
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(2): 324-334 (2018)
Evaluation of anti-hyperglycaemic potential of the
ethanolic leaf extract of
Quisqualis indica
Jyoti Verma*, Devender Arora and Ajeet Singh
Department of Biotechnology, G B Pant Engineering College, Pauri Garhwal, Uttarakhand, India
ABSTRACT
The present study was conducted to evaluate the in vitro inhibitory effect of ethanolic extract of Quisqualis indica
leaves on the digestive enzyme -amylase and to characterize the compounds responsible for it as a means of man-
aging hyperglycaemia. The presence of various phytochemicals such as avonoids, phenols, alkaloids, tannins may
be responsible for the plant biological activities. The ethanolic leaf extract of the plant was further subjected to
-amylase inhibitory assay where it showed the dose dependent inhibitory effect on the -amylase enzyme when
compared with the positive control acarbose. GC-MS analysis of the ethanolic plant leaf extract revealed the pres-
ence of phytol in the highest concentration and other compounds like linolenic acid, pentadecanoic acid and 9,
12-linoleic acid in moderate concentrations which were further evaluated for their biological activities using PASS
and compared with the biological activity pro le of the anti-diabetic drug acarbose. The various modes of action
of these compounds include -amylase inhibition, -glucosidase inhibition, insulin inhibition, etc. In silico stud-
ies were also done using AutoDock to study the compound’s minimum free energy of stabilization in complex with
the -amylase enzyme. Further Ligplot
+
was used to study the presence of hydrogen bonding in the complex. Drug
parameters of the identi ed compounds in the extract were evaluated and compared with the acarbose. The results
obtained were successfully compared to the pharmacological and toxicological activity information available for the
studied compounds.
KEY WORDS: AUTODOCK VINA, DIABETES MELLITUS, GC-MS ANALYSIS, PASS,
QUISQUALIS INDICA
, LIGPLOT
+
V.1.4.3.
324
ARTICLE INFORMATION:
*Corresponding Author: vermajyoti983@gmail.com
Received 19
th
March, 2018
Accepted after revision 19
th
June, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.1/20

Jyoti Verma, Devender Arora and Ajeet Singh
INTRODUCTION
Diabetes mellitus is a chronic heterogeneous endocrine
disorder characterized by elevated blood glucose level
resulting in serious metabolic disturbances in carbohy-
drate, protein and fat metabolism causing premature
fatality (Patel et al. 2011; Warjeet 2011). After cancer,
cardiovascular and cerebrovascular disease, it is the
third most life-threatening disease posed to the health
of mankind (Chauhan et al. 2010). Annually, a rise of
4-5 % in number of diabetic patients is observed (Wag-
man and Nuss 2001). It is caused due to insuf cient
insulin production or psychological unresponsiveness
to insulin. Pancreatic -amylase enzyme is an endoglu-
canase that catalyzes the internal -1, 4 glycosidic bond
hydrolysis in starch and other polysaccharides to yield
maltose and maltotriose polysaccharides. Inhibition of
this enzyme can help to manage the disorder by lower-
ing the level of glucose released in the blood. There is
considerable evidence that lipid peroxidation owing to
free radical activity causes induction of oxidative stress
that plays a crucial role in the onset of the abnormal
condition. Alteration in anti-oxidant enzymes, impaired
glutathione metabolism and decreased ascorbic acid lev-
els are the main causes of disturbance of anti-oxidant
defence system (Patel et al. 2011). This leads to accu-
mulation of advanced glycation products (AGEs) and
sorbitol concentration that cause complications such as
retinopathy, neuropathy and renal dysfunction (Sa et
al. 2014). The physical symptoms include cycle of heavy
thirst and frequent sugar loaded urination along with
presence of sugar in mucus, sweat and breath. In the
past recent years, natural pharmacologically bioactive
compounds derived from terrestrial and marine organ-
isms had received considerable attention to cure poten-
tially vulnerable diseases due to their lesser or virtually
no side effects as compared to synthetic drugs (Yuan
et al. 2016).
Recently many R&D based pharmaceutical company
are employing more time and money on development of
herbal therapies rather than formulating synthetic drugs
due to their unpredictable adverse effects. Ayurveda,
which is the renowned traditional system of medicines
native to India, had always promoted the use of plant
biodiversity for several therapeutic uses due to their ease
of availability and low cost of production (Patwardhan
and Hopper 1992). Plant-derived molecules (PDMs) could
be chemically elaborated to generate novel leads and
to screen molecules from drug-like libraries and hence
can be proved effective to systematically extract unique
molecular scaffolds. Quisqualis indica, commonly
known as ‘rangoon creeper’ is an important medicinal
plant of the Indian subcontinent, Africa and Indo Malay-
sian region (Joshi 2002). It is a vine with pink and white
owers. It is also used as anthelmenic by the inhabitants
of North Annan to expel parasitic worms (helminths)
and other internal parasites from the body (Kirtikar and
Basu 2006). It has also been reported that the extract of
owers of Q. indica exhibit hypoglycaemic and hypo-
cholesterolemic activity against animal models (Bairagi
et al. 2012). Various parts of the plant are used individu-
ally or mixed with other ingredients as a cure for ail-
ments like anti atulence, coughs, diarrhea (Khare 2007),
body pains, toothache (Padua et al. 1999).
With the available therapeutic knowledge, scien-
tists have been thriving hard to explore the safe and
effective treatment of the disease which is yet to be
achieved. Clinically various oral anti-diabetic drugs are
used such as biguanides that increase glucose uptake,
sulfonylureas that increase insulin secretion and diges-
tive enzyme inhibitors that delay complex carbohydrate
digestion and absorption. Large scale research is being
done worldwide, exploiting the known ethno-botanical
knowledge and phytochemical interpretations that could
be an effective approach for the diabetes treatment. In
the designing of drug, the in silico analysis using bioin-
formatics tools is a boon for the researchers as they min-
imize the time and labour employed during the work.
PASS is one such tool that uses algorithms based on
the physico-chemical methods, that predicts the possible
activity of the drug against a target using the intrinsic
property of the compounds (Filimonov et al. 1995; Fili-
monov and Poroikov 1996). In 1972, the National Regis-
tration System of New Chemical Compounds organized
in the USSR formulated the computer program PASS
which was suggested by researcher V. Avidon (Burov et
al. 1990; Poroikov et al. 2003).
Computational based approaches, such as molecular
docking, hydrogen bonding analysis, evaluation of drug
parameters have been widely used in the modern drug
discovery to explore drug-receptor interactions. For
designing of novel inhibitors, molecules from a data-
base of organic compounds should be screened based
on steric and electrostatic complementarity with the
binding pocket of protein. Molecular docking simulation
studies were performed using AutoDock Vina. Dock-
ing of the individual compounds with the -amylase
enzyme which is the key enzyme involved in the regula-
tion of the metabolic pathway was done using Autodock
Tools 1.5.4 package. Ligplot
+
v.1.4.3 software can help in
re ned assessment of docked complexes and in obtain-
ing detailed information on protein-ligand interaction
(Laskowski and Swindells 2011).
Chemicalize.org
beta
software tool by ChemAxon was
used to study the drug like activities of the compounds
identi ed through GC-MS analysis. Therefore, combina-
tion of these methods can be used to study mechanisms
of drug-receptor interactions, and provide structural
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF 325

Jyoti Verma, Devender Arora and Ajeet Singh
insights by which molecules interact within binding
pocket of the receptor. Further experimental evaluation
and validation are required to establish the clinical sig-
ni cance of leads obtained that are promising candi-
dates.
MATERIALS AND METHODS
Plant material and preparation of extract
The plant leaves of Quisqualis indica Linn. were obtained
from Forest Research Institute (FRI), Dehradun, India.
The plant was botanically identi ed at FRI. Leaves were
thoroughly cleaned with tap water to remove any dust
particles and further shade dried for 3 weeks to make
them crisp and completely devoid of moisture. The dried
leaves were nely grinded and 10 g of the powdered
material was extracted with 250 mL of ethanol pro-
cured from Merck, India using Soxhlet apparatus for 48
hrs. The extract was concentrated using vacuum rotary
evaporator at 78 °C. The extract was ltered through
Whatmann lter paper No.1 and stored at 4 °C until
use. Rest of the chemicals were of analytical grade. Pre-
liminary screening: Phytochemical screening of the leaf
extract was done using the methods described by Trease
and Evans (1996), and Sofowora (2006).
In vitro
-amylase inhibitory assay
The assay was carried out according to the standard pro-
tocol (Hansawasdi et al. 2000), with slight modi cations.
2 mg of starch azure was suspended in 0.2 mL substrate
solution containing 0.5 M Tris-HCl buffer (pH 6.9) and
0.01 M CaCl
2
. The substrate solution in tubes were boiled
for 5 min and then preincubated at 37 °C for 5 min.
Ethanol extract of Q. indica was dissolved in DMSO to
obtain concentrations of 10, 20, 40, 60, 80, and 100 μg/
mL. Then, 0.2 mL of plant extract of particular concen-
tration was added to the substrate solution in respective
tubes. Further, 0.1 mL of porcine pancreatic -amylase
procured from Hi-Media in Tris-HCl buffer (2 units/mL)
was added to the tube containing the plant extract and
substrate solution. The reaction was carried out at 37 °C
for 10 min. The reaction was terminated by addition of
0.5 mL of 50 % acetic acid in each tube. The reaction
mixture was centrifuged at 3000 rpm for 5 min at 4
°C. The absorbance of resulting supernatant was meas-
ured at 595 nm using spectrophotometer. Acarbose, a
known -amylase inhibitor was used as a standard drug
control. The experiments were done in triplicates. The
-amylase inhibitory activity was calculated by using
following formula:
The -amylase inhibitory activity = [{(Ac+) – (Ac–)}
– {(As – Ab)}/ {(Ac+) – (Ac–)}] × 100,
where Ac+, Ac-, As, and Ab are de ned as the absorb-
ance of 100 % enzyme activity (only solvent with
enzyme), 0 % enzyme activity (only solvent without
enzyme), a test sample (with enzyme), and a blank (a
test sample without enzyme), respectively. The percent-
age inhibitory effects of acarbose and plant extract on
-amylase activity were determined. Statistical analysis
was performed using Microsoft Excel. All values were
expressed as mean ± standard deviation.
GC-MS was carried out at Advanced Instrumenta-
tion Research Facility (AIRF) at Jawaharlal Nehru Uni-
versity (JNU), New Delhi, India. The analysis was done
on GCMS-QP2010 Ultra under the following condi-
tions to study the phytochemical components present
in the extract. Column-Rtx-5 MS (30 m X 0.25 mm i.d.
X 0.25μm lm thickness) was used. 2 μL of the plant
sample was injected in split mode at a constant column
ow rate of 1.21 mL/ min with linear velocity 40.9 cm/
sec ow control mode and purge ow of 3.0 mL/ min.
The column temperature was programmed to 100 °C, ion
source temperature at 220 °C and the injection tempera-
ture was 260
°C. Total GC-MS running time was 45 min.
Inbuilt libraries WILEY8.lib and NIST11.lib were used
for the identi cation and comparison of the organic
compounds. The name, molecular weight and structure
of the phytochemical components of the extract were
ascertained.
The activity list comprises of names of pharmaco-
therapeutic effects as well as names of mechanism of
action. The compounds present in high concentration in
the plant leaf extract were studied using PASS for their
biological activity prediction, whose structures were
drawn using MarvinSketch v5.10.0 and compared with
acarbose. The mean accuracy of prediction is about 85
% in leave-one-out cross-validation (LOOCV), hence it
is reasonable using this tool to nd and optimize new
lead compounds which is a crucial step in pharmaceu-
tical research and development process. The chemical
structures of molecules were drawn and edited using
MarvinSketch v.5.10.0 software (https://www.chemaxon.
com), an advanced chemical structure editor. The struc-
tures were saved in 3D MOL2 format. Individual MOL2
les were converted into PDBQT format (acceptable for-
mat for AutoDock Vina package (Trott and Olson 2010)),
using the python script ‘prepare_ligand4.py’ available
in Autodock Tools 1.5.4 package (Morris et al. 2008).
During this conversion, appropriate charges were added
to ligands. The commercially available drug acarbose as
well as the compounds present in high concentration in
the extract were docked with enzyme -amylase using
software AutoDock Vina to analyze their free energy.
Further presence of hydrogen bonding were analyzed
using software Ligplot
+
v.1.4.3 software and molecular
interactions between protein and ligands were predicted.
326 EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Jyoti Verma, Devender Arora and Ajeet Singh
Drug parameters were studied using chemicalize.org
beta
by ChemAxon.
RESULTS AND DISCUSSION
The plant leaf extract was found to contain the phy-
toconstituents like avonoids, saponins, tannins, phe-
nols, alkaloids and quinones upon preliminary screen-
ing among which avonoids, tannins and phenols are
reported to play a crucial role in the management of
diabetes mellitus (Table 1, S. No. 1-10). The percentage
inhibitory activity exhibited by the extract and com-
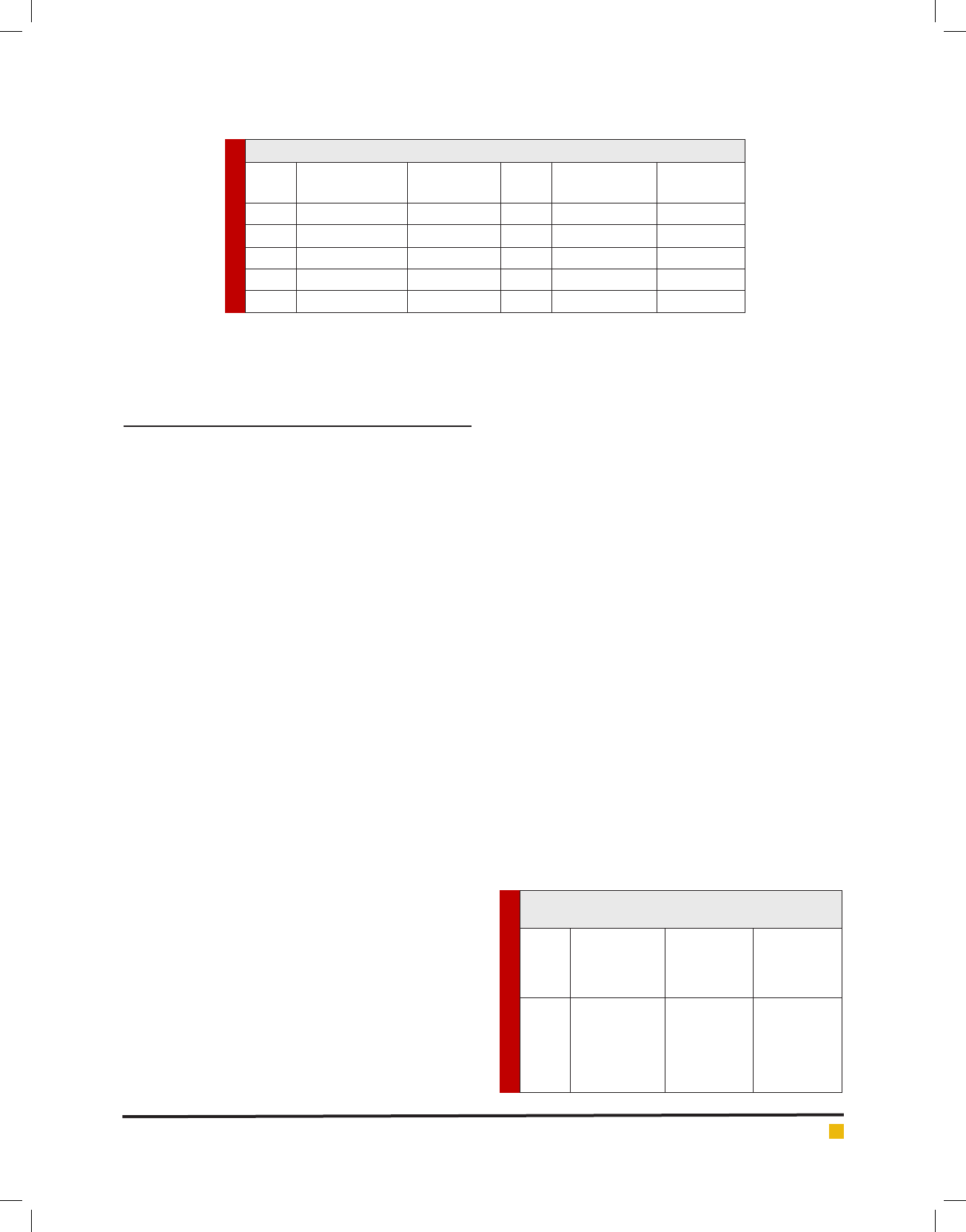
parison with acarbose is shown in Table 2. (S. No. 1-6).
Acarbose at concentration 100 μg/mL showed 71.79 ±
0.55 % inhibitory effects on the -amylase activity. The
ethanolic leaf extract of Q. indica at a concentration 100
μg/mL exhibited 56.40 ± 2.35 % of -amylase inhibi-
tory activity. The plant extract showed potent -amylase
inhibitory activity in a dose dependent manner as com-
pared with acarbose (Fig. 1). Acarbose was used as a
positive control which is a secondary metabolite which
belongs to the class of drugs called digestive enzyme
inhibitors. It is obtained via a multistep batch fermenta-
tion process from bacterium Actinoplanes species SE50.
It is approved for treating type II diabetic patients. This
reveals that the extract exhibited a comparable inhibi-
tory effect on the -amylase enzyme as compared to the
standard drug acarbose.
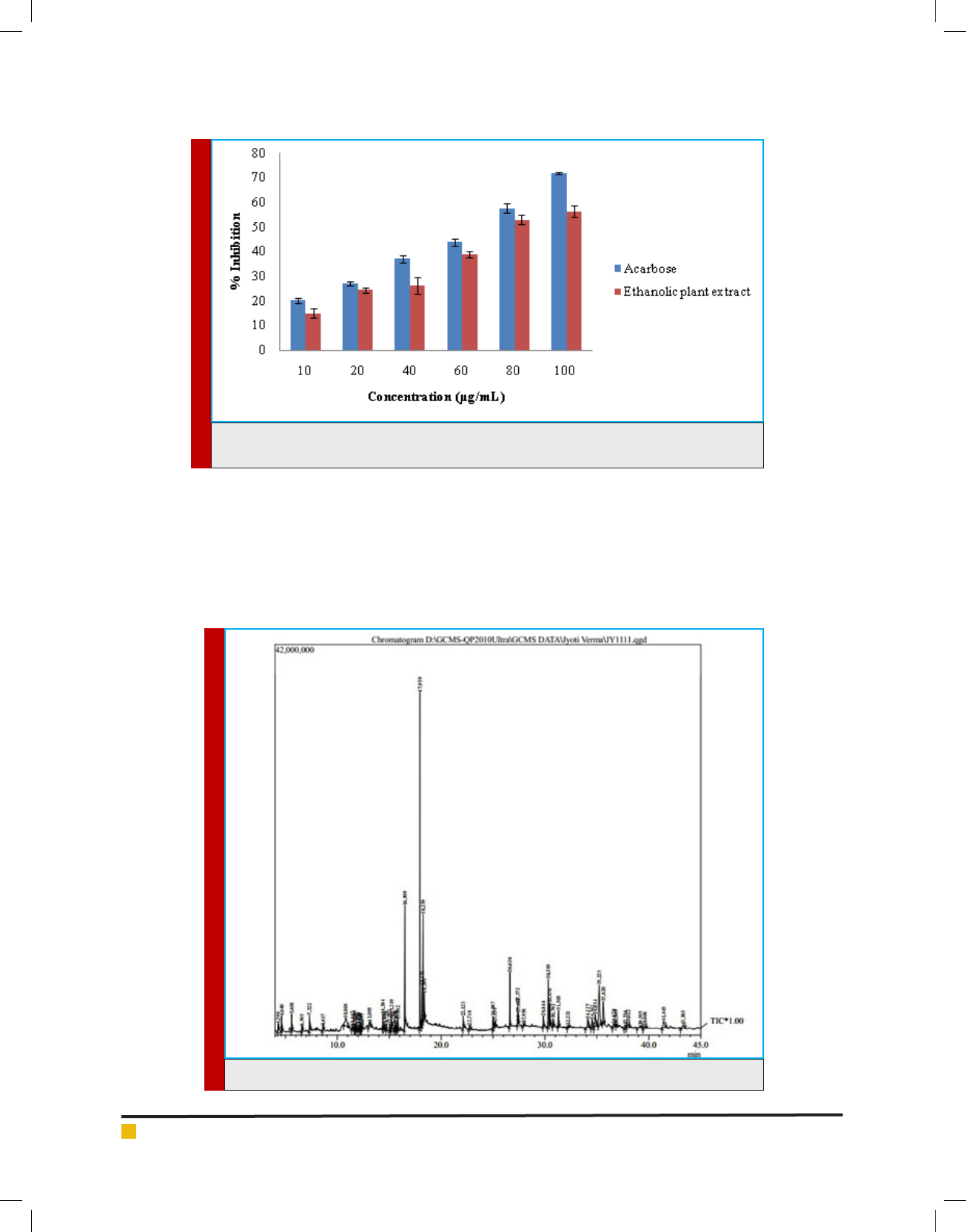
Although the presence of avonoids and phenols
contribute to the anti-diabetic activity, the speci c bio-
active components of the plant extract were studied
through GC-MS analysis (Fig. 2). The peaks clearly show
that compounds like phytol, linolenic acid, pentadeca-
noic acid and 9, 12-linoleic acid were present in subse-
quently higher concentrations. GC-MS study of the Q.
indica leaf extract has shown the presence of a number
of phytochemical constituents which confer the medici-
nal property to the plant (Table No 3).
This study depicted the presence of 55 compounds.
Among these, 21 compounds with their biological activ-
ities have been reported earlier. Compounds like lauric
acid, 9, 12-linoleic acid, linolenic acid, vitamin E, stig-
masterol, -sitosterol are reported to posses hypocho-
lesterolemic activity. Compounds like neophytadiene,
squalene, heptacosanol, solanesol, -tocopherol, vita-
min E, stigmasterol, -sitosterol, fucosterol are reported
to have anti-oxidant activity. Both of these activities
play a direct role in the management of diabetes mellitus
by blocking the sugar metabolism. Phytol also known as
phytanic acid is the test compound to be studied which
was present in the highest concentration in the plant
Q. indica leaves. Phytol is an acyclic diterpene alcohol
molecule which is a precursor of synthetic vitamin E
and K1 (Thomas and Netscher 2007; Daines et al. 2003).
It was rst obtained by chlorophyll hydrolysis and now
obtained in the process of chlorophyll separation from
alfalfa. It is reported as transcription factors peroxisome
proliferator activated receptor (PPAR-) and retinoid X
receptor (RXR) activator. It is already reported to be a
cholesterol lowering agent in patients with type II dia-
betes, obesity and cardiovascular diseases. It is also
reported to possess anti-in ammatory as well as meta-
bolic properties.
The predicted biological activity spectrum of the
compounds by PASS contributing to their anti-hyper-
glycaemic potential is shown in Table 4 (S. No. 1-5).
The PASS result is obtained in the form of names of
biological activity whose probability value ranges from
0.000 to 1.000. Only activity types for which Pa>Pi,
are considered possible. Pa and Pi are the probability
measures for the compound to be active and inactive
respectively for the respective activities in the biologi-
Table 2. -amylase inhibitory effects of Q. indica leaf
extract in comparison with standard drug acarbose
S. No. Concentration
(μg/mL)
% inhibition
by acarbose
% inhibition
by ethanolic
leaf extract
of
Q. indica
1.
2.
3.
4.
5.
6.
10
20
40
60
80
100
20.19 ± 0.96
27.04 ± 0.98
37.17 ± 1.47
43.91 ± 1.46
57.68 ± 1.92
71.79 ± 0.55
14.86 ± 1.93
24.35 ± 0.88
26.15 ± 3.35
38.97 ± 1.17
53.07 ± 2.03
56.40 ± 2.35
Table 1. Phytochemical screening of ethanolic leaf extract of Q. indica
S. No. Phytochemicals + = present;
- = absent
S. No. Phytochemicals + = present;
- = absent
1. Anthraquinones - 6. Tannins +
2. Flavonoids + 7. Terpenoids -
3. Reducing sugar - 8. Phenols +
4. Saponins + 9. Alkaloids +
5. Steroids - 10. Quinones +
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF 327
Jyoti Verma, Devender Arora and Ajeet Singh
FIGURE 1. Percentage of -amylase inhibitory effects of acarbose (standard
drug) and ethanolic leaf extract of Q. indica
FIGURE 2. Chromatogram of the ethanolic leaf extract of Q. indica by GC-MS
cal activity spectrum. The various predicted metabolic
pathways of phytol involved in control of diabetes
include dextranase inhibition, glycerol-3-phosphate
dehydrogenase inhibition, -glucuronidase inhibition,
diabetic neuropathy treatment, etc. It shows 7 possible
pathways known to play a role in diabetes management
that can be further studied using wet lab experiments
to explore its potential to be used as drug for treatment
of diabetes. Other compounds like stigmasta-5, 23-dien-
3-ol, squalene, stearic acid, tetracontane, heptacosa-
nol, stigmasterol were also predicted for the presence
of their biological activities based on their chemical
328 EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Jyoti Verma, Devender Arora and Ajeet Singh
Table 3. Phytochemical components identi ed as major percentage in the ethanolic extract of leaves of Q.
indica by GC-MS
R.Time Mol. Wt. Formula Name Area% Activity already reported
16.509 242 C
15
H
30
O
2
Pentadecanoic acid 8.73 -
17.959 296 C
20
H
40
O (E)-Phytol 18.07 anti-microbial, anti-cancer, anti-in ammatory,
anti-diuretic, immunostimulatory, anti-diabetic
a
18.173 280 C
18
H
32
O
2
9,12-Linoleic acid 3.48 anti-in ammatory, nematicide, insectifuge,
hypocholesterolemic, cancer preventive,
hepatoprotective, anti-histaminic, anti-acne,
anti-arthritic, anti-eczemic, 5- reductase
inhibitor, anti-androgenic, anti-coronary
b
18.259 278 C
18
H
30
O
2
Linolenic acid 9.52 preventheart attacks, lowershigh blood
pressure,lowers cholesterol, reverse “hardening
of the blood vessels (atherosclerosis)
c
, treatment
ofrheumatoid arthritis(RA),multiple sclerosis
(MS),lupus,anti-diabetic, treatment of
chronic obstructive pulmonary disease (COPD),
migraineheadache,skin cancer,depression,
allergic and in ammatory conditions such
aspsoriasisandeczema
d
a
Venkata et al. 2012;
b
Sermakkani and Thangapandian 2012;
c
Brouwer et al. 2004;
d
Christensen et al. 2000
Table 4. Biological activity spectrum of standard drug acarbose and peak compounds identi ed by GC-MS
S. No. Compound Pa Pi Biological activity
1. Acarbose 0,972
0,962
0,958
0,943
0,779
0,752
0,691
0,681
0,636
0,611
0,512
0,508
0,499
0,447
0,333
0,000
0,000
0,000
0,000
0,002
0,005
0,005
0,002
0,009
0,001
0,002
0,003
0,003
0,016
0,002
Sucrose -glucosidase inhibitor
4--glucanotransferase inhibitor
-glucosidase inhibitor
-amylase inhibitor
-amylase inhibitor
Anti-diabetic
Fructan -fructosidase inhibitor
-glucosidase inhibitor
-glucuronidase inhibitor
-galactosidase inhibitor
Amylo--1,6-glucosidase inhibitor
Isoamylase inhibitor
Oligo-1,6-glucosidase inhibitor
Galactose oxidase inhibitor
-L-fucosidase inhibitor
2. Phytol 0,541
0,491
0,465
0,401
0,369
0,373
0,349
0,018
0,013
0,020
0,030
0,006
0,112
0,010
Dextranase inhibitor
Sorbitol-6-phosphate 2-dehydrogenase inhibitor
Glycerol-3-phosphate dehydrogenase inhibitor
Fructan -fructosidase inhibitor
-N-acetylgalactosaminidase inhibitor
Diabetic neuropathy treatment
-glucuronidase inhibitor
3. Linolenic acid 0,902
0,852
0,826
0,597
0,574
0,515
0,490
0,491
0,467
0,466
0,447
0,464
0,430
0,003
0,004
0,004
0,015
0,011
0,004
0,006
0,013
0,006
0,012
0,016
0,051
0,021
Dextranase inhibitor
Cholesterol antagonist
Antihypercholesterolemic
Insulin promoter
Fructan -fructosidase inhibitor
-glucuronidase inhibitor
-amylase inhibitor
Sorbitol-6-phosphate 2-dehydrogenase inhibitor
1,2- -L-fucosidase inhibitor
Anti-diabetic (type II)
Galactose oxidase inhibitor
-glucuronidase inhibitor
Diabetic neuropathy treatment
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF 329

Jyoti Verma, Devender Arora and Ajeet Singh
0,409
0,405
0,411
0,363
0,348
0,346
0,344
0,316
0,308
0,301
0,336
0,012
0,018
0,041
0,015
0,004
0,007
0,016
0,005
0,009
0,009
0,116
Anti-diabetic symptomatic
-N-acetylglucosaminidase inhibitor
Anti-diabetic
Antioxidant
Nitric oxide scavenger
-glucosidase inhibitor
Cholesterol synthesis inhibitor
Diabetic nephropathy treatment
-D-fucosidase inhibitor
-amylase inhibitor
Insulysin inhibitor
4. Pentadecanoic acid 0,957
0,753
0,739
0,723
0,699
0,611
0,593
0,583
0,540
0,544
0,453
0,422
0,400
0,387
0,359
0,318
0,315
0,323
0,001
0,004
0,004
0,007
0,002
0,013
0,004
0,003
0,028
0,037
0,012
0,011
0,004
0,011
0,011
0,005
0,027
0,070
Dextranase inhibitor
Insulin promoter
Fructan -fructosidase inhibitor
Cholesterol antagonist
-glucuronidase inhibitor
Antihypercholesterolemic
-amylase inhibitor
-amylase inhibitor
-glucuronidase inhibitor
Insulysin inhibitor
Diabetic neuropathy treatment
Anti-diabetic symptomatic
-glucosidase inhibitor
Cholesterol synthesis inhibitor
Pancreatic disorders treatment
Nitric oxide scavenger
Free radical scavenger
Anti-diabetic
5. 9,12-Linoleic acid 0,923
0,836
0,801
0,573
0,574
0,490
0,491
0,482
0,447
0,438
0,464
0,377
0,373
0,348
0,334
0,403
0,314
0,301
0,315
0,002
0,004
0,005
0,003
0,011
0,006
0,013
0,008
0,016
0,018
0,051
0,005
0,013
0,004
0,004
0,086
0,021
0,009
0,027
Dextranase inhibitor
Cholesterol antagonist
Antihypercholesterolemic
-glucuronidase inhibitor
Fructan -fructosidase inhibitor
-amylase inhibitor
Sorbitol-6-phosphate 2-dehydrogenase inhibitor
Anti-diabetic symptomatic
Galactose oxidase inhibitor
Diabetic neuropathy treatment
-glucuronidase inhibitor
-glucosidase inhibitor
Cholesterol synthesis inhibitor
Nitric oxide scavenger
Diabetic nephropathy treatment
Insulysin inhibitor
Antioxidant
-amylase inhibitor
Free radical scavenger
structures that could play a role in the management of
diabetes.
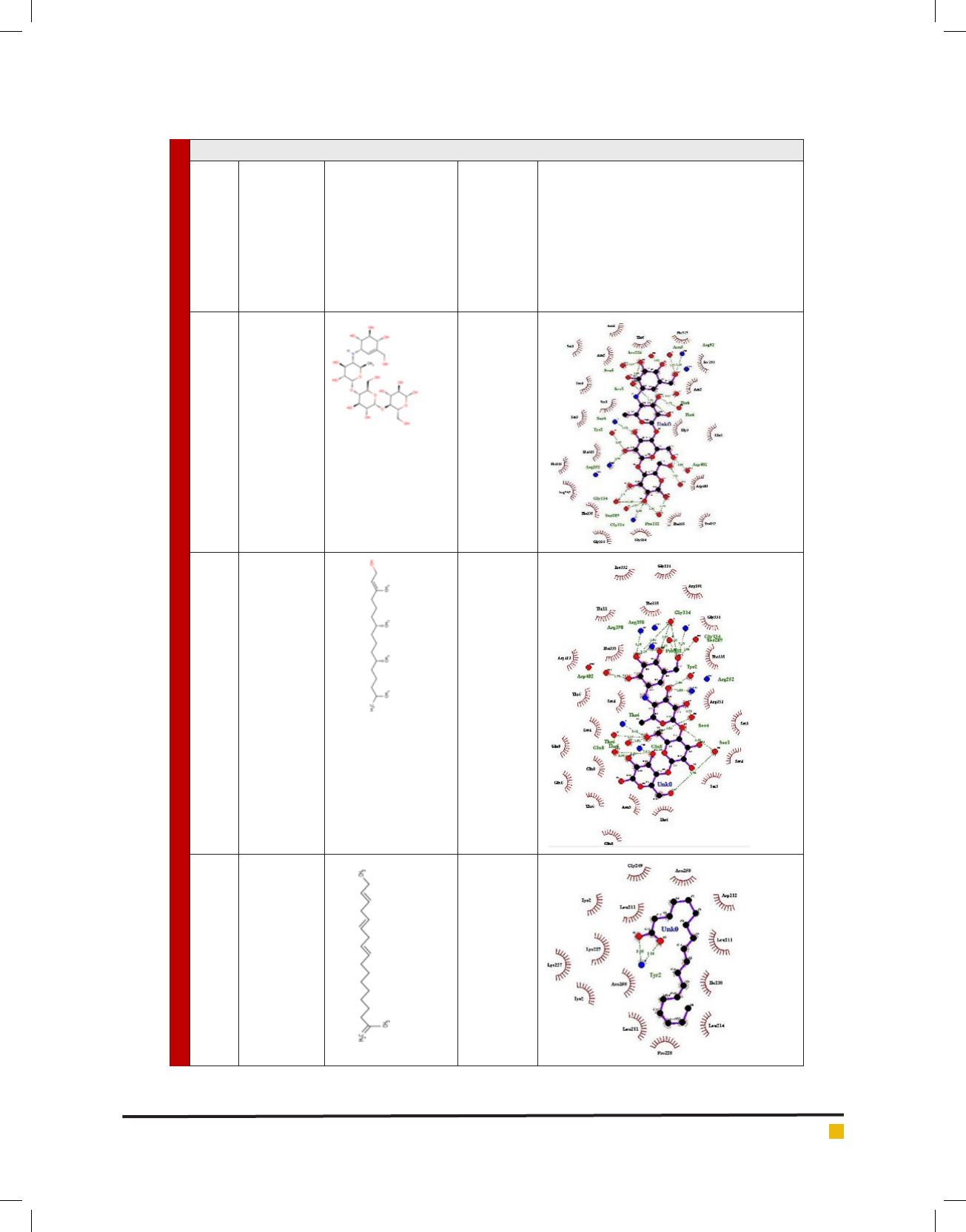
Binding simulation studies revealed stable complexes
of enzyme and the respective compounds with their
energy minimization values shown in Table 5 (S. No.
1-5). AutoDock studies have revealed the minimum free
energy needed to stabilize the complex of the -amylase
enzyme with the studied compounds. Interestingly, in
our docking studies, we found that phytol could be a
potent -amylase inhibitor with quite a strong binding
af nity and made remarkable inhibitory interactions
with critical residues. It was observed that the chemical
interactions established between phytol and -amylase
binding pocket residues were the most stable as com-
pared with other studied compounds like linolenic acid,
pentadecanoic acid and 9, 12-linoleic acid. Hydrogen
bondings were visualized using Ligplot
+
v.1.4.3 software
to study the stability of the complex of the enzyme and
the target compounds. Drug parameters of the target
compounds were analyzed and compared with acarbose,
330 EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Jyoti Verma, Devender Arora and Ajeet Singh
Table 5. Computational analysis of peak compounds identi ed by GC-MS and anti-diabetic drug acarbose
S. No. Compound
name
Chemical structure
drawn using
MarvinSketch
v5.10.0
Af nity
calculation
with
enzyme
-amylase
(kcal/
mol) using
AutoDock
Vina
Hydrogen bonding visualization with
enzyme -amylase using Ligplot+ v.1.4.3
1. Acarbose -8.3
2. Phytol -8.2
3. Linolenic acid -4.7
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF 331

Jyoti Verma, Devender Arora and Ajeet Singh
4. Pentadecanoic
acid
-4.4
5. 9,12-Linoleic
acid
-4.2
as shown in table 6 (S. No. 1-6). The molecular mass,
polar surface area and molar refractivity of phytol, lino-
lenic acid, pentadecanoic acid and 9, 12-linoleic acid
were found to be in range in compliance with Lipinski
rule. The results of the conducted study clearly indicate
the anti-hyperglycaemic property of the plant Q. indica.
All the test compounds studied above may contribute to
the anti-diabetic potential of the plant.
CONCLUSION
In the context of already known mechanisms central to
diabetes and its complications, we proposed that bioac-
tive compounds of Q. indica could be prospected for,
in search of potential -amylase inhibitors. To the best
of our knowledge, this is the rst report of screening
-amylase inhibitor from Q. indica leaves for allevia-
tion of diabetes and related complications. The molecu-
lar interaction patterns observed in the conducted study
may enable the designing of novel drug structures with
considerable inhibitory action. We believe that the study
conducted could provide leads for designing novel drug
inhibitors of -amylase with better ef cacy and com-
paratively lesser side-effects. Such a formulation could
be exploited for the development of an effective dosage
form for drugs that inhibit the digestive enzymes, help-
ing to attain maximum therapeutic ef cacy at reduced
dose and minimum toxicity.
Table 6. Evaluation of drug parameters of peak compound identi ed by GC-MS analysis and comparison with
the anti-diabetic drug acarbose
S. No. Drug parameters Acarbose Phytol Linolenic acid Pentadecanoic acid 9,12 linoleic acid
1. Mass 646 296 267 227 267
2. Formula C
25
H
43
NO
18
C
20
H
40
OC
18
H
30
O
2
C
15
H
30
O
2
C
18
H
32
O
2
3. Log P -7.61 -7.04 -6.06 -5.81 -6.42
4. Polar Surface Area 321.17 20.23 37.30 37.30 37.30
5. Molar Refractivity 136.524780 95.561760 79.009491 69.104492 80.161491
6. Lipinski Rule of 5 No No No No No
332 EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Jyoti Verma, Devender Arora and Ajeet Singh
ACKNOWLEDGMENTS
This study was conducted in the Department of Bio-
technology, G. B. Pant Engineering College (GBPEC),
Pauri Garhwal (Uttarakhand), India. Authors gratefully
acknowledge the assistance provided by Dr. Ajai Kumar
(system analyst) at AIRF-JNU and Dr. Ritu Chowdhary.
Jyoti Verma is thankful to TEQIP-II (Technical Education
Quality Improvement Programme, Government of India)
for nancial assistance.
CONFLICT OF INTEREST
Authors have no con ict of interest regarding the pub-
lication of paper.
REFERENCES
Ariful HM, Azmal IH, Tridib KP, Mariz S, Himel NK (2010) A
survey of medicinal plant usage by folk medicinal practition-
ers in two villages by the Rupsha river in Bagerhat District,
Bangladesh. American-Eurasian J Sustainable Agri 4(3): Pages
349-356.
Bairagi VA, Sadul N, Senthilkumar KL, Y Ahire (2012) Anti-
diabetic potential of Quisqualis indica Linn in rats. Int J Pharm
Phytopharmacol Res 1(4): Pages 166-171.
Brouwer IA, Katan MB, Zock PL (2004) Dietary alpha-linolenic
acid is associated with reduced risk of fatal coronary heart
disease, but increased prostate cancer risk: a meta-analysis. J
Nutr 134(4): Pages 919-922.
Burov YV, Poroikov VV, Korolchenko LV (1990) National sys-
tem for registration and biological testing of chemical com-
pounds: facilities for new drugs search. Bull Natl Center for
Biologically Active Compounds (Rus.) 1: Pages 4-25.
Chakraborty AK, Shrivastava A, Garg S, Shrivastava TP (2015)
Pharmacognostic and phytochemical study of whole plant of
Quisqualis indica Linn. International Journal of Medicine and
Pharmaceutical Research 3(2): Pages 970-977.
Chauhan A, Sharma PK, Srivastava P, Kumar N, Dudhe R
(2010) Plants having potential anti-diabetic activity: A review.
Der Pharmacia Lettre 2(3): Pages 369-387.
Christensen JH, Christensen MS, Toft E, Dyerberg J,Schmidt
EB (2000) Alpha-linolenic acid and heart rate variability. Nutr
Metab Cardiovasc Dis 10(2): Pages 57-61.
Colditz GA (2000) Changing dietary patterns and cancer pre-
vention: alpha-linolenic acid health risks and bene ts. Cancer
Causes Control 11(8): Pages 677-678.
Connor WE (1999) Alpha-linolenic acid in health and disease.
Am J Clin Nutr 69(5): Pages 827-828.
Daines A, Payne R, Humphries M, Abell A (2003) The syn-
thesis of naturally occurring vitamin K and vitamin K ana-
logues.Current Organic Chemistry7(16): Pages 1625-1634.
Ding Z, Lu Y, Lu Z, Lv F, Wang Y, Bie X, Wang F, Zhang
K (2010). Hypoglycaemic effect of comatin, an anti-diabetic
substance separated from Coprinus comatus broth, on alloxan-
induced-diabetic rats. Food Chem 121(1): Pages 39-43.
Djousse L, Arnett DK, Pankow JS, Hopkins PN, Province MA,
Ellison RC (2005) Dietary linolenic acid is associated with a
lower prevalence of hypertension in the NHLBI Family Heart
Study. Hypertension 45: Pages 368-373.
Filimonov DA, Poroikov VV (1996) PASS: Computerized pre-
diction of biological activity spectra for chemical substances.
In: Bioactive compound design: Possibilities for industrial use,
Oxford: BIOS Scienti c Publishers 47-56 http://www.ibmh.
msk.su/PASS
Filimonov DA, Poroikov VV, Borodina Y, Gloriozova T (1999)
Chemical similarity assessment through multilevel neighbor-
hoods of atoms: de nition and comparison with the other
descriptors. J Chem Inf Comput Sci 39: Pages 666-670.
Filimonov DA, Poroikov VV, Karaicheva EI, Kazaryan RK, Bou-
dunova AP, Mikhailovsky EM, Rudnitskih AV, Goncharenko
LV, Burov YV (1995) Computer-aided prediction of biological
activity spectra of chemical substances on the basis of their
structural formulae: computerized system PASS. Experimental
and Clinical Pharmacology (Rus) 8(2): Pages 56-62.
Guidelines on registration of traditional medicines in the WHO
African Region (2004). Brazzaville: World Health Organization
Regional Of ce for Africa, Background and Purpose: 40.
Hansawasdi C, Kawabata J, Takanori K (2000)
-amylase
inhibitors from Roselle (Hibiscus sabdariffa Linn.) Tea. Biosci
Biotechnol Biochem 64: Pages 1041-1043.
Joshi SG (2002) Medicinal plants, 1st edn. Mohan Primlani for
Oxford and IBH publishing Co pvt Ltd, Delhi, Page 141.
Khare CP (2007) Indian Medicinal Plants. An Illustrated Dic-
tionary Berlin/Heidelberg: Springer-Verlag. Pages 649-650.
Kirtikar KR, Basu BD (2006) Indian medicinal plant, 2nd edn.
Prashant Gahlot at valley offset publishers, New delhi, Page
1037.
Laskowski R, Swindells M (2011) LigPlot
+
: multiple ligand-
protein interaction diagrams for drug discovery. Journal of
Chemical Information and Modeling 51: Pages 2778-2786.
Morris GM, Huey R, Olson AJ (2008) Using AutoDock for
ligand-receptor docking. Curr Protoc Bioinformatics Chapter
8:8.14.
Padua LS, Bunyapraphatsara N, Lemmens RM (1999) Plant
resources of South-East Asia in medicinal and poisonous
plants. Source Backhuys Publications Leiden In: the Nether-
lands 12(1): Pages 255-259.
Patel DK, Kumar R, Laloo D, Hemalatha S (2011) Evaluation of
phytochemical and antioxidant activities of the different frac-
tions of Hybanthus enneaspermus (Linn.) F. Muell (Violaceae).
Asian Pac J Trop Med 4(5): Pages 391-396.
Patel DK, Kumar R, Prasad SK, Sairam K, Hemalatha S (2011)
Anti-diabetic and in vitro antioxidant potential of Hybanthus
enneaspermus (Linn.) F. Muell in streptozotocin-induced dia-
betic rats. Asian Pac J Trop Biomed 1(4): Pages 316-322.
Patwardhan B, Hopper M (1992). Ayurveda and future drug
development. J Altern Complement Med 3: Pages 9-11.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF 333

Jyoti Verma, Devender Arora and Ajeet Singh
Poroikov VV, Filimonov DA, Ihlenfeldt WD, Gloriozova TA,
Lagunin AA, Borodina YV, Stepanchikova AV, Nicklaus MC
(2003) J Chem Inf Comput Sci 43(1): Pages 228-236.
Sa SZ, Qvist R, Kumar S, Batumalaie K, Ismail ISB (2014)
Molecular Mechanisms of Diabetic Retinopathy, General Pre-
ventive Strategies, and Novel Therapeutic Targets. BioMed
Research International PMC4106080
Sermakkani M, Thangapandian V (2012) GC-MS analysis of
Cassia italica leaf methanol extract. Asian J Pharm Clin Res
5(2): Pages 90-94.
Sofowora A (2006) Medical plants and traditional medicine in
Africa. Spectrum Books, Ibadan, Nigeria.
Thomas, Netscher (2007) Synthesis of vitamin E. Vitamins and
hormones 76: Pages 155-202.
Trease GE, Evans WC (1996) Pharmacognosy. WB Saunders,
Philadelphia, Pa, USA
Trott O, Olson A (2010) AutoDock Vina: Improving the speed
and accuracy of docking with a new scoring function, ef cient
optimization, and multithreading. Journal of Computational
Chemistry 31: Pages 455-461.
Venkata RB, Samuel LA, Pardha SM, Narashimha RB, Naga
VKA, Sudhakar M, Radhakrishnan TM (2012). Antibacterial,
antioxidant activity and GC-MS analysis of Eupatorium odo-
ratum. Asian J Pharm Clin Res 5(2): Pages 99-106.
Wagman AS, Nuss JM (2001) Current therapies and emerg-
ing targets for the treatment of diabetes. Curr Pharm Des 7(6):
Pages 417-450.
Warjeet SL (2011) Traditional medicinal plants of Manipur as
anti-diabetics. J Med Plants Res 5(5): Pages 677-687.
Wetwitayaklung P, Phaechamud T, Keokitichai S (2007) The
study of antioxidant activities of edible ower. In: Proceeding
of International Workshop on Medicinal and Aromatic Plants,
Chiang Mai: Thailand 75.
Yuan H, Ma Q, Ye L, Piao G (2016) Traditional Medicine and
Modern Medicine from Natural Products. MDPI Molecules
21(559): Pages 1-18.
334 EVALUATION OF ANTI-HYPERGLYCAEMIC POTENTIAL OF THE ETHANOLIC LEAF BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS