Agricultural
Communication
Biosci. Biotech. Res. Comm. 11(2): 335-346 (2018)
Transcript analysis of the known moisture stress
responsive gene orthologs among different genotypes
of Little millet,
Panicum sumatrense
Banda Sushmitha, Patil Arun H, Mahima Dubey and Girish Chandel*
Department of Plant Molecular Biology and Biotechnology, Indira Gandhi Agricultural University, College of
Agriculture, Raipur, C.G, 492012, India
ABSTRACT
Among different abiotic stresses (cold, temperature, salinity, drought, oxidative stress etc.) moisture stress is the
most important limiting factor for crop production and is becoming an increasingly severe problem in many regions
of the world. The aim of the current study is to identify some keys genes that are responsible for drought tolerant
related traits, in the selected genotypes of Little millet (BL-8, MM-23, MM-10, BL-15-1, RLM-37, OLM-203, BL-4,
JK-8). Genotypes belonging to diverse genetic background were grown under stress and control conditions for the
identi cation of moisture stress tolerant traits. A set of known moisture stress related gene orthologs were selected for
expression analysis using semi quantitative RT-PCR analysis. Expression analysis of these drought responsive gene
orthologs (Amino-transferase, Thionin-osthi, Aquaporin, Synaptotagmin, CDPK, Scythe protein, Ta NAC-2, Ec NAC-
67, NAM, U2-SnRNP, Hv NAC, Os NAC-29-2, MPK 17-1, DQP1, DQP 2, DQP 3, DQP 4, DQP 6 ) had given a differential
expression under moisture stress as compared to controlled traits. Majority of these genes were up-regulated in the
genotypes RLM-37, MM-23, MM-10, BL-4, BL-8 and BL-15-1 under moisture stress condition and these ndings was
found to be in correlation with the estimated biochemical traits (Proline, Chlorophyll, Carbohydrate and Protein). This
can be taken as a base for drought tolerance response of the crop, which may be useful for further validation studies
of the candidate genes for drought tolerance in the millet species as well as other crop plants.
KEY WORDS: ABIOTIC STRESS, DROUGHT, SEMI QUANTITATIVE RT-PCR
335
ARTICLE INFORMATION:
*Corresponding Author: ghchandel@gmail.com
Received 19
th
April, 2018
Accepted after revision 22
nd
June, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.1/21

336 TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Sushmitha, Arun and Dubey
INTRODUCTION
The exceptional tolerance of millets toward diverse abi-
otic stresses including drought, salinity, light and heat
makes them a tractable system to study their stress
responsive traits at the cellular, molecular and physi-
ological levels (Bandyopadhyay et al., 2017). Several
morpho-physiological and biochemical studies in millets
have shown their stress adaptation strategies. Little mil-
let is grown to a limited extent in India, up to altitudes
of 2,100 m. It occurs wild in northern India and south
East Asia. It belongs to the subfamily Panicoideae, tribe
Paniceae, genus Panicum, species P. sumatrense, with
chromosome number 36 (tetraploid) (Hiremath et al.,
1990). Little millet is a domesticated form of the weedy
species Panicum psilopodium (De Wet et al., 1983a).
Introgression of genes between the two species is com-
mon (Hiremath et al., 1990). Little millet is comparable
to other cereals in terms of ber, fat, carbohydrates, and
protein, and rich in phytochemicals including phenolic
acids, avonoids, tannins, and phytate (Pradeep and
Guha 2011). Improved varieties of small millets could
play a role in the “New Green Revolution”- a term coined
to re ect novel strategies which will be required to deal
with complex challenges in developing nations includ-
ing increasing population and ever diminishing arable
land. Like many other small millets, it is drought toler-
ant, pest and salt resistant,( Sivakumar et al., 2006b,
Herder et al., 2010, Bhaskara and Panneerselvam 2013,
Ajithkumar et al., 2014 Tang et al., 2017, Jaiswal et al.,
2018).
Nagarjuna et al., (2016) have reported the identi ca-
tion and characterisation of an abiotic stress responsive
protein kinase called CBL Interacting Protein Kinase
(EcCIPK31-like) from drought tolerant crop, nger mil-
let. Where, the upregulation was reported for rst time
under salinity, desiccation, oxidative and temperature
stresses at seedling level in nger millet. The stress
responsive nature of EcCIPK31-like to diverse stresses
indicates that the gene could regulate multiple cellular
tolerance traits and its further functional validation can
highlight the relevance in abiotic stress. Similarly, it has
been reported that Kodo millet is known to be highly
drought and salt tolerant crop as ascertained by antioxi-
dants and antioxidant enzymes levels. cDNA library was
constructed from 6 days’ drought stressed seedlings. 5
ESTs differentially expressed under drought stress were
characterized by DDRT-PCR and their expression pro-
le was assessed by real time RT-PCR. Drought stress
in Kodo millet led to the characterization of three up-
regulated ESTs compared to two down-regulated, (Sid-
dappa et al 2016).
Experimental results by Hittalmani, et al. (2017)
revealed that, from whole genome sequencing and
assembling process of ML-365 nger millet cultivar
yielded 1196 Mb covering approximately 82% of total
estimated genome size. Transcriptome analysis of low
moisture stress and non-stress samples revealed the
identi cation of several drought-induced candidate
genes, which could be used in drought tolerance breed-
ing. This genome sequencing effort had strengthened
the plant breeders for allele discovery, genetic mapping,
and identi cation of candidate genes for agronomically
important traits.
In a study, physiological and transcriptomic compari-
sons between drought tolerant S. italica cultivar ‘Yugu1’
and drought-sensitive ‘An04’ were conducted by Tang,
et al. (2017). They identi ed 20 candidate genes that
contributed to germination and early seedling drought
tolerance in S.italica. Finally their analysis provided a
comprehensive picture of how different S.italica gen-
otypes respond to drought, and may be used for the
genetic improvement of drought tolerance in Poaceae
crops.
Jaiswal, et al., (2018) reported de novo assembly-based
transcriptomic signature of drought response induced by
irrigation withdrawal in pearl millet. They found 19,983
differentially expressed genes, 7,595 transcription fac-
tors, gene regulatory network having 45 hub genes
controlling drought response. They also reported 34652
putative markers (4192 simple sequence repeats, 12111
SNPs and 6249 InDels). This Study had revealed the role
of purine and tryptophan metabolism in ABA accumula-
tion mediating abiotic response in which MAPK acts as
major intracellular signal sensing drought.
The molecular biology of Little millet has been
explored to a limited extent. Little millet is perhaps the
least studied of the small millet species and there is much
that requires investigation, including the establishment
of a genetic map and sequenced genome. It is important
to dissect the transcriptome information under stress
condition for the identi cation and characterization of
the key genes for moisture stress tolerance. The identi-
ed genes which were up-regulated under the moisture
stress condition, can be taken as a base for drought tol-
erance response of the crop, which may be useful for
further validation studies of the candidate genes for
drought tolerance mechanism in the millet species as
well as other crop plants.
MATERIAL AND METHODS
Sowing of Little millet (Panicum sumatrense) was done
in trays. Moisture stress was imposed after 30 days of
sowing, at the vegetative stage before panicle initiation
for a set of eight Little millet genotypes under the con-
trolled environmental conditions as shown in gure 1.

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES 337
Sushmitha, Arun and Dubey
Temperature was maintained around 30±2. Plants were
watered normally once in a day before the stress impo-
sition and the leaf samples are harvested when the soil
moisture content in the stress trays as reached below
10%. The harvested samples were stored immediately
in liquid nitrogen for RNA isolation. The RWC was cal-
culated based on the formula suggested by Barr and
Weatherley (1962) as follows:
Where FW = fresh weights of leaf taken immediately
after excision TW = Turgid weight of leaf DW = Dry
weight of leaf dried at 70 °C for 48 h. Leaf carbohy-
drate content was estimated by phenol sulphuric acid
method proposed by Krishnaveni et al., (1984). The total
carbohydrate present in the sample solution was calcu-
lated as given below using the standard graph. Absorb-
ance corresponds to 8 ml of the test = ‘x’ mg of glucose.
100 ml of the sample solution contains = x / Sample
volume X 10 mg of glucose. Leaf proline content was
estimated by Acid ninhydrin method described by Bates
et al., (1973). Free proline content in the sample was
estimated by referring to a standard curve made from
known concentrations of Proline by taking following
formula:
Leaf Chlorophyll content was estimated by acetone
method developed by Arnon (1949). The amount of
Chlorophyll present in leaf sample was calculated by
using following equation:
The values were expressed in milligram per gram of fresh
weight. Where, A = Absorbance at speci c wavelength,
V = nal volume of chlorophyll extract in 80% acetone,
Wt = fresh weight of tissue extract. Leaf Protein content
was estimated as per the method given by Lowry et al.,
(1951). From the standard graph the amount of protein
in the unknown solution was calculated. The amount
of protein present in the unknown solution is mg (g
of protein). The effect of moisture stress under stress
and control condition in genotypes of Little millet was
analysed statistically by calculating factorial CRD using
OP-STAT, an online computerized software developed at
BHU. RNA was isolated using TRIzol (Invitrogen, USA)
and the concentration was determined using Nanodrop
spectrophotometer ND-1000® (Nanodrop technologies
USA). cDNA was prepared by using BIORAD iScript
cDNA synthesis kit as per manufacturer’s instructions.
Semi-Quantitative RT-PCR reactions were carried out
in 20 µl of the solutions using gene speci c primers and
Actin gene primer as internal control. The reaction was
performed by adding following components in order
into the PCR tubes for ampli cation: cDNA of 2.0 µl,
10X PCR buffer of 2.0 µl, (2Mm) dNTP of mix 1.0 µl,
Primer Forward of 1.0 µl, Primer Reverse of 1.0 µl, (5U/
ul) Taq polymerase of 0.2 µl, Nanopure water 1,500 ng/
µl of 11.8 µl. Ampli cations were performed by a cycles
of 2 min at 95
0
C followed by 35 cycles each of 15 sec
at 95
0
C, 30 sec at 56-62
0
C, and 30 sec at 68
0
C and nal
extention of 1 min at 72
0
C.
FIGURE 1. Plant morphology of Eight Little millet Genotypes (RLM-37, OLM-203, MM-10,
MM-23, JK-8, BL-4, BL-8, BL-15-1) under Control and Stress condition at Vegetative stage
before Panicle Initiation.
Sushmitha, Arun and Dubey
338 TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Separation of ampli ed fragments was carried out
using Bio-Rad gel electrophoresis assembly. PCR ampli-
cation products were analyzed by Agarose gel elec-
trophoresis on 1.5% agarose gel stained with Ethidium
Bromide solution (0.5 g/ml). The gel was run in 1X TBE
buffer at 70-80 Volts for 45 minutes to 1.5 h. Standard
ladders of 100bp size were used. The resultant PCR prod-
uct was then resolved on 1.5% Agarose gel followed by
digitalization of uorescence data to numerical values
using GelQuant.NET Analyze. The relative expression of
genes was expressed in terms of fold change (Increase/
Decrease) under water stress with respect to their
control.
RESULTS AND DISCUSSION
Wide variation for Relative water content values was
recorded in stress tissue compared to control one’s among
all tested Little millet genotypes. RWC of leaf samples
ranged from 14.711% to 67.9% in stress plant leaf tis-
sues and 67.821% to 95.073% in control plant leaf tissues.
The drought tolerant Little millet genotype OLM-203 has
the highest RWC value (67.9%) in stress tissue followed
by MM-23 (64.83%), JK-8 (64.255%), RLM-37 (64.028%),
MM-10 (52.966%), BL-4(35.48%). whereas, susceptible
genotypes BL-15-1 (14.711%), BL-8 (14.194%) showed the
minimum RWC in stress tissues. Two Little Millet geno-
types RLM-37 and OLM-203 had shown lower decrease
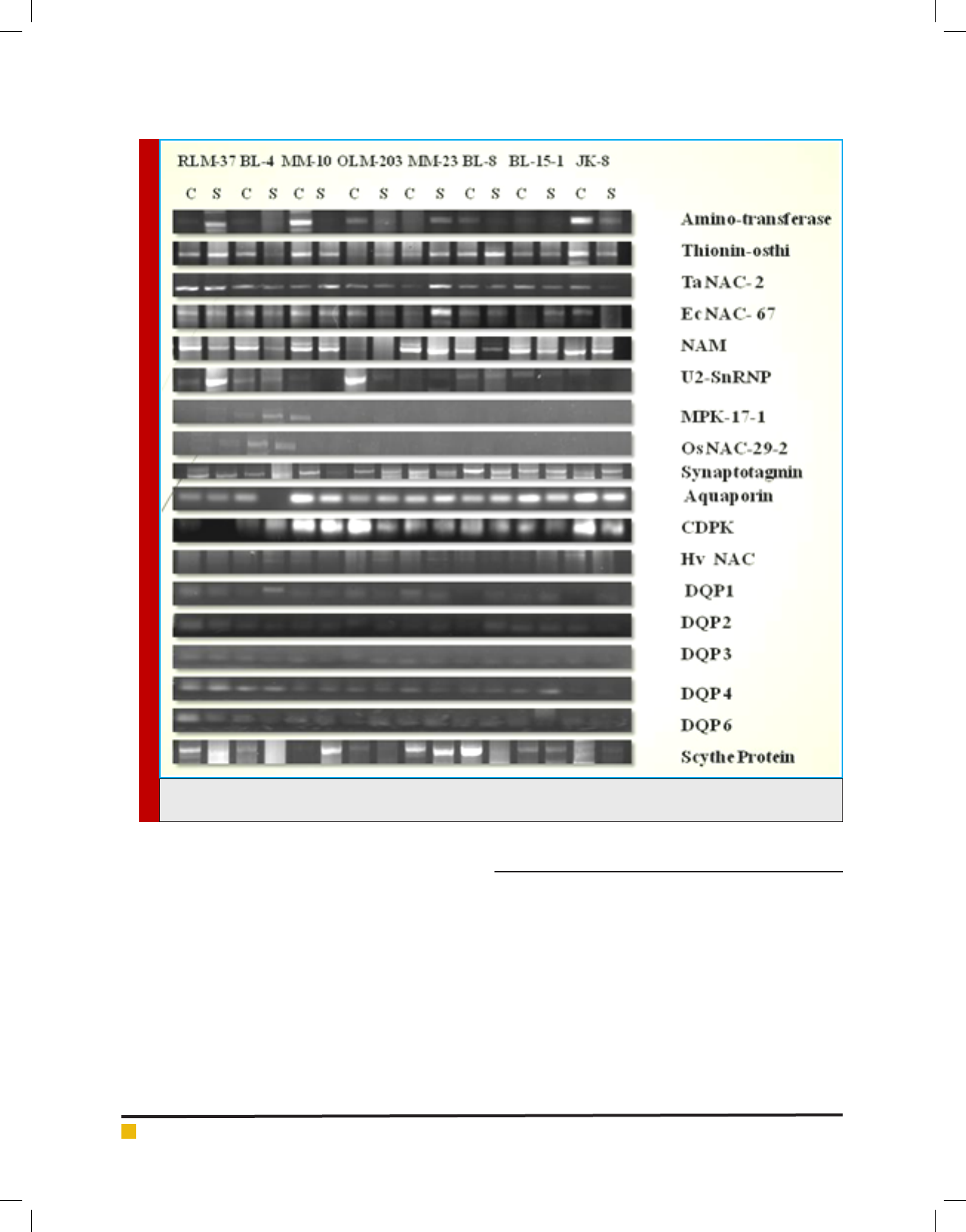
FIGURE 2. Semi quantitative RT- PCR Analysis of 18 Drought Responsive genes for Eight Genotypes of Little millet (BL-
8, MM-23, MM-10, BL-15-1, RLM-37, OLM-203, BL-4, JK-8) under Control and Stress condition.

Sushmitha, Arun and Dubey
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES 339
in RWC with values of 0.141% and 13.85% respectively
as given in table 1. This clearly indicates that the two
genotypes have the ability to retain more water during
moisture stress which helps in sustaining the metabolic
and physiological activities of plants. A wide variation for
proline content was recorded in stress tissue compared to
that of control one’s for eight diverse Little millet geno-
types (BL-8, MM-23, MM-10, BL-15-1, RLM-37, OLM-
203, BL-4, JK-8). The proline content ranged from 0.192
to 7.869 µ mole/tissue under stress; whereas under con-
trol condition proline content ranged from 0.015 to 0.204
µmole/tissue. A signi cant increase in proline has been
observed in response to water stress, favouring osmotic
adjustment. When comparing fold increase in proline
content under stress over control among eight genotypes
BL-15-1 was found with (63.460) higher fold increase fol-
lowed by BL-8 (52.789), MM-10 (42.200), BL-4 (13.264),
MM-23 (9.121), JK-8 (3.514), OLM-203 (1.670) and RLM-
37 (1.343) as given in table 1. Enhanced proline accu-
mulation in leaf tissues of plants exposed to water stress
condition is considered as one of the major trait for the
phenotypic characterization of plants for abiotic stress
tolerance (Zhu et al., 2006)
.
A wide variation for chlorophyll content was recorded
in stress tissues for eight Little millet genotypes (BL-8,
MM-23, MM-10, BL-15-1, RLM-37, OLM-203, BL-4,
JK-8). Chlorophyll a, Chlorophyll b and Total Chlorophyll
content ranged from 0.783 to 2.441 mg/tissue, 0.403 to
1.332 mg/tissue, 1.330 to 3.811 mg/tissue respectively
for stress leaf tissue where as under control condition
it ranged from 1.223 to 3.075 mg/tissue, 0.597 to 3.006
mg/tissue, 1.819 to 6.047 mg/tissue respectively. The
genotype MM-10 had the highest fold decrease of 2.011
mg/tissue followed by BL-4 (1.583), BL-15-1 (1.463),
BL-8 (1.368), JK-8 (1.334), OLM-203 (1.229), MM-23
(1.221) RLM-37 (1.042) in the total chlorophyll content,
Where as in case of chlorophyll a, the genotype MM-23
showed highest fold decrease of 2.00 followed by BL-4
(1.749), BL-15-1 (1.641), MM-10 (1.619), JK-8 (1.571),
BL-8 (1.319), OLM-203 (1.208), RLM-37 (1.016) and in
chlorophyll b, the genotype JK-8 showed the highest
fold decrease of 3.00 followed by MM-10 (2.665), BL-8
(1.481), MM-23 (1.360), BL-4 (1.314), OLM-203 (1.270),
BL-15-1 (1.159), RLM-37 (1.149) as given in the table 1.
The carbohydrate content ranges from 234.221
to 612.222 mg/tissue under stress condition whereas
153.907 to 302.313 mg/tissue under control condition.
BL-15-1 (2.705) had the highest fold increase followed
by MM-23 (2.202), BL-4 (1.671), MM-10 (1.633), OLM-
203 (1.522), JK-8 (1.502), RLM-37 (1.274), and BL-8
(1.043) as given in table 1. The wide variation for pro-
tein content was recorded in stress tissues for eight Little
millet genotypes (BL-8, MM-23, MM-10, BL-15-1, RLM-
37, OLM-203, BL-4, JK-8). The protein content ranged
from 0.040 to 0.586 mg/tissue under stress condition,
whereas 0.027 to 0.080 mg/tissue under control condi-
tion. BL-4 (8.746) had the highest fold increase followed
by BL-8 (8.457), MM-10 (3.604), JK-8 (2.875), OLM-204
(1.821), BL-15-1 (1.750), RLM-37 (1.481), MM-23 (1.431)
as given in table 1.
Expression pattern of drought stress responsive genes in
little millet genotypes under moisture stress condition
Semi quantitative RT-PCR was performed to analyze the
expression pattern of eighteen differentially expressed
transcripts in Little millet under moisture stress ver-
sus control condition. The genes include Amino-trans-
ferase, Thionin-osthi, Aquaporin, Synaptotagmin, CDPK,
Table 1. Percentage change (Fold Increase or Decrease) in the RWC, Proline, Chlorophyll, Carbohydrate
and Protein content for Eight Genotypes of Little millet (BL-8, MM-23, MM-10, BL-15-1, RLM-37,
OLM-203, BL-4, JK-8) under Control and Stress condition.
Genotype
Decrease in
RWC %
Fold increase
proline
Fold decrease
Total Chlorophyll
Fold increase
Carbohydrate
Fold increase
Protein
RLM-37 13.85 1.343 1.042 1.274 1.481
OLM-203 0.141 1.670 1.229 1.522 1.821
JK-8 27.275 3.514 1.334 1.502 2.875
MM-23 28.702 9.121 1.221 2.202 1.431
MM-10 14.855 42.200 2.011 1.633 3.604
BL-4 39.63 13.264 1.583 1.671 8.746
BL-8 56.82 52.789 1.368 1.043 8.457
BL-15-1 80.362 63.460 1.463 2.705 1.750

Sushmitha, Arun and Dubey
340 TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Table 2. List of Eighteen Genes, with their Primer sequences, GenBank Acc. No./Locus ID’s and
Annealing temperatures used for Expression Analysis for Eight Genotypes of Little millet (BL-8, MM-23,
MM-10, BL-15-1, RLM-37, OLM-203, BL-4, JK-8) under Control and Stress condition.
Gene
GenBank Acc.
No./ Locus ID
Forward and Reverse sequence (5’-3’) Tm
Amino
Transferase
LOC_Os08g39300
CTGAGTGGAGTGGAGATGGT
GTTCGTTGTGCTTCAGATCC
610C
Thionin osthi
GT090938 TCAACGCTGCTCTGGGAAAT
GGCTTGGTCGCAACTCTCAA
580C
Synaptotagmin GT090932 TCTTGCAAGGTGCCAAATCTG GGCTGTGGCGTCCACTTAA 580C
U2 Sn RNP GT090867 TGTGACCGACTTCCGTGAAG CCACGGTTGCAGCTGTTCT 590C
Scythe protein GT090877 CCAGACACTAGCAGCACACATG CATCCCTTGCTCTGTTTGCA 590C
Aquaporin
GT090849
CCCGTTCAAGAGCAGGTCTTA CCTGTTTGGACTGGCATCTCA 610C
CDPK GT090918 CAGAATTGACAGAGAATGAAATCCGATGGTTCCGCTGTTGTCAATA 580C
Os NAC 29-2 NC_029266 AAAGAAGGAGCAACGTGCATTCTTGTGGATTCTGCACAGC 560C
MPK 17-1 GT090884 TGTCGATGGATTGTCTGAAAAAGT TGCCGCGGTCTTTGGA 560C
Ta NAC-2 JN621240 GATTTGGTCGGGATTTCAGA GCTCCATCATCGTCTCCTCT 570C
Ec NAC-67 KU500625 CACTGCAAAGGAGGAGGAAG CTTCTTGGGCACCATCATCT 580C
Hv NAC JX855805
CTACGACGACATCCAGAGCA
GTCATCCATTCCGCTTCTGT
580C
NAM LOC_Os03g21060
CAAGACCAACTGGATCATGC
TTCTTGTAGATCCGGCACAG
620C
DQP1 LOC_Os08g36920
AGTACATGATCCGATTCGAC
GTCCTGTAGCCGGAGATGAC
65.40C
DQP 2 LOC_Os11g26760
GTGAAGGAGGAGCACAAGAC
TTGATCTTCTCCTTGATTCC
640C
DQP 3 LOC_Os01g44390
CGATGTCGGTGAGCTCGT
GGTCTCGATGCGCTTGAC
63.50C
DQP 4 LOC_Os03g20550
ATCAATCACACCATCTAGGC
GTATCTGGGGAAATTACAGTTG
610C
DQP 6 LOC_Os04g49550
GAGCTAGAGAGGAAGACGATG
ATGATGACGATGTCCCTGTC
64.10C
Scythe protein, Ta NAC-2, Ec NAC-67, NAM, U2-Sn
RNP, Hv NAC, Os NAC-29-2, MPK 17-1, DQP-1, DQP-
2, DQP-3, DQP-4, DQP-6. Semi quantitative RT-PCR
analysis showed differential expression of these eight-
een transcripts in Little millet genotypes under stress
with respect to the control condition. The results are dis-
cussed below in detail. Note: The increase or decrease in
the Fold value was calculated my measuring the band
intensity and size using GelQuantNET software.
You J., Hu H., Xiong L.(2012) have con rmed that
OsOAT is a direct target gene of the stress-responsive
NAC transcription factor SNAC2 (Li et al., 2008). In addi-
tion, OsOAT over expressing plants show signi cantly
increased tolerance to oxidative stress mainly through
enhancing ROS-scavenging capacity and pre-accumula-
tion (You et al., 2012). The RT-PCR of Amino-transferase
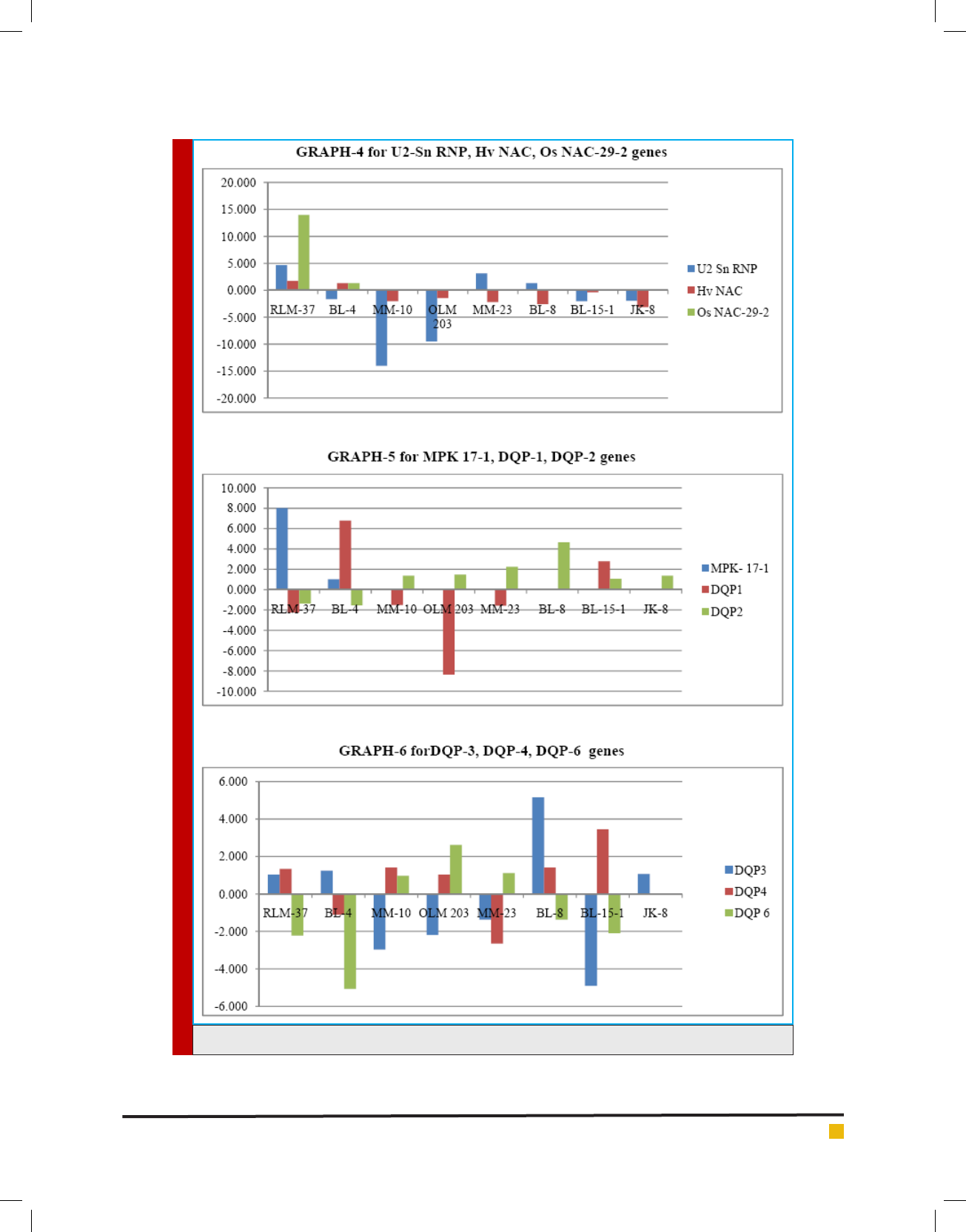
showed up-regulation in the transcript level by 2.25,
5.65 fold in the genotypes OLM-203, MM-23 respec-
tively as shown in graph 1. The transcriptional analysis
revealed that PvOAT was strongly induced by drought
stress and it has also been reported that the expression
of PvOAT was higher in leaves than that in root and
stem of common bean (Phaseolus vulgaris L.) by drought
stress (Ji-bao et al., 2016). Thus the up-regulation of this
transcript under water stress suggests that it may play a
key role in identi cation of different transcription fac-
tors which are responsible for different drought tolerant
mechanisms in Little millet.
Thionin Osthi belongs to oxidative stress category
of genes. A report by Yamakawa et al. (2007) reveals

Sushmitha, Arun and Dubey
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES 341
that this gene showed 2.0 fold up-regulation under high
temperature stress in Medicago truncatula. The RT-PCR
of Thionin-osthi in this study showed up-regulation
in the transcript level by 10.15, 16.6 fold in the geno-
types RLM-37, MM-23 respectively as shown in graph
1. Hence its induction in moisture stress in Little millet
may be attributed due to the presence of the cis acting
elements, suggesting an important role of this gene in
combating oxidative stress.
Aquaporin belongs to major intrinsic protein super
family which functions as a membrane channel. Over
expression of a Panax ginseng tonoplast, aquaporin
enhances drought and salt tolerance ability in trans-
genic Arabidopsis plants (Yanhui et al., 2007). But the
RT-PCR of Aquaporin showed a negligible up-regulation
in the transcript level by 1.16, 1.26, 1.42 fold in the gen-
otypes BL-8, MM-23, OLM-203 respectively as shown
in graph 1.
It has been shown that Synaptotagmin imparts cal-
cium dependent freezing tolerance via membrane reseal-
ing and also loss of function of this gene reduces cell
viability and plasma membrane integrity in Arabidop-
sis (Yamazaki et al., 2008). RT-PCR of Synaptotagmins
showed an up-regulation in the transcript level by 2.92
fold in the genotype JK-8 as shown in graph 2. Identi -
cation and up-regulation of Synaptotagmin under mois-
ture stress indicates its role in stress signal transduction
and tolerance which needs to be further elucidated.
Calcium-dependent protein kinases play important
role in signalling pathways for various stress responses
(Ray et al., 2007,
Sheen J. 1996). The RT-PCR of CDPK
showed up-regulation in the transcript level by 1.21,
5.25 fold in the genotypes MM-10, BL-4 respectively as
shown in graph 2. In several previous studies, induc-
tion and expression of CDPK(s) have been reported to be
higher in tolerant cultivars in different abiotic stresses
(Kawasaki et al., 2001, Li et al., 2008). The CIPK family
of 26 protein kinases regulates the function of several
ion transporters near the cell membrane to restore ion
homeostasis under stress situations (Chaves-Sanjuan
et al., 2014).
The differentially induced expression of OsCIPK genes
by different stresses and the examples of improved
stress tolerance of the OsCIPK transgenic rice suggest
that rice CIPK genes have diverse roles in different stress
responses and some of them may possess potential use-
fulness in stress tolerance improvement of rice (Xiang
et al., 2007). Thus, suggesting its putative role in cell
signalling pathway and in combating drought stress.
Scythe protein has been observed as a novel reaper-
binding apoptotic regulator in vertebrates. Research by
Thress et al., suggests that the Scythe protein might
work by regulating the folding and activity of the mol-
ecules that make up the signaling pathway that controls
apoptosis (Thress et al., 1998). The RT-PCR of Scythe
protein showed up-regulation in the transcript level by
2.01, 29.20 fold in the genotypes MM-23 and MM-10
respectively and a negligible level of up-regulation was
found in BL-4 by 1.31 fold as shown in graph 2.NAC
(NAM, ATAF, and CUC) is a plant speci c gene family
of transcription factors. A few NAC genes from Arabi-
dopsis and Brassica have been reported to be responsive
in various environmental stresses (Shao et al., 2015).
Over expression of various NAC genes have also been
reported to signi cantly improve drought tolerance in
transgenic rice Shao et al., (2015).
RT-PCR of NAM showed a negligible up-regulation
in the transcript level by 1.07, 1.07, 1.20 fold in the
genotyes BL-8, BL-15-1, MM-23 respectively, Where as
a signi cant up-regulation was found in the genotype
OLM-203 by 3.75 fold as shown in graph 3. The RT-PCR
analysis of the up-regulation of this transcript under
moisture stress suggests that it may play an important
role in the cross-linking of different signalling pathways
in Little millet.
In wheat, NAC TFs are known to be involved in pro-
cesses such as senescence and nutrient remobilization as
well as responses to abiotic and biotic stresses, ranging
from stripe rust to abiotic stresses including drought and
salt tolerance (Xia et al., 2010a). Out of four genes Ta
NAC-2, Ec NAC-67, Hv NAC, Os NAC-29-2, The RT-PCR
analysis of Ta NAC-2 showed up-regulation in the tran-
script level by 2.35, 8.66 fold in the genotypes MM-10,
MM-23 respectively and RT-PCR analysis of Ec NAC-
67 showed up-regulation in the transcript level by 4.44,
10.76 fold in the genotypes BL-15-1, MM-23 respectively
as shown in graph 3. The RT-PCR analysis of Hv NAC
showed negligible up-regulation in the transcript level
by 1.30, 1.75 in the genotypes RLM-37, BL-4 respec-
tively and RT-PCR analysis of Os NAC-29-2 showed a
signi cant up-regulation in the transcript level by 13.99
fold in the genotype RLM-37 as shown in graph 4.
Alternative splicing takes place in highly specialized
structures within nucleus called spliceosomes consist-
ing of ve small nuclear ribonucleoprotein particles,
SnRNPs (U1, U2, U4/6, and U5) and other non-SnRNPs
(Reddy ASN. 2001). RT-PCR of U2-SnRNP showed an
up-regulation in the transcript level by 3.12, 4.65 fold
in the genotypes MM-23, RLM-37 respectively shown
in graph 4. Similar gene induction of U2-SnRNP has
been reported among tolerant and susceptible cultivars
of Foxtail millet (Charu Lata et al., 2010). These results
suggest that U2-SnRNP may play a signi cant role in
alternative splicing in Little millet and thus regulating
gene expression.
MAP kinase signaling is one of the most important
and conserved pathways in most cellular process as
well as environmental stress responses (Lee et al., 2008,

Sushmitha, Arun and Dubey
342 TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
GRAPHS 1-6. Shows the Fold Increase/Decreaseamong Eight Genotypes of Little millet (RLM-37, BL-4, MM-10,
OLM-203, MM-23, BL-8, BL-15-1, JK-8) due to the effect of Moisture stress on Gene Expression under Control
and Stress condition.
Sushmitha, Arun and Dubey
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES 343
GRAPHS 1-6. (Continued)

Sushmitha, Arun and Dubey
344 TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Moustafa et al., 2008). The RT-PCR analysis of MPK
17-1 showed an up-regulation in the transcript level by
7.99 fold in the genotype RLM-37 as shown in graph
5. MAP kinase gene has been reported to be induced
due to dehydration, salinity and hyper-osmotic stresses
(Moustafa et al., 2008). The activation of MPK 17-1 gene
in moisture stress suggests that it may play an important
role in the cross-linking of different signalling pathways
to activate plant defense mechanisms in Little millet.
The AP2/EREBP genes play crucial roles in plant
growth, development and biotic and abiotic stress
responses and is one of the largest and speci c transcrip-
tion factor (TF) families in plants. Liu and Zhang have
reported in G.hirsutum that, 151 non-repeated genes of
the DREB and ERF subfamily genes were responsive to
different stresses: 132 genes were induced by cold, 63
genes by drought and 94 genes by heat (Liu and Zhang
2017). The RT-PCR of DQP 1 showed an up-regulation
in the transcript level by 2.78, 6.79 fold in the genotypes
BL-15-1, BL-4 respectively as shown in graph 5.
Three of the four rice genes [(OsBIERF 1–4) Oryza
sativa benzothiadiazole (BTH)-induced ethylene respon-
sive transcriptional factors (ERF)] with a single con-
served ERF domain were found to be up-regulated by
salt, cold, drought, wounding as well as in an incompat-
ible interaction between rice and fungal pathogen sug-
gesting their role in biotic and abiotic stress (Jisha et al.,
2015). In studies dealing with drought stress, Pelah et
al. found a correlation between drought tolerance and
accumulation of dehydrin proteins in Populus popularis
(Pelah et al., 1997). The RT-PCR of DQP 2 showed an
up-regulation in the transcript level by 2.23, 4.65 fold
in the genotypes MM-23, BL-8 respectively. There was
a negligible up-regulation found in the genotypes JK-8,
MM-10, OLM-203 by 1.37, 1.37, 1.47 fold respectively
as shown in graph 5.
A total of 44.67% and 47.21% MYB genes were
found up and down-regulated in Arabidopsis under cold
stress, respectively in the case of drought stress, many
MYB genes have been isolated and demonstrated to be
involved in drought responses in plants (Mmadi et al.,
2017). The transcriptional activation of cuticular wax
biosynthesis by MYB96 contributed to drought resist-
ance in Arabidopsis thaliana (Seo et al., 2011). The RT-
PCR of DQP 3 showed a signi cant up-regulation in the
transcript level by 5.15 fold in BL-8 genotype as shown
in graph 6. Altogether, these evidences demonstrated the
versatility and importance of this gene family in plants.
Members of the large family of WRKY transcription
factors are involved in a wide range of developmental
and physiological processes, most particularly in the
plant response to biotic and abiotic stress. RT-PCR anal-
ysis of Yu Y., Wang N., Hu R, Xiang F. showed that in
whole soybean plant, 66 GmWRKYs exhibited distinct
expression patterns in response to salt stress (Yu et al.,
2016). The RT-PCR of DQP 4 showed a negligible up-
regulation in the transcript level by 1.33, 1.40, 1.41 fold
in the genotypes RLM-37, BL-8, MM-10 respectively and
a signi cant level of up-regulation in the transcript was
observed in the genotype BL-15-1 by 3.44 fold as shown
in graph 6.
In a study by Liu K. et al. showed over-expression
of OsCOIN protein, a RING nger protein in transgenic
rice lines signi cantly enhanced their tolerance to cold,
salt and drought, accompanied by an up-regulation of
OsP5CS expression and an increase of cellular proline
level (Liu et al., 2007). Salt and drought-induced RING
FINGER1 (SDIR1), is involved in abscisic acid (ABA)-
related stress signal transduction in Arabidopsis thali-
ana (Zhang et al., 2007). SDIR1 is expressed in all tissues
of Arabidopsis and is up-regulated by drought and salt
stress, but not by ABA (Zhang et al., 2007). The RT-PCR
of DQP 6 showed an up-regulation in the transcript level
by 2.61 fold in the genotype OLM-203 and a negligi-
ble level of up-regulation was noticed in the genotype
MM-23 by 1.11 fold as shown in graph 6.
Out of 18 transcripts under control and stress condi-
tion for eight genotypes of Little millet, a signi cant
level of up-regulation was observed among the follow-
ing:
• Genotype MM-23 showed a higher level of up-reg-
ulation for the genes Aminotransferase, Ta NAC-2
Ec NAC-67 and Thionin osthi by 5.65, 8.66, 10.76,
16.60 fold respectively.
• Genotype RLM-37 showed a signi cant up-regula-
tion for the genes U2 Sn RNP, MPK-17-1, Thionin
osthi and Os NAC-29-2 by 4.65, 7.99, 10.15 and
13.99 fold respectively
• Genotype BL-4 showed an up-regulation in the
transcript level by 5.25 and 6.79 fold for the genes
CDPK and DQP1 respectively.
• Genotype MM-10 showed a greater level of up-
regulation for the gene scythe protein by 29.20
fold.
• Genotype BL-8 showed an up-regulation for the
genes DQP 2 and DQP 3 by 4.65 and 5.15 fold
respectively.
• BL-15-1 genotype showed a signi cant up-regu-
lation for the transcript Ec NAC-67 by 4.44 fold.
CONCLUSION
Current study helps us to identify the key genes expressed
in response to moisture stress in the selected Little millet
genotypes. Induction of transcripts Amino-transferase,
Thionin-osthi, Aquaporin,Synaptotagmin, CDPK, Scythe
protein, Ta NAC-2, Ec NAC-67, NAM, U2-Sn RNP, Hv
NAC, Os NAC-29-2, MPK 17-1, DQP1, DQP 2, DQP 3,

Sushmitha, Arun and Dubey
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES 345
DQP 4, and DQP 6 suggests that these genes may impart
drought avoidance capacity to the tolerant genotypes.
Genes which were up-regulated suggests their func-
tion in positive regulation in adaptation of the moisture
stress under the drought condition, this can be taken as
a base for drought tolerance response of the crop, which
may be useful for further validation studies of the can-
didate genes for drought tolerance in the millet species
as well as other crop plants.
ACKNOWLEDGMENTS
Seed material was provided by ZARS, Jagdalpur, and
KVK Shivpuri. Department of Biotechnology, Ministry
of Science and Technology, Govt. of India are thankfully
acknowledged for providing the nancial support.
REFERENCES
Ajithkumar I.P., Panneerselvam R., (April 2014) ROS scaveng-
ing system, osmotic maintenance, pigment and growth sta-
tus of Panicum sumatrense roth. Under drought stress. Cell
Biochem Biophys. Vol. 68, No 3: Pages 587-95 doi:10.1007/
s12013-013-9746-x.
Bandyopadhyay T, Muthamilarasan M, Prasad M. (2017) Millets
for Next Generation Climate-Smart Agriculture. Frontiers in
Plant Science Vol. 8: Pages 1266. doi:10.3389/fpls.2017.01266.
Bhaskara J., Panneerselvam R. (2013) Accelerated reactive
oxygen scavenging system and membrane integrity of two
Panicum species varying in salt tolerance. Cell Biochem Bio-
phys. Vol. 67, No 3: Pages 885-92.
Chaves, M.M., Maroco J.P., Pereira J.S. (Jan 2003) Understand-
ing plant responses to drought-from genes to the whole plant.
Functional Plant Biology Vol. 30: Pages 239-264 doi: 10.1071/
FP02076
Charu Lata, Sahu P.P., Prasad M. (Mar 2010) Comparative tran-
scriptome analysis of differentially expressed genes in foxtail
millet (Setaria italica L.) during dehydration stress. Biochemi-
cal and Biophysical Research Communications Vol. 393 No 4:
Pages 720–727 doi: 10.1016/j.bbrc.2010.02.068.
Chaves-Sanjuan A. et al. (2014) Structural basis of the regula-
tory mechanism of the plant CIPK family of protein kinases
controlling ion homeostasis and abiotic stress. Proc. Natl.
Acad. Sci. USA Vol. 111, No 42: Pages 4532–4541.
Den Herder G., Van Isterdael G., Beeckman T., De Smet I. (Nov
2010) The roots of a new green revolution. Trends Plant Sci.
Vol 15, No 11: Pages 600-7.
De Wet J. M. J., Prasada Rao K. E., Brink D. E., (1983a). Sys-
tematics and domestication of Panicum sumatrense (Grami-
nae). J. d’agriculture Tradit. Bot. appliquée Vol. 30, No 2: Pages
159–168.
Hiremath S. C. Patil G. N. V. Salimath S. S. (1990). Genome
homology and origin of Panicum sumatrense (Gramineae). Cyt-
ologia (Tokyo) Volume 55 No 2: Pages 315-319 doi: 10.1508/
cytologia.55.315.
Ji-bao CHEN, Yuan-nan CAO, Zhao-yuan ZHANG, Shu-min
WANG, Jing WU, Lan-fen WANG (2016). Cloning of the OAT
gene and the correlation between its expression and drought
tolerance in Phaseolus vulgaris L. Journal of Integrative Agri-
culture Vol. 15, No 5: Pages 973-982 doi:10.1016/S2095-
3119(15)61283-7.
Jisha V., Dampanaboina L., Vadassery J., Mithöfer A., Kap-
para S., Ramanan R. (2015) Overexpression of an AP2/ERF
Type Transcription Factor OsEREBP1 Confers Biotic and Abi-
otic Stress Tolerance in Rice. PLOS ONE Vol. 10, No 6: Page
e0127831 doi:10.1371/journal.pone.0127831.
Kawasaki S., Borchert C., Deyholos M., et al., ( April 2001)
Gene expression pro les during the initial phase of salt stress
in rice, Plant Cell Vol. 13, No 4: Pages 889–905.
Lee J.S., Huh K.W., Bhargava A., Ellis B.E. (Dec 2008) Com-
prehensive analysis of protein– protein interactions between
Arabidopsis MAPKs and MAPK kinases help de ne potential
MAPK signaling modules. Plant Signaling Behav. Vol. 3 No 12:
Pages 1037–1041.
Li A., Wang X., Leseberg CH., Jia J., Mao L. (2008) Biotic and
abiotic stress responses through calcium-dependent protein
kinase (CDPK) signaling in wheat (Triticum aestivum L.) Plant
Signaling Behav. Vol. 3 No 9: Pages 654–656.
Liu C., Zhang T. (Jan 2017) Expansion and stress responses of
the AP2/EREBP superfamily in cotton. BMC Genomics Vol. 18
No 1:Page 118.
Liu K., Wang L., Xu Y., Chen N., Ma Q., Li F., Chong K. (Sep
2007) Overexpression of OsCOIN, a putative cold inducible
zinc nger protein, increased tolerance to chilling, salt and
drought, and enhanced proline level in rice. Planta Vol. 226,
No 4: Pages 1007-16. doi: 10.1007/s00425-007-0548-5.
Mmadi M.A., Dossa K., Wang L., et al., (Dec 2017) Functional
Characterization of the Versatile MYB Gene Family Uncovered
Their Important Roles in Plant Development and Responses
to Drought and Waterlogging in Sesame. Genes.Vol. 8 No 12:
Page E362. doi:10.3390/genes8120362.
Moustafa K., Vos D.L., Leprince A., Savoure A., Lauriere C.
(2008) Analysis of the Arabidopsis Mitogen-activated protein
kinase families: organ speci city and transcriptional regulation
upon water stresses. Scholarly Research Exchange Vol. 2008
No 2008 12 pages Article ID 143656 doi:10.3814/2008/143656.
Nagarjuna, K. N., Parvathi, M. S., Sajeevan, R. S., Pruthvi, V.,
Mamrutha, H. M., Nataraja, K. N. (2016). Full-length cloning
and characterization of abiotic stress responsive CIPK31-like
gene from Finger millet, a drought-tolerant crop. Current Sci-
ence, Vol.111 No 5: Pages 43-45.
Nisha Chhabra and Amarjeet Kaur (Part A 2017) Studies on
physical and engineering characteristics of maize, pearl millet
and soybean. Journal of Pharmacognosy and Phytochemistry
Vol. 6 No 6: Pages 01-05.
Pelah D., Wang W., Altman A., Shoseyov O., Bartels D. (1997)
Differential accumulation of water stress related proteins,

Sushmitha, Arun and Dubey
346 TRANSCRIPT ANALYSIS OF MOISTURE STRESS GENE RESPONSIVE ORTHOLOGS OF LITTLE MILLET GENOTYPES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
sucrose synthase and soluble sugars in Populus species that
differnin their water stress response. Physiol Plant Vol. 99 No
1: Pages 153–159 doi:10.1111/j.1399-3054.
Pradeep SR., Guha M., (Jun15 2011) Effect of processing meth-
ods on the nutraceutical and antioxidant properties of Little
millet (Panicum sumatrense) extracts. Food Chem. Vol. 126,
No 4: Pages 1643-7
Ray S., Agarwal P., Arora R., et al., (2007) Expression analy-
sis of calcium-dependent protein kinase gene family during
reproductive development and abiotic stress conditions in rice
(Oryza sativa L. ssp. indica), Mol. Genet. Genomics Vol. 278,
No 5: Pages 493–505 DOI: 10.1007/s00438-007-0267-4.
Reddy ASN. (2001) Nuclear pre mRNA splicing in
plants, Crit. Rev. Plant Sci. Vol. 20 No 6: Pages 523–571
doi:abs/10.1080/20013591099272.
Sarika Jaiswal, Tushar, J., Antala, Mandavia, M.K., Meenu Chopra,
Rahul Singh Jasrotia, Rukam S. Tomar, Jashminkumar Kheni,
Angadi, U.B., Iquebal, M.A., Golakia, B.A., Anil Rai and Dinesh
Kumar (2018). Transcriptomic signature of drought response in
pearl millet (Pennisetum glaucum (L.) and development of web-
genomic resources. Nature, 8: Article number-3382.
Seo P.J., Lee S.B., Suh M.C., Park M.J., Go Y.S., Park C.M. (2011)
The MYB96 Transcription Factor Regulates Cuticular Wax Bio-
synthesis under Drought Conditions in Arabidopsis. The Plant
Cell Vol.23 No 3: Pages 1138-1152.
Shao H., Wang H., Tang X. (2015) NAC transcription factors
in plant multiple abiotic stress responses: progress and pros-
pects. Frontiers in Plant Science Vol. 6: Page 902. doi:10.3389/
fpls.2015.00902.
Sha Tang, Lin Li, Yongqiang Wang, Qiannan Chen, Weny-
ing Zhang, Guanqing Jia, Hui Zhi, Baohua Zhao & Xianmin
Diao. (2017). Genotype-speci c physiological and transcrip-
tomic responses to drought stress in Setaria italica (an emerg-
ing model for Panicoideae grasses) Nature, 7: Article num-
ber-10009.
Shailaja Hittalmani, Mahesh, H.B., Meghana Deepak Shirke,
Hanamareddy Biradar, Mohanrao, A. (2017). Genome and
Transcriptome sequence of Finger millet (Eleusine coracana
(L.) Gaertn.) provides insights into drought tolerance and
nutraceutical properties. BMC Genomics, 18 : 465.
Sheen J. (1996) Ca2+-dependent protein kinases and stress sig-
nal transduction in plants, Science Vol. 274, No 5294: Pages
1900–1902.
Shinozaki, K., Yamaguchi-Shinozaki K., Seki M. (Oct 2003).
Regulatory network of gene expression in the drought and cold
stress responses. Curr Opin Plant Biol Vol. 6 No 5: Pages 410-417.
Siddappa, N., Raghu ,G. K., and Devaraj Varadahally, R. (2016).
Identi cation of Drought-Responsive Transcripts in Kodo Mil-
let (Paspalum scrobiculatum L.) International Journal of Inno-
vative Research & Development, Vol.5 No 11: Pages 140-143.
Sivakumar S., Mohan M., Franco O. L., Thayumanavan B.,
(2006b). Inhibition of insect pest -amylases by little and n-
ger millet inhibitors. Pestic. Biochem. Physiol. Vol.85: Pages
155–160 doi:10.1016/j.pestbp.2005.11.008
Thress K., Henzel W., Shillinglaw W., Kornbluth S. (1998)
Scythe: a novel reaper-binding apoptotic regulator. The
EMBO Journal Vol. 17 No 21: Pages 6135-6143. doi:10.1093/
emboj/17.21.6135.
Xia N., Zhang G., Liu X.-Y., Deng L., Cai G.-L., et al., (2010a)
Characterization of a novel wheat NAC transcription factor
gene involved in defense response against stripe rust pathogen
infection and abiotic stresses. Mol. Biol. Rep. Vol. 37 No 8:
Pages 3703–3712 doi: 10.1007/s11033-010-0023-4.
Xiang Y., Huang Y., Xiong L. (Jul 2007) Characterization
of stress responsive CIPK genes in rice for stress tolerance
improvement. Plant Physiol. Vol. 144 No 3: Pages 1416–1428
DOI: https://doi.org/10.1104/pp.107.101295.
Xiong, L., Schumaker K.S., Zhu J.-K. (2002). Cell signaling
during cold, drought, and salt stress. The plant cell Vol. 14:
S165-S183.
Yamaguchi-Shinozaki, K., Shinozaki K. (2006) Transcrip-
tional regulatory networks in cellular responses and toler-
ance to dehydration and cold stresses. Annual Review of
Plant Biology Vol. 57: Pages 781-803 doi:10.1146/annurev.
arplant.57.032905.105444.
Yamazaki T., Kawamura Y., Minami A., Uemura M (2008)
Calcium-dependent freezing tolerance in Arabidopsis involves
membrane resealing via synaptotagmin SYT1, Plant Cell Vol.
20 No 12: Pages 3389–3404, DOI:10.1105/tpc.108.062679.
Yanhui Peng, Wuling Lin, Weiming Cai, Rajeev Arora (2007)
Overexpression of a Panax ginseng tonoplast aquaporin alters
salt tolerance, drought tolerance and cold acclimation ability
in transgenic Arabidopsis plants, Planta Vol. 226, No 3: Pages
729-40 DOI 10.1007/s00425-007-0520-4.
You J., Hu H., Xiong L. (2012) An ornithine -aminotransferase
gene OsOAT confers drought and oxidative stress tolerance
in rice. Plant Science Vol 197: Pages 59–69, doi: 10.1016/j.
plantsci.2012.09.002.
Yu Y., Wang N., Hu R., Xiang F. (Jun 2016) Genome-wide iden-
ti cation of soybean WRKY transcription factors in response
to salt stress. Springer Plus Vol 5 No 1: Page 920. doi:10.1186/
s40064-016-2647-x.
Zhang Y., Yang C., Li Y., et al. (2007) SDIR1 Is a RING Finger
E3 Ligase That Positively Regulates Stress-Responsive Absci-
sic Acid Signaling in Arabidopsis. The Plant Cell Vol 19 No
6:Pages 1912-1929 doi:10.1105/tpc.106.048488.
Zhu, H., Ding G.H., Fang K., Zhao F.G., and Qin P. (2006) New
perspective on the mechanism of alleviating salt stress by sper-
midine in barley seedlings. Plant Growth Regulation Vol 49 No
2-3: Pages 147-156 doi:10.1104/pp.107.105882.