mechanisms of
endotoxin neutralization by cationic amphiphilic peptides.
Biophys. J. 100, 2652–2661. doi: 10.1016/j.bpj.2011.04.041
Kahn J N, Hsu M, Racine F, Giacobbe R, Motyl M (2006).
Caspofungin susceptibility in Aspergillus and non-Aspergillus
molds: inhibition of glucan synthase and reduction of ˜-D-
1, 3 glucan levels in culture. Antimicrob Agents Chemother;
50:2214–16. https://doi.org/10.1128/aac.01610-05.
Kang, H. K., Kim, C., Seo, C. H., and Park, Y. (2017). The thera-
peutic applications of antimicrobial peptides (AMPs): a pat-
ent review. J. Microbiol. 55, 1–12. doi: 10.1007/s12275-017-
6452-1
Kasetty, G., Kalle, M., Morgelin, M., Brune, J. C., and
Schmidtchen, A. (2015). Anti-endotoxic and antibacterial
effects of a dermal substitute coated with host defense pep-
tides. Biomaterials 53, 415–425. doi: 10.1016/j.biomateri-
als.2015.02.111
Kazemzadeh-Narbat, M., Kindrachuk, J., Duan, K., Jenssen, H.,
Hancock, R. E., and Wang, R. (2010). Antimicrobial peptides
on calcium phosphate-coated titanium for the prevention of
implant-associated infections. Biomaterials 31, 9519–9526.
doi: 10.1016/j.biomaterials.2010.08.035
Krause DS, Reinhardt J, Vazquez JA, Reboli A, Goldstein BP,
Wible M, Henkel T. (2004). Phase 2, randomized, dose-rang-
ing study evaluating the safety and ef cacy of anidulafungin
in invasive candidiasis and candidemia. Antimicrob Agents
Chemother.48 (6):2021–4. Doi: 10.1128/AAC.48.6.2021-
2024.2004.
Krishnan S, Manavathu EK, Chandrasekar PH. (2005). A com-
parative study of fungicidal activities of voriconazole and
amphotericin B against hyphae of Aspergillus fumigatus. J
Antimicrob Chemother. J Antimicrob Chemother. 2005 Jun;
55(6):914-20. DOI: 10.1093/jac/dki100
Kubicek-Sutherland, J. Z., Lofton, H., Vestergaard, M., Hjort,
K., Ingmer, H., and Andersson, D. I. (2017). Antimicrobial pep-
tide exposure selects for Staphylococcus aureus resistance to
human defence peptides. J. Antimicrob. Chemother. 72, 115–
127. doi: 10.1093/jac/dkw381
Kuhn DM, George T, Chandra J, Mukherjee P.K, Ghannoum
M.A (2002). Antifungal susceptibility of Candida bio lms:
unique ef cacy of amphotericin B lipid formulations and
echinocandins. Antimicrob Agents Chemother; 46:1773–80.
https://doi.org/10.1128/aac.46.6.1773-1780.2002.
Lakshmaiah Narayana, J., and Chen, J. Y. (2015). Antimicrobial
peptides: possible anti-infective agents. Peptides 72, 88–94.
doi: 10.1016/j.peptides.2015.05.012
Lakshminarayanan, R., Liu, S., Li, J., Nandhakumar, M., Aung,
T. T., Goh, E., etal. (2014). Synthetic multivalent antifungal
peptides effective against fungi. PLoS ONE 9:e87730. doi:
10.1371/journal.pone.0087730
Landon C, Sodano P, Hetru C, Hoffman J, Ptak M. (1997).
Solution structure of drosomycin, the rst inducible antifun-
gal protein from insects. Protein Sci; 6:1878-1884. https://doi.
org/10.1002/pro.5560060908.
Lata, S., Mishra, N. K. & Raghava, G. P. S. (2010) AntiBP2:
improved version of antibacterial peptide prediction. BMC Bio-
inform. 11 (Suppl 1),S19.
384 ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Narjis Fathima Mirza etal.
Lei Shao; Jian Li; Aijuan Liu; Qing Chang; Huimin Lin; Dai-
jie Chen. (2013). Ef cient Bioconversion of Echinocandin B to
Its Nucleus by Overexpression of Deacylase Genes in Different
Host Strains. Applied and Environmental Microbiology. 79 (4):
1126–1133. doi:10.1128/AEM.02792-12.
Lemaitre B, Reichhart J-M, Hoffmann JA. (1997). Drosophila
host defense: differential induction of antimicrobial peptide
genes after infection by various classes of microorganisms.
Proceedings of the National Academy of Sciences; 94:14614-9.
https://doi.org/10.1073/pnas.94.26.14614.
Leuschner C, Hansel W. (2004). Membrane disrupting lytic
peptides for cancer treatments. Current pharmaceutical design;
10:2299-310. https://doi.org/10.2174/1381612043383971.
Liu, B. & Long, R(2016). iDHS-EL: Identifying DNase I hyper-
sensi-tivesites by fusing three different modes of pseudo
nucleotide composition into an en-semble learning framework.
Bioinform. 32, 2411–2418.
Liu, Z., Xiao, X. & Yu, D. J (2016). pRNAm-PC: Predicting
N-methyl-adenosine sites in RNA sequences via physical-
chemical properties. Anal. Biochem. 497, 60–67.
López-Garcia, B., Lee, P. H., Yamasaki, K., and Gallo, R. L.
(2005). Anti-fungal activity of cathelicidins and their potential
role in Candida albicans skin infection. J. Invest. Dermatol.
125, 108–115. doi: 10.1111/j.0022-202X.2005.23713.x
Lyu, Y., Yang, Y., Lyu, X., Dong, N., and Shan, A. (2016). Anti-
microbial activity, improved cell selectivity and mode of action
of short PMAP36-derived peptides against bacteria and Can-
dida. Sci. Rep. 6:27258. doi: 10.1038/srep27258
Mandard N, Sodano P, Labbe H, Bonmatin JM, Bulet P, Hetru
C, etal.. (1998). Solution structure of thanatin, a potentbacte-
ricidal and fungicidal insect peptide, determined from proton
two‐dimensional nuclear magnetic resonance data. European
Journal of Biochemistry; 256:404-10. https://doi.org/10.1046/
j.1432-1327.1998.2560404.x.
Mohammad Omer Faruck, Faridah yusof, Silvia Chowdhury.
(2015).an overview of antifungal peptides derived from insect.
Peptides. 18 Jun 2015; 80:80-88. https://doi.org/10.1016/j.pep-
tides.2015.06.001.
Montgomery, C. P., Daniels, M. D., Zhao, F., Spellberg, B.,
Chong, A. S., and Daum, R. S. (2013). Local in ammation
exacerbates the severity of Staphylococcus aureus skin infec-
tion. PLoS ONE 8:e69508. doi: 10.1371/journal.pone.0069508
Murdoch D, Plosker GL. (2004). Anidulafungin. Drugs; 64:
2249–58.https://doi.org/10.2165/00003495-200464190-
00011.
Nappi A, Vass E. (2001). Cytotoxic reactions associated with
insect immunity. Phylogenetic perspectives on the verte-
brate immune system: Springer; 2001. p. 329-48. https://doi.
org/10.1007/978-1-4615-1291-2_33.
Ordonez, S. R., Amarullah, I. H., Wubbolts, R. W., Veldhuizen,
E. J., and Haagsman, H. P. (2014). Fungicidal mechanisms of
cathelicidins LL-37 and CATH-2 revealed by live-cell imaging.
Antimicrob. Agents Chemother. 58, 2240–2248. doi: 10.1128/
AAC.01670-13
Osorio, D., Rondon-Villarreal, P. & Torres, R (2015). Peptides:
A package for data mining of antimicrobial peptides. The R
Journal. 7(1),4–14.
Pettengell K, Mynhardt J, Kluytis T, SoniP. (1999). A multi-
center study to determine the minimal effective dose of FK
463 for the treatment of esophageal candidiasis in HIV-posi-
tive patients. Abstracts of the39th Interscience Conference on
Antimicrobial Agents and Chemotherapy.San Francisco, CA
1999: 1421.
Pfaller MA, Boyken L, Hollis RJ, Messer SA, Tendolkar S,
Diekema DJ (2005). In Vitro Activities of Anidulafungin against
More than 2,500 Clinical Isolates of Candida spp., Including
315 Isolates Resistant to Fluconazole. J Clin Microbiol. 43 (11):
5425–7. Doi:10.1128/JCM.43.11.5425-5427.2005.
Pfaller MA, Diekema DJ, Boyken L, Messer SA, Tendolkar S,
Hollis RJ, Goldstein BP. (2005). Effectiveness of anidulafungin
in eradicating Candida species in invasive candidiasis. Anti-
microb Agents Chemother. 49 (11): 4795–7. Doi: 10.1128/
AAC.49.11.4795-4797.2005.
Popovic, S., Urban, E., Lukic, M., and Conlon, J. M. (2012).
Peptides with antimicrobial and anti-in ammatory activities
that have therapeutic potential for treatment of acne vulgaris.
Peptides 34, 275–282. doi: 10.1016/j.peptides.2012.02.010
Porto, W. F., Souza, V. A., Nolasco, D. O. & Franco, O. L (2012).
In silico identi cation of novel hevein-like peptide precursors.
Peptides. 38, 127–136.
Qin Gu, Yang Yang, Qiming Yuan, Guangming Shi, Liming
Wu, Zhiying Lou,Rong Huo, Huijun Wu, Rainer Borriss and
Xuewen Gao. (2017). Bacillomycin D produced by Bacillus
amyloliquefaciens is involved in the antagonistic interaction
with the plant pathogenic fungus Fusarium graminearum.
American Society for Microbiology.21 July 2017, doi: 10.1128/
AEM.01075-17.
Ravi C, Jeyashree A, Devi KR. (2011). Antimicrobial peptides
from insects: An overview. Research in Biotechnology. 2011; 2.
Rees JA, Moniatte M, Bulet P. (1997). Novel antibacterial
peptides isolated from a European bumblebee, Bombus pas-
cuorum (Hymenoptera, apoidea). Insect biochemistry and
molecular biology; 27:413-22. ttps://doi.org/10.1016/s0965-
1748(97)00013-1.
Schelenz S, Barnes R, Kibbler C, Jones B, Denning D. (2009).
Standards of care for patients with invasive fungal infections
within the United Kingdom: a national audit. Journal of Infec-
tion; 58:145-53. https://doi.org/10.1016/j.jinf.2008.12.006.
Shai, Y. (1995). Molecular recognition between membrane-
spanning polypeptides. TIBS 20:460–464. https://doi.
org/10.1016/s0968-0004(00)89101-x.
Sorensen, K. N., K.-H. Kim, and J. Y. Takemoto. (1996). In vitro
antifungal and fungicidal activities and erythrocyte toxicities
of cyclic lipodepsin peptides produced by Pseudomonas syrin-
gae pv. syringae. Antimicrob.Agents Chemother. 40:2710–2713.
Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW,
Scheld WM, etal.. (2008). The epidemic of antibiotic resistant
infections: a call to action for the medical community from
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS 385

Narjis Fathima Mirza etal.
the Infectious Diseases Society of America. Clinical Infectious
Diseases; 46:155-64. https://doi.org/10.1086/524891.
Tabbene Olfa, Di Grazia Antonio, Azaiez Sana, Ben Slimene
Imen, Elkahoui Salem, Alfeddy Mohamed Najib, Casciaro
Bruno, Luca Vincenzo, Limam Ferid, Mangoni Maria Luisa;
Synergistic fungicidal activity of the lipopeptide bacillomy-
cin D with amphotericin B against pathogenic Candida spe-
cies, FEMS Yeast Research, Volume 15, Issue 4, 1 June 2015,
fov022, https://doi.org/10.1093/femsyr/fov022.
Tan NH, Zhou J. (2006). Plant cyclopeptides. Chem Rev. 2006
Mar; 106(3):840-95. https://doi.org/10.1021/cr040699h.
Thakur, N., Qureshi, A. & Kumar, M (2012). AVP pred: collec-
tion and prediction of highly effective antiviral peptides. Nucl.
Acids. Res. 40,W199–204.
Vazquez JA. (2005). Anidulafungin: A New Echinocandin
with a Novel Pro le, Clin Ther; 27(6):657-73. https://doi.
org/10.1016/j.clinthera.2005.06.010.
Velazhahan, R., Radhajeyalakshmi, R., Thangavelu, S. Muth-
ukrishnan.(2001). An Antifungal Protein Puri ed from Pearl
Millet Seeds Shows Sequence Homology to Lipid Trans-
fer Proteins. Biologia Plantarum; 44: 417. https://doi.
org/10.1023/A:1012463315579
Veltri, D., Shehu, A. & Kamath, U (2017). Improving recogni-
tion of antimicrobial peptides and target selectivity through
machine learning and genetic programming. IEEE/ACM
Trans. Comput. Biol. Bioinform. 14(2):300-313. doi: 10.1109/
TCBB.2015.2462364.
Vouldoukis I, Shai Y, Nicolas P, Mor A. (1996).Broad spectrum
antibiotic activity of skin- PYY. FEBS Lett; 380: 237- 240.
https://doi.org/10.1016/0014-5793(96)00050-6.
Wang, G., Li, X. & Wang, Z (2016). APD3: the antimicrobial
peptide database as a tool for research and education. Nucl.
Acids Res. 44(D1),D1087–1093.
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP,
Edmond MB. (2004). Nosocomial blood stream infections in US
hospitals: analysis of 24,179 cases from a prospective nation-
wide surveillance study. Clinical Infectious Diseases; 39:309-
17. https://doi.org/10.1086/421946.
Xiao, X., Wang, P., Lin, W. Z., Jia, J. H. & Chou, K. C (2013).
iAMP-2L: A two- level multi-label classi er for identifying
antimicrobial peptides and their functional types. Anal. Bio-
chem. 436(2), 168–177 .
XinZhao, Zhi-jiangZhou, YeHanZhan-zhongWang, JieFan,
Hua-zhiXia. (2013). Isolation and identi cation of anti-
fungal peptides from Bacillus BH072, a novel bacterium
isolated from honey; [598-606]. https://doi.org/10.1016/j.
micres.2013.03.001.
Zaas AK, Alexander BD. (2005). Echinocandins: role in anti-
fungal therapy. Exp Opin Pharmacother. 2005; 6:1657–68.
https://doi.org/10.1517/14656566.6.10.1657.
Zasloff M. (2002). Antimicrobial peptides of multicellular organ-
isms. Nature. 2002; 415:38-95. https://doi.org/10.1038/41538
Zhao, X., Wu, H., Lu, H., Li, G. & Huang, Q (2013). LAMP: A
database linking antimicrobial peptides. PLoS ONE. 8(6), e66557.
386 ANTIFUNGAL PEPTIDES: BIOSYNTHESIS, PRODUCTION AND APPLICATIONS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS