
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 11(2): 195-207 (2018)
In-silico
identi cation of phytohormone pathway genes
in
Camellia sinensis
and expression analysis under
combined water and herbivore stress
Madhurjya Gogoi
1,2
*, Hemanta Saikia
1
, Sangeeta Borchetia
1
, Raj Narain Singh Yadav
3
and
Tanoy Bandyopadhyay
1
1
Department of Biotechnology, Tea Research Association, Tocklai Tea Research Institute, Jorhat-785008,
Assam, India
2
Centre for Biotechnology and Bioinformatics, School of Science and Engineering, Dibrugarh University,
Dibrugarh-786004, Assam, India
3
Department of Life Sciences, School of Science and Engineering, Dibrugarh University, Dibrugarh-786004,
Assam, India
ABSTRACT
Tea (Camellia sinensis) is a popular beverage worldwide. Abiotic and biotic stresses due to recent climate change have
signi cant effect on yield of tea. Plant hormones such as abscisic acid (ABA), jasmonic acid (JA), salicylic acid (SA)
and ethylene (ET) plays an important role in regulating plant defense responses to different kind of stresses. In this
study homologous phytohormone genes of ABA, JA, SA and ET pathway in tea plant were identi ed from the public
domain transcriptomic database and the expression of the rate-limiting genes of phytohormone pathway were ana-
lyzed in tea plants subjected to combined water and herbivore stress to understand the interaction among the stress-
induced phytohormone pathways genes. Vegetatively propagated TV1 clones of tea plant were subjected to three
level of water stress treatments: 1) well watered control 2) mild water stress 3) severe water stress for three months
and then infested with Hyposidra talaca (looper caterpillar). The constitutive expression (without infestation) of the
rate-limiting genes of ABA and ET pathway were positively regulated by water stress whereas JA and SA pathway
genes were negatively regulated. On looper caterpillar infestation (induced expression) water stressed plants showed
signi cant decrease in expression of the rate-limiting phytohormone genes except ACC synthase. Our study showed
that on herbivore infestation well watered plants have higher capacity to induce the phytohormone genes and water
195
ARTICLE INFORMATION:
*Corresponding Author: madhurjyag@gmail.com
Received 28
th
March, 2018
Accepted after revision 16
th
June, 2018
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC / Clarivate Analytics USA and
Crossref Indexed Journal
NAAS Journal Score 2018: 4.31 SJIF 2017: 4.196
© A Society of Science and Nature Publication, Bhopal India
2018. All rights reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/11.1/2

196
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Madhurjya Gogoi et al.
stress played a major role in regulation of gene expression than herbivore infestation. The presence of an initial water
stress not only affected the tea plant constitutive defense but also signi cantly altered the phytohormone defense
gene expression towards subsequent herbivore stress. The water stressed tea plant with weak induced expression of
defense associated phytohormone genes may be at a higher risk for incidence of pest and pathogen attack compared
to well watered plants.
KEY WORDS:
CAMELLIA SINENSIS
, EXPRESSION ANALYSIS, HERBIVORE INFESTATION,
HYPOSIDRA TALACA
,
IN SILICO
IDENTIFICATION;
PHYTOHORMONE PATHWAY
INTRODUCTION
Tea, Camellia sinensis is a major economic crop world-
wide and its young leaves are used for preparing bever-
age. Huge losses in tea leaf yield is incurred due to the
present climate change scenario. As the climatic change
event is expected to increase the incidence of water
shortage and outburst of insect population, the mono-
culture cultivation of tea may face severe crop loss in
recent future. Drought is one of the major abiotic stress
that in uences the quality and productivity of crops by
growth inhibition, increase in organic solutes concen-
tration and changes in the endogenous phytohormones
content (Wijeratne et al., 2007; Bhagat et al., 2010,
Aimar et al. 2011, Chen and Chen, 2012 and Anderegg
et al. 2015).
Biotic stressors such as insects and pathogens, also
contribute signi cantly towards the enormous dam-
age to the crops (Hammond-Kosack and Jones, 2000).
Plants have both inherent and adopted mechanisms to
cope with the environmental stresses by producing cer-
tain proteins and secondary metabolites that are toxic
or have repellant effect on the biotic agents (Rani and
Jyothsna, 2010; War et al., 2011a; War et al., 2011b; War
et al., 2012). Abiotic and biotic stresses in plants trigger
the activation of a number of phytohormone pathway
genes which simultaneously activate other intercon-
nected defense network to help plants sustain the stress
period (Fraire-Velá zquez et al., 2011). The primary phy-
tohormones abscisic acid (ABA), jasmonic acid (JA), sali-
cylic acid (SA), ethylene (ET) are involved as messengers
triggering the speci c defense pathways against envi-
ronmental stress and may act individually or in combi-
nations depending upon the stress perceived, (Atkinson
and Urwin, 2012, Verma et al., 2016; Wani et al., 2016).
ABA is produced in response to water-de cit stress
(Osakabe et al., 2013). Enhanced accumulation of ABA
in Arabidopsis thaliana seedlings has been reported
under drought conditions (Huang et al., 2008). Exog-
enous application of ABA delay wilting and is reported
to induce drought tolerance in plants (Lu et al., 2009).
ABA functions both synergistically and antagonistically
with JA, SA and ET signaling pathways which play a
dominant role during biotic stress (Chen and Yu, 2014).
Under the combination of abiotic and biotic stresses,
ABA mostly acts as an antagonist to JA/SA/ET mak-
ing the plant susceptible to disease and pathogen attack
(Rejeb et al., 2014). However, a positive interaction has
also been observed, whereby an increase in ABA level
under abiotic stress results in stomatal closure which
prevents the entry of biotic agents and protects the
plants from both biotic and abiotic stresses (Melotto et
al., 2006). SA, an endogenous growth regulator, induces
systemic acquired resistance (SAR) in plants against dif-
ferent pathogens, particularly microbes and serves as a
signal molecule by producing pathogenesis related (PR)
proteins (Gao et al., 2015; Verma et al., 2016).
SA is also involved in plant response to different abi-
otic stresses such as drought, temperature variations,
heavy metals and osmotic stress (Rivas-San Vicente and
Plasencia, 2011). JA, a key regulator of plant response
to pathogens and insects, is involved in both direct and
indirect defenses of plants to herbivory (Creelman and
Mullet, 1995). JA also participates in plant’s response to
drought and salinity (Riemann et al., 2015). ET, the gas-
eous phytohormone for defense, helps in both direct and
indirect response of plants to abiotic and biotic stresses.
The effects of ET can be transitory or long lived as its
biosynthesis shows a diurnal rhythm and controls its
own biosynthesis (Eyidogan et al., 2012; Gamalero and
Glick, 2012; Verma et al., 2016).
Depending on the type of stress perceived by the
plant, different signaling pathways are activated which
synergistically or antagonistically in uence the type of
response generated. The interactions among the different
signal transduction pathways are considered as crosstalk
between the pathways which helps the plants to sustain
the stress period (Rejeb et al., 2014). In the event of cli-
matic change where both the abiotic and biotic stress will
co-occur, it is largely unknown how the phytohormone
based plant defense network would behave as majority
of the studies so far considered single stress factor either
abiotic or biotic at a time. It also remain unpredictable
how the presence of an initial stress affects the plant
defense network on perception of a subsequent stress.
As the frequency and extent of drought as well as insect
infestation are projected to increase due climate change
it is essential to understand the response of plants to
combined stress conditions. Knowledge of the molecular
mechanisms underlying these effects is very limited. The

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES 197
Madhurjya Gogoi et al.
molecular study of the stress-related hormonal genes
and their interactions would help to understand the syn-
chronization of plant constitutive and induced defense
responses to insect infestation in plants under abiotic
stress. Tea plant faces water stress event round the year
and also plethora of insect infestation. Looper cater-
pillar infestation stand out to be the most destructive
insect infestation in terms of crop loss. The water stress
may change the overall metabolism of the tea plant and
alter its defense interaction with the biotic agents. It will
be vital to know the interaction of the phytohormone
defense gene network in perception of combined water
and herbivore stress.
In this study, a comparative genomics approach was
undertaken to mine the phytohormone pathway genes
of C.sinensis and the expression pattern of the rate lim-
iting genes of the four phytohormone pathway (ABA,
JA, SA, ET) was analyzed in a clone (TV1) of tea plant
which was subjected to different regime of water stress
(abiotic stress) treatment with subsequent insect infesta-
tion (looper caterpillar). This study will help to better
understand the stress-induced phytohormones defense
interaction at transcriptional level in tea plant under
combined water and herbivore stress.
MATERIALS AND METHODS
IN SILICO
IDENTIFICATION OF PHYTOHORMONE
PATHWAY GENES IN
C. SINENSIS
The genes or transcripts involved with ABA, JA, SA and
ET phytohormone pathway in C. sinensis were mined
from different databases using Arabidopsis sequences as
reference. The Arabidopsis full-length coding sequences
(CDS) were collected from the TAIR database (https://
www.arabidopsis.org/) and subsequently the sequences
were subjected to BLASTN with C. sinensis Expressed
Sequence Tags (EST), Transcriptome Shotgun Assembly
(TSA) and Non-Redundant (NR) nucleotide databases of
NCBI. The sequences showing signi cant similarity with
an E-value ≤ 1e
-15
were selected and assembled using
CAP3 program to remove redundancy and get consensus
sequences. Each of those sequences were screened for
the presence of open reading frame (ORF) using NCBI
ORF Finder (Wheeler et al., 2003) and sequences with
the longest ORF having both start and a stop codon
were sorted out. The sequences were further subjected to
BLASTX with NCBI NR (Non-Redundant) protein data-
base to con rm their annotation. Based on the BLAST
annotation and alignment results, the sequences found
similar to Arabidopsis reference sequences and nearby
plant species sequences were retained and others were
ltered out. The best representative sequences were sub-
jected to blast and functional classi cation following
the Gene Ontology (GO) scheme using BLAST2GO suite
(Conesa and Götz, 2008). The transcripts were classi ed
into the major GO categories, namely, cellular compo-
nent, molecular function and biological process. Further
the rate-limiting gene sequences of the phytohormone
pathways were used in expression study.
MOTIF AND DOMAIN IDENTIFICATION
MEME and MAST programs (Bailey and Elkan, 1994;
Bailey and Gribskov, 1998) were used for the identi ca-
tion of the motif cluster present in the phytohormone
pathway gene sequences. MEME program performs motif
discovery on DNA, RNA or protein datasets. Whereas,
MAST program searches sequences for matches to a set
of motifs and sorts the sequences by the best combined
match to all motifs. The sequences from C. sinensis and
Arabidopsis were analyzed together for the easy identi-
cation of common motifs between them. The motif dis-
covery mode was set to normal. The maximum number
of motif was set to 20. Domain search was performed
using CD-search tool available at the conserved domain
database (CDD) of National Center (https://www.ncbi.
nlm.nih.gov/Structure/cdd/wrpsb.cgi).
SELECTION OF GENE FOR qRT PCR AND
PRIMER DESIGN
The rate limiting gene/enzyme of each phytohormone
pathway was searched from literature references and
the expression of the corresponding genes was analyzed
across different treatments. For the ABA pathway, the
reaction catalyzed by 9-cis-epoxycarotenoid dioxyge-
nase (NCED) i.e- the oxidative cleavage of neoxanthin
is considered as the rate-limiting step (Tan et al., 1997;
Qin and Zeevaart, 1999) and chosen for our study. In the
ET biosynthesis pathway, the conversion of S-AdoMet
to 1-aminoacyclopropane 1-carboxylate (ACC) by ACC
synthase is taken as the rate-limiting step (Wang et al.,
2002). Allene oxide synthase (AOS), the rst enzyme
in the branch pathway leading to the formation of JA
act as rate-limiting step in JA biosynthesis (Harms et
al., 1995; Sivasankar et al., 2000). In the SA pathway,
isochorismate synthase (ICS) acts as the rate-limiting
enzyme (Serino et al., 1995; Gaille et al., 2003).Thus
NCED gene for ABA, ACC synthase gene for ET, AOS
gene for JA and ICS gene for SA pathway was chosen
for gene expression analysis. The primers for the cor-
responding genes were designed using primer3 (http://
frodo.wi.mit.edu/primer3/) software. The primers were
designed as such that the product length was within
100-200 base pair. List of the primers used in the study
is provided in Supplementary Table 1.

Madhurjya Gogoi et al.
198
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
EXPERIMENTAL SET UP AND SAMPLE
COLLECTION
Two years old vegetatively propagated TV1 clones of
tea plant were collected from the nursery of Tocklai Tea
Research Institute, TRA, Jorhat. The plants from nursery
were replanted in black poly-sleeves (18 cm diameter
and 23cm height) with eld soil (sandy loam, pH 4.8-
5.1, bulk density 1.3-1.4 Mg m
-3
, single super phophate
0.5 kg. m
−3
of soil) and allowed to acclimatize for 30
days in natural environmental condition with suf cient
irrigation. After the acclimatization period the plants
were transferred to a polyhouse and were allowed to
acclimatize within the polyhouse for 10 days before
starting the stress experiments. The plants were covered
with nets for protecting it from external pest infestation.
Thereafter the plants were subjected to three level of
water stress treatment: 1) well-watered control; 2) mild
water stress; 3) severe water stress. The plants in each
drought stress level received same amount of water and
watered simultaneously. The well-watered control plants
received water every 3rd day such that the soil remained
constantly moistened. Mild drought stressed plants were
watered once the soil water content drops to 7-8% and
received 40-50 % of water supplied to the well-watered
control plants. The severe water stressed plants were
watered when the soil water content reaches 4-5 % and
received around 15–20% the amount of water supplied
to well-watered control plants. At the end of the three
months the plants were rehydrated with small amount of
water overnight in all the treatment and then subjected
to Hyposidra talaca (looper caterpillar) infestation.
Rehydration of plants was done to ensure that the
gene expression of plants re ect the effect of pulsed
water stressed treatment which is often faced by tea
plant in natural environment rather than continuous
drought. The leaves were collected before insect infesta-
tion i.e - at 0 hours (0 TPI), and after insect infestation
i.e - 24, 48 hours (24 TPI, 48 TPI) in all the treatments.
The leaves collected at 0 TPI were taken as undamaged
control. As only the non-damaged control tissue was
measured at the initial time point (time = 0 hrs), the
main effect of time represents time post herbivory infes-
tation or time post infestation and is designated at TPI.
For each treatment and for each time point there were
three biological replicates. Once the plants were used to
collect sample they were discarded and not used further
in the experiment. For all the treatment the third leaf of
tea plant from top was collected and immediately stored
at -80
0
C to prevent any enzymatic activity.
RNA ISOLATION AND cDNA PREPARATION
Total RNA was extracted from 100 mg of each sample
according to the protocol of Zaman et al., 2016. RNA
integrity was determined using a 1% agarose gel and
concentration was quanti ed using an Eppendorf Bio-
photometer (Eppendorf, Hamburg, Germany). After veri-
fying the integrity of the RNA, equal concentration of
RNA from each sample was used for rst strand cDNA
preparation using QuantiTect Rev. Transcription Kit(Cat
No./ID:205311, QIAGEN, Germany).
QUANTITATIVE REAL-TIME PCR ANALYSIS
Four important genes known to be involved in the rate-
limiting step of the phytohormone biosynthesis pathway
(ABA, JA, ET, SA) in tea were selected based on the com-
parative in-silico identi cation of homologous genes. The
expression of the selected four genes was studied in tea
plant subjected to three level of water stress treatments
with subsequent insect infestation. Quantitative Real-
Time PCR was performed in a Roche Light Cycler 480 real
time machine (Roche, Germany) using QuantiTect SYBR
Green PCR Kit (Cat No./ID:204145, QIAGEN, Germany).
The reverse transcribed rst strand cDNA of each sample
was used as template in the assay and ampli ed by gene-
speci c primers. The PCR was performed in 10 μl reaction
volume and prepared according to the protocol mentioned
in the kit manual. In short, 3.5 μl of supplied PCR grade
water was mixed with 0.5 μl of forward and reverse primer
and 5 μl of ‘QuantiTect SYBR Green I Master Mix’ to get
a nal volume of 9.5 μl. Finally, 0.5 μl of template was
added. The relative expression levels of all the genes were
calculated using the ddCt method. The raw Ct values were
normalized against Ribulose-1, 5-bisphosphate carboxy-
lase/oxygenase housekeeping gene. The log 2 fold change
values for all the samples was calculated relative to well-
watered control sample at 0 TPI.
STATISTICAL ANALYSIS
The transcript relative expression values were log 2 fold
transformed and analyzed with a 3 3 (T x TPI) mixed
model analysis of variance (ANOVA) followed by post
hoc pairwise comparisons with Bonferroni adjustment
for multiple testing. “T” stands for water stress treat-
ment and “TPI” stands for time post infestation. All
the expression data are expressed as mean ± standard
deviation (SD). Each expression value is the mean of
three biological replicates. Data were analyzed using
IBMSPSS Statistics, Version 20.0.
RESULTS AND DISCUSSION
IDENTIFICATION AND ANALYSIS OF
PHYTOHORMONE PATHWAY GENES
Gene mining of the four phytohormone pathways (ABA,
JA, SA and ET) resulted in a large number of C. sinen-

Madhurjya Gogoi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES 199
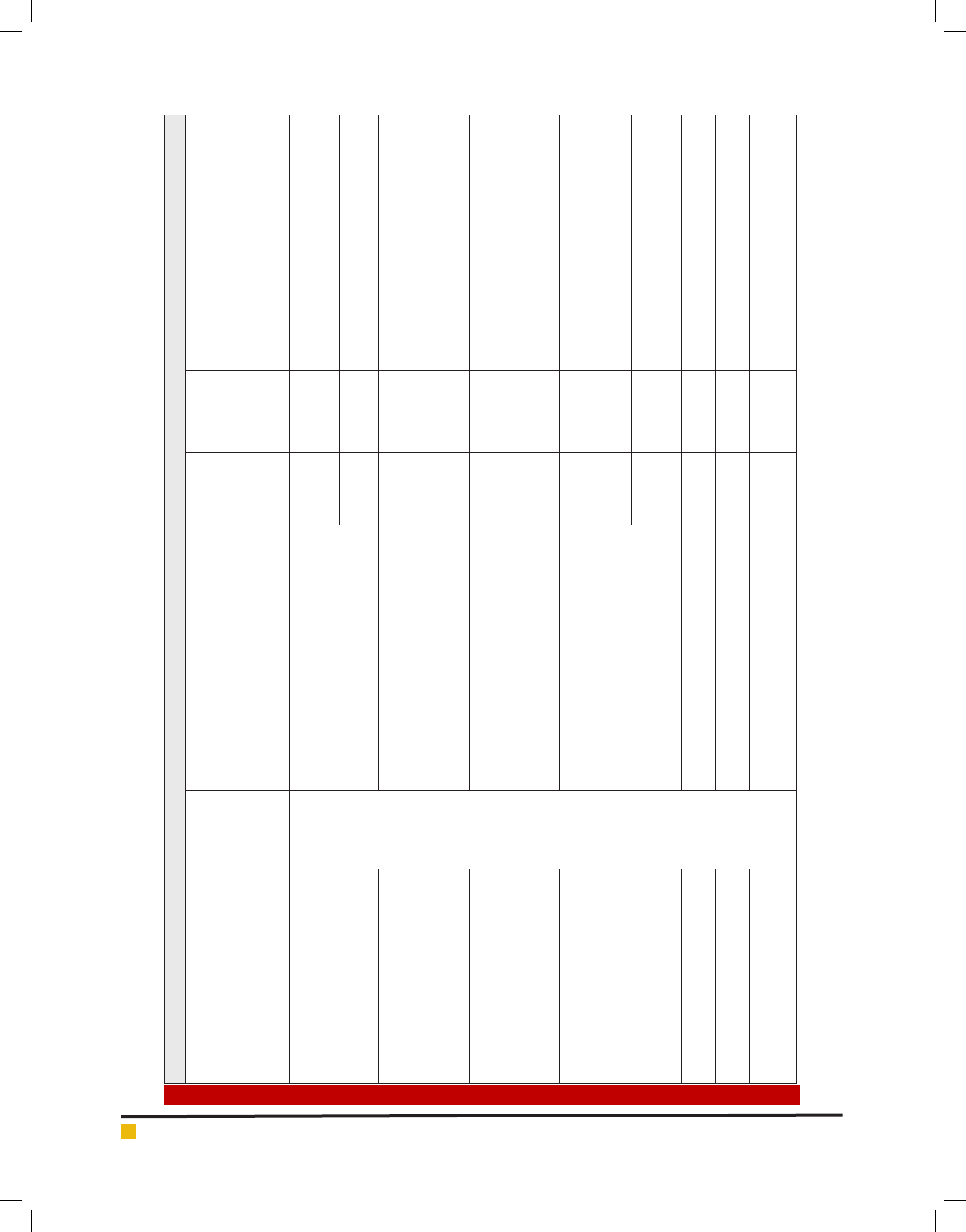
sis homologues for each gene (Table1). CAP3 cluster-
ing removed the redundancy of the sequences, still a
signi cant number of homologues were retained after
clustering in many instances. In the ABA pathway few
genes like ABA 8’-hydroxylase, 9-cis-epoxycarotenoid
dioxigenase (NCED) etc. were represented by more than
one homologue of C. sinensis having signi cant similar-
ity and they possessed most of the common motifs pre-
sent in corresponding Arabidopsis reference sequences
(Table 1, Fig.1 (A, B)). Homologue gene mining of JA
pathway resulted in the identi cation of nine genes and
each gene best representative homologue is listed in
Table1. For the SA pathway a total of four genes were
mined but only three gene homologues were retained.
The C. sinensis homologues obtained for salicylic acid
carboxyl methyltransferase gene had an E-value >1e
-
15
and hence it was not considered for further study.
Two full-length sequences of ET pathway genes namely
1-aminocyclopropane-1-carboxylate synthase (ACCS)
and 1-aminocyclopropane-1-carboxylate oxidase
(ACCO) were obtained based on keyword search in the
NCBI NR nucleotide database.
Further the result of Blast2GO program with the
details of sequence similarity, GO classi cation, enzyme
list, InterPro scan domain etc. for the four pathway
genes are provided in Supplementary Table 2. MEME/
MAST search, for the putative functional and common
motif occurrence showed that most of the motifs are
conserved among the C. sinensis homologues and its
Arabidopsis counterpart except in few cases, where it
was seen that few motifs were missing in C. sinensis
homologues. This may be due to the presence of par-
tial transcript sequences of C. sinensis. Few representa-
tive genes displaying the occurrence of common motifs
between Arabidopsis and C. sinensis homologues are
shown in Fig.1 (A, B), Supplementary Fig. 1 (A-D) &
Supplementary Fig. 2 (A-D). The result of the functional
domain identi cation using CD-search tool showed the
presence of conserved domains between Arabidopsis and
Camellia sinensis homologues (Supplementary Table 3).
The presence of conserved common motif and domain in
sequential pattern between the phytohormone pathway
genes of C. sinensis and Arabidopsis clearly con rms
their identity and support the results of our comparative
genomics approach.
Gene expression of the rate-limiting genes 9-cis-
epoxycarotenoid dioxygenase (NCED), Allene oxide syn-
thase (AOS), 1-aminocyclopropane-1-carboxylate syn-
thase (ACC synthase) and Isochorismate synthase (ICS)
of ABA, JA, ET and SA biosynthesis pathway respec-
tively was studied at different time point in the three
water stress treatment with subsequent insect infesta-
tion. The relative gene expression values discussed here
are expressed in terms of log 2 Fold Change and the well
watered treatment at 0 TPI is taken as control for calcu-
lation of relative gene expression fold change for other
time points and treatments.
Considering the expression of rate limiting genes at 0
TPI (without insect infestation) the expression of NCED
gene of ABA pathway in case of mild and severe water
stress treatment was higher than well watered plants.
Mild water stress plant showed a log 2 FC value of 1.31
while severe stress plant showed a value of 1.62 (Fig.2
(A)). In mild water stress plants the AOS gene of JA
pathway had almost similar expression value with con-
trol and the difference was not statistically signi cant.
Whereas in severe water stress plants there was signi -
cant down regulation of AOS expression with a log 2
FC value of -2.49 compared to control plants (P< 0.05,
Fig.2 (B)). ICS gene of SA biosynthesis pathway showed
higher transcript accumulation in mild water stress plant
with a value of 2.84 whereas in severe stressed plants it
was down-regulated with a value of -4.04 compared to
control ( P< 0.05, Fig.2 (C)). In case of ET pathway, with
the increase in water stress intensity the expression of
ACC synthase gene also increased proportionally with a
value of 1.88 and 2.59 in mild and severe water stress
treatment respectively (Fig.2 (D)). The transcript expres-
sion at 0 TPI represent the constitutive expression of tea
plant and it mainly re ect the effect of the water stress
treatment on the expression of the phytohormone path-
way genes. The expression at 24 TPI and 48 TPI mainly
represent the induced expression of the tea plant after
insect infestation under different levels of water stress
treatment.
At 24 TPI, the expression of NCED gene in well-
watered plants increased signi cantly (log 2 FC: 2.13)
compared to control at 0 TPI. However the expression at
48 TPI was somewhat less (log 2 FC: 1.42) compared to
24 TPI. In the mild stress plants there was up-regulation
at 24 TPI followed by down-regulation at 48 TPI with a
value of -0.04. The severe stress plant showed a decline
in expression pattern with the increase of time (1.62 at
0 TPI, 1.38 at 24 TPI and 0.04 at 48 TPI). AOS gene
of JA pathway at 24 TPI showed signi cant induction
in expression (log 2 FC 3.05, P<0.05) of well-watered
plants. As the time elapsed the gene expression declined
at 48 TPI (Fig.2 (B)). However the expression was still
signi cantly higher compared to 0 TPI. Mild and severe
stressed plant followed the same trend with increase of
transcript accumulation at 24 TPI and then a decline at
48 TPI. The expression of ICS gene in case of control and
severe stressed plants increased at 24 TPI and decreased
at 48 TPI whereas in mild stressed plant the expression
declined both at 24 TPI and 48 TPI (Fig.2 (C)). Expres-
sion of ACC Synthase of ET pathway increased at 24 TPI
and then declined at 48 TPI in all the water stress condi-
tions (Fig.2 (D)). Two way ANOVA showed that for the
Madhurjya Gogoi et al.
200
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
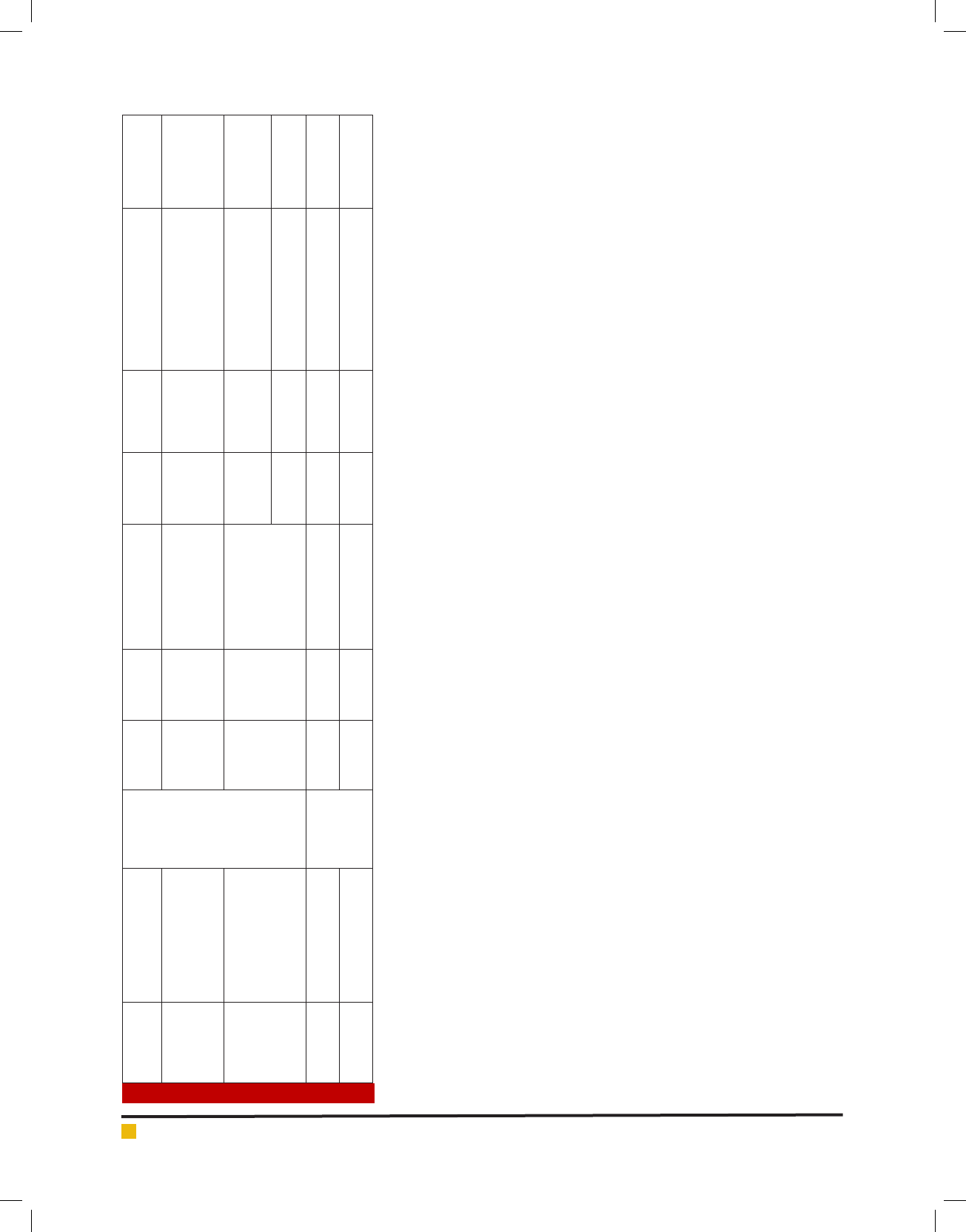
Table 1. Results of in-silico mining of Camellia sinensis phytohormone genes..
Arabidopsis
reference
sequence
accession
numbers
Sequence Name
Associated
Pathway
Total
No. of
Homologs
(EST + NR
+ TSA)
before
CAP3
Total
sequences
after CAP3
assembly
(Contig +
singleton)
Camellia sinensis
homologous sequences
retained after
processing
CAP3
(Contig,
singleton)
Number
Accession No.s
of Contigs &
singletons
Blast Annotation
BlastX similarity
percentage
and best hit
organism
AT4G19230,
AT2G29090,
AT5G45340,
AT3G19270
ABA 8’-hydroxylase
(ABA8ox)
Abscisic acid
pathway
22 5 ABA 8’-hydroxylase
(ABA8ox) [Singleton1,
Singleton2]
Singleton1 HP733304.1 ABA 8’-hydroxylase 86% Citrus
sinensis
Singleton2 HP764411.1 PREDICTED: abscisic acid
8’-hydroxylase 4
83% Vitis
vinifera
AT1G52400 ABA glucosidase 12 6 ABA glucosidase
[Contig1]
Contig1 KA279844.1,
KA279587.1,
HP733896.1,
GH710784.1,
GH710770.1,
FE942881.1
beta-glucosidase-like protein 99% Camellia
sinensis
AT2G27150 abscisic aldehyde oxidase
(ABAO)
6 1 abscisic aldehyde
oxidase (ABAO)
[Contig1]
Contig1 KA288987.1,
HP767578.1,
KA286688.1,
KA282538.1,
HP727479.1,
HP770168.1
PREDICTED: aldehyde oxidase
4-like
87% Vitis
vinifera
AT1G16540 molybdenum cofactor
sulfurase
3 2 molybdenum cofactor
sulfurase [Contig1]
Contig1 HP742704.1,
JK475554.1
PREDICTED: molybdenum
cofactor sulfurase-like
70% Vitis
vinifera
AT4G18350,
AT3G14440,
AT1G30100,
AT3G24220,
AT1G78390
9-cis-epoxycarotenoid
dioxigenase (NCED)
18 4 NCED [Singleton1,
Contig2]
Singleton1 HP727751.1 9-cis-epoxycarotenoid
dioxygenase 1
88% Diospyros
kaki
Contig2 HP765237.1,
BJ999395.1
putative 9-cis epoxycarotenoid
dioxygenase
88% Daucus
carota subsp.
sativus
AT1G67080 neoxanthin synthase
(NSY)
2 1 NSY[Contig1] Contig1 KA283257.1,
HP702818.1
neoxanthin synthase 73% Citrus
sinensis
AT1G52340 xanthoxin dehydrogenase
(XD)
7 6 XD[Contig1] Contig1 HP701207.1,
JK341963.1
short chain alcohol
dehydrogenase
79% Citrus
sinensis
AT5G67030 zeaxanthin epoxidase
(ZEP)
4 1 ZEP[Contig1] Contig1 HP760589.1,
HP739212.1,
HP727722.1
zeaxanthin epoxidase 1 82% Vitis
vinifera

Madhurjya Gogoi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES 201
AT3G45140 13-lipoxygenase/LOX2 Jasmonic acid
pathway
20 5 LOX2[Contig1] Contig1 KA280187.1,
DY523322.1,
HP756288.1,
FE942952.1
lipoxygenase 99% Camellia
sinensis
AT4G16760,
AT5G65110,
AT1G06290,
AT3G51840,
AT2G35690
acyl-CoA oxidase 38 13 ACX[Contig4, Contig5] Contig4 FE943071.1,
KA288172.1,
KA280456.1,
HP709220.1,
HP724420.1 ,
HP740917.1,
HS399721.1,
KA280456.1
Peroxisomal acyl-coenzyme A
oxidase 1-like
70% Nicotiana
tomentosiformis
Contig5 HP754805.1,
KA298085.1,
KA300729.1
acyl-coenzyme A oxidase 2 85%
Brassica oleracea
var. oleracea
AT3G25760,
AT3G25770,
AT3G25780
allene oxide cyclase 46 5 AOC[Singleton1,
Singleton2]
Singleton1 HP769131.1 allene oxide cyclase 74% Camellia
sinensis
Singleton2 KA282377.1 allene oxide cyclase 99% Camellia
sinensis
AT5G42650 allene oxide synthase
(CYP74A1)
4 3 AOS[Contig1] Contig1 KA283779.1,
HP717071.1
cytochrome P450 allene oxide
synthase
77% Populus
trichocarpa
AT5G07010 hydroxyjasmonic acid
sulfotransferase
5 4 hydroxyjasmonic
acid sulfotransferase
(AtST2a)[Contig1]
Contig1 KA297677.1,
HP755751.1
PREDICTED: avonol
sulfotransferase-like
74% Vitis
vinifera
AT1G19640 jasmonic acid carboxyl
methyltransferase
1 NA NA KA286401.1 KA286401.1 Jasmonate O-methyltransferase,
putative
67% Ricinus
communis
AT2G46370 jasmonic acid-amino acid
synthase
6 4 JAR[Contig1] Contig1 HP705426.1,
KA286035.1
JAR1-like protein 80% Nicotiana
attenuata
Keyword
Search
OPC-8:0-CoA ligase NA NA NA NA FS957551.1 OPC-8:0-CoA ligase NA
AT1G20510 OPC-8:0-CoA ligase 6 4 OPCL1[Contig1] Contig1 HP750023.1
,KA293135.1,
KA286488.1
4-coumarate--CoA ligase-like 5 76%
Sesamum
indicum
AT2G06050 OPDA reductase 9 2 OPR3[Contig1] Contig1 HP743074.1,
KA280376.1,
HS399901.1,
HP725651.1,
HP746582.1,
HS398253.1,
HS398153.1,
KA302045.1
PREDICTED:
12-oxophytodienoate reductase
3
82% Vitis
vinifera
Madhurjya Gogoi et al.
202
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
NCED gene expression there was signi cant main effect
for time post infestation and interaction effect between
water stress treatment and time post infestation whereas
for AOS, ICS and ACC synthase gene expression both
the main effect (water stress treatment, time post infes-
tation) and there interaction effect was signi cant (Fig
2 A- D). The homogeneity and speci city of the sin-
gle PCR product was determined by the melting curve
and melting peak of the four analyzed genes and are
provide in Supplementary Fig. 3 (A-D), Supplementary
Fig. 4 (A-D) For supplementary data please see:https://
drive.google.com/drive/folders/1qVqzAqW1IEg-
kKnh6RA75zkdVIUxx0xH?usp=sharing.
It can be seen that as the water stress (0 TPI) increased
the transcript abundance of NCED gene also increased
(Fig.2 (A)). Water stress is known to increase the expression
of NCED gene followed by accumulation of ABA (Shino-
zaki and Yamaguchi-Shinozaki, 2007; Wang et al., 2009).
The function of ABA in the control of stomata closure and
the responses to abiotic stress is well-established (Mittler
and Blumwald, 2015). ABA integrates various stress sig-
nals and is known to controls stress responses during water
de cit stress (Raghavendra et al., 2010; Ye et al., 2012).
After insect infestation the expression of NCED gene in
well watered plant showed higher induction than mild and
severe stressed plants (24 TPI) and then there was a drop in
transcript level at 48 TPI for all treatment with well watered
plant retaining the highest transcript abundance.
When the expression folds were analyzed for statisti-
cal signi cance main effect of water stress treatment (T)
on NCED gene expression was not statistically signi cant
(P> 0.05). However the main effect of time post infesta-
tion (TPI) and interaction effect (TxTPI) was signi cant
(P<0.05). Herbivore infestation and the interaction of water
stress treatment and time post infestation signi cantly
regulated the expression differences. The strong induc-
tion of NCED expression in well-watered and mild stressed
plants along with the increase in AOS gene expression of
JA pathway at 24 TPI (Fig. 2(A) , Fig.2(B)) strongly sug-
gest the synergistic role of ABA and JA signaling pathway
genes in plant herbivore defense. On the other hand severe
stressed plant produced a smaller induction for both NCED
and AOS gene. It is very likely that water stress severity
negatively affected the induced response of the ABA and
JA biosynthesis pathway genes on herbivore attack. ABA
have been reported to interact with JA signaling and ena-
bles N. attenuataplants to mount a full defense response
against chewing herbivores (Dinh and Baldwin, 2013).
The expression of AOS, JA pathway rate-limiting
gene, was signi cantly down-regulated in severe water
stressed plants with a value of -2.49 compared to
well watered control plants (0 TPI, P<0.05, Fig. 2 (B)).
Irrespective of water treatment on herbivore infesta-
tion AOS gene expression increased at 24 TPI for all
AT1G18870,
AT1G74710
isochorismate synthase Salicylic acid
pathway
5 2 ICS[Contig1] Contig1 HP734410.1,
KA297692.1,
isochorismate synthase,
putative
69% Ricinus
communis
AT2G23620,
AT2G23600,
AT2G23560,
AT4G37150
methyl salicylate esterase 13 6 Methyl_salicylate_
esterase [Singleton1]
Singleton1 KA281093.1 PREDICTED: polyneuridine-
aldehyde esterase
63% Vitis
vinifera
AT2G43840,
AT2G43820
salicylic acid
glucosyltransferase
14 8 salicylic acid
glucosyltransferase
[Singleton1, Contig2]
Singleton1 KA286158.1 PREDICTED: UDP-
glycosyltransferase 74F2
71% Vitis
vinifera
Contig2 HP768085.1,
KA296966.1
PREDICTED: UDP-
glycosyltransferase 74E1-like
80% Vitis
vinifera
Keyword
Search
1-aminocyclopropane-1-
carboxylate synthase
Ethylene
pathway
NA NA NA NA EF205149.1 1-aminocyclopropane-1-
carboxylate synthase
NA
Keyword
Search
1-aminocyclopropane-1-
carboxylate oxidase
NA NA NA NA DQ904328.1 ACC oxidase NA

Madhurjya Gogoi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES 203
FIGURE 1. (A) Schematic representation of occurrence and distribution of motifs in the ABA
8’-hydroxylase sequences of Arabidopsis and Camellia sinensis - MEME program output (B)
Schematic representation of motifs and sorting of Arabidopsis and Camellia sinensis ABA
8’-hydroxylase sequences by the best combined match to all motifs - MAST program output.
FIGURE 2. Expression of 9-cis-epoxycarotenoid dioxygenase (NCED) gene of ABA pathway (B)
Expression of Allene oxide synthase (AOS) gene of JA pathway (C) Expression of Isochoris-
mate synthase (ICS) gene of SA pathway (D) Expression of 1-aminocyclopropane-1-carboxylate
synthase (ACC synthase) gene of ET pathway. Signi cant main effect of water stress treatment
and time post infestation are indicated by T and TPI respectively, while signi cant interaction
are indicated by TxTPI (P<0.05). All the expression data are calculated relative to well watered
control sample at 0 TPI and are expressed as mean ± standard deviation (SD). Each expression
value is the mean of three biological replicates.
the plants with control showing maximum transcript
abundance. It is noteworthy to mention that severe
stressed plant had maximum induction value when the
induced expression fold change of individual treatment
at 24 TPI is calculated relative to its own expression
at 0 TPI. Wound induced elevated level of AOS gene
expression was found to correlate with the increase in
endogenous JA content (Wilmowicz et al., 2016). JA is
mainly involved in biotic stress response and its role
biotic defense is well established. The detailed molecular
mechanisms of the role of jasmonates for drought stress
signaling are still unclear (Riemann et al., 2015).Plant

Madhurjya Gogoi et al.
204
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
responses to combined abiotic stresses and biotic stress
are largely controlled by different signaling pathways
that may interact and inhibit one another (Suzuki et
al., 2014). In our study the expression of AOS gene of
JA biosynthesis pathway is clearly suppressed in severe
water stressed plants (0 TPI, 24 TPI). High transcript
accumulation of AOS gene in controls and mild water
stressed plants compared to severe stressed plants indi-
cates that water availability play an important role in
stronger constitutive and induced JA pathway defense.
Plants under increasing drought stress may be at dan-
ger of herbivore infestation with lower activation of JA
pathway defense. In some plants, it has been reported
that a speci c abiotic stress enhanced the resistance of
plants to biotic stress (Rouhier and Jacquot, 2008). How-
ever, in most cases, prolonged exposure of plants to abi-
otic stresses, such as drought resulted in the weakening
of plant defenses (Mittler and Blumwald, 2010). Plants
under combinations of abiotic and biotic stresses may
prioritize responses to address the potentially more dam-
aging abiotic stress (Atkinson et al., 2013).
Increase in ICS gene expression at 0 TPI in mild water
stress points towards the role of SA pathway in moderate
water stress condition. SA signi cance has been increas-
ingly recognized in enhanced plant abiotic stress-toler-
ance via SA-mediated control of major plant-metabolic
processes (Khan et al., 2015). Studies extensively found
and reviewed the role of SA pathway in the improve-
ment of plant abiotic stresses tolerance such as drought
(Horváth et al., 2007;Pal et al., 2013; Fayez and Bazaid,
2014; Miura and Tada, 2014). In induced response (24 TPI)
well watered plant maintained higher ICS gene expres-
sion followed by mild and severe water stressed plant. It is
known that upon insect attack usually two signaling path-
ways Salicylic acid (SA) and Jasmonic acid (JA), mediate
plant responses. Severe water stress negatively affected
the ICS gene expression at constitutive and induced level.
The lower expression of SA biosynthesis pathway gene
in water stress tea plant may increase their susceptibil-
ity to herbivores and necrotrophic pathogens. A signi -
cant amount of literature mentioned that the induction of
the SA signaling pathway suppresses JA signaling (Niki
et al., 1998; Preston et al., 1999; Koornneef et al., 2008a,
2008b).
However, in our study, we have seen a completely dif-
ferent picture where SA and JA associated gene expres-
sion increased co-currently in control and mild stressed
plants. Whereas in case of severe stressed plant both the
genes were signi cantly down-regulated. Thus JA and SA
pathway associated gene expression may not always act
antagonistically and may act in synchrony according to
the type and severity of stress it is undergoing. In tea
cultivation, water stress severity is an important factor
which needs to be taken care to avoid disastrous pest and
pathogen attack due to weakening of SA and JA associ-
ated plant defense.
ACC synthase expression showed maximum peak in
sev ere stressed plants at 0 TPI. The increased constitu-
tive expression in water stressed plants may be asso-
ciated with ET signaling role in osmotic stress adjust-
ment. ET signaling is known to act as an important
controller of the hormone-regulated defense pathways
in biotic stress (Broekgaarden et al., 2015) as well as
helping plants to adjust to drought stress (abiotic stress)
by increasing the compatible solutes accumulation (Cui
et al., 2015). Pairwise comparison showed that induced
expression value was not signi cant between control
and severe stressed plant (P >0.05, Fig.2 (D)). However it
was signi cant between control and mild stressed plant
(P< 0.05, Fig.2 (D)). When the fold change at 24 TPI in
all treatment is calculated relative to its own expression
at 0 TPI the increase in well-watered fold induction in
maximum. Thus the increase of ACC synthase expression
in response to herbivore infestation points towards ET
signaling role in biotic stress signaling and well-watered
plant have higher capacity of transcript induction on
insect infestation. Ethylene transcripts mediated role in
plant stress and pathogen responses have been already
reported in literature (Abeles et al., 1992; O’Donnell et
al., 1996). In rice plant, ACC oxidase and ACC synthase
transcript up regulation in response to feeding by the
brown planthopper, Nilaparvata lugens (Stal) have been
documented (Zhang et al., 2004).
Overall from our study it has been seen that water
stress positively affected ABA and ET pathway genes
with increase in constitutive expression. However JA and
SA pathway genes known to be involved with herbivore
and pathogen defense signaling were negatively affected
in severe stressed plants. In case of induced expression
(24 TPI), severe water stressed plants showed signi cant
decrease in expression of the rate-limiting phytohor-
mone genes compared to well water plants except ACC
synthase gene. The crosstalk among the phytohormone
pathway transcriptional defense in combination with
other intrinsic defense mechanism will ultimately gov-
ern the of tea plant response.
The presence of abundant antagonistic or synergistic
interactions among the pathways provide the plant with an
extensive regulatory potential for the activation of speci c
defenses (Vos et al., 2013). When combination of water and
herbivory stress was applied, it seems to be most likely that
plant transcriptional defense machinery was largely con-
trolled by water stress severity and herbivory stress regula-
tion of phytohormone genes was overpowered by water
stress gene regulation. Thus our results clearly shows that
well-watered plants on insect infestation will have higher
capacity to induce the expression of phytohormone genes
than water stressed plants.

Madhurjya Gogoi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES 205
CONCLUSION
It can be concluded that the presence of an initial water
stress not only affected the tea plant constitutive defense
but also signi cantly altered the phytohormone defense
gene expression towards subsequent herbivore stress. On
prolonged drought events tea plant with weak induced
defense may face higher incidence of pest and pathogen
attack.
CONFLICT OF INTEREST
The authors declare that they have no con ict of
interest.
ACKNOWLEDGEMENTS
The authors are grateful to the Director, Tocklai Tea
Research Institute (TTRI), Tea Research Association
(TRA) for providing the necessary facilities to conduct
the research work. The authors also acknowledge the
support and facilities provided by Director In-Charge,
Centre for Biotechnology and Bioinformatics, School of
Science and Engineering, Dibrugarh University for car-
rying out the research activity.
REFERENCES
Abeles, F. (1992) Ethylene in Plant Biology. New York, NY:
Academic Press.
Aimar, D., Calafat, M., Andrade, A. M., Carassay, L., Abdala,
G. I. and Molas, M. L. (2011) Drought tolerance and stress hor-
mones: from model organisms to forage crops. In: H. K. N. Vas-
anthaiah, and D. Kambiranda (eds) Plants and environment.
Rijeka, Croatia: INTECH, pp. 137-164.
doi: 10.5772/24279
Anderegg, W. R. L., Hicke, J. A., Fisher, R. A., Allen, C. D.,
Aukema, J., Bentz, B., Hood, S., Lichstein, J. W., Macalady,
A. K. and McDowell, N. (2015) Tree mortality from drought,
insects, and their interactions in a changing climate. New Phy-
tol, 208(3), 674-683. doi: 10.1111/nph.13477.
Atkinson, N. J. and Urwin, P. E. (2012) The interaction of plant
biotic and abiotic stresses: from genes to the eld. Journal
of Experimental Botany, 63(10), 3523-3543. doi: 10.1093/jxb/
ers100.
Atkinson, N. J., Lilley, C. J. and Urwin, P. E. (2013) Identi -
cation of Genes Involved in the Response of Arabidopsis to
Simultaneous Biotic and Abiotic Stresses. Plant Physiology,
162(4), 2028-2041. doi: 10.1104/113.222372.
Bailey, T. L. and Elkan, C. (1994) Fitting a mixture model by
expectation maximization to discover motifs in biopolymers,
in. California: Proceedings of the Second International Confer-
ence on Intelligent Systems for Molecular Biology. AAAI Press,
Menlo Park, 28-36.
Bailey, T. L. and Gribskov, M. (1998) Combining evidence using
p-values: application to sequence homology searches. Bioin-
formatics, 14(1), 48-54. doi: 10.1093/bioinformatics/14.1.48.
Bhagat, R. M., Deb Baruah, R. and Sa que, S. (2010) Climate
and tea [Camellia sinensis (L.) O. Kuntze] production with
special reference to north eastern India: A review. Journal of
Environmental Research And Development, (4), 1017-1028.
Broekgaarden, C., Caarls, L., Vos, I. A., Pieterse, C. M. and Van
Wees, S. C. (2015) Ethylene: traf c controller on hormonal
crossroads to defense. Plant Physiology, p. 01020.2015. doi:
10.1104/15.01020.
Chen, L. and Yu, D. (2014) ABA Regulation of Plant Response to
Biotic Stresses. In: D.P. Zhang, (ed.)Abscisic acid: Metabolism,
transport and signaling. Dordrecht, Netherlands: Springer, pp.
409-429. doi: 10.1007/978-94-017-9424-4_20
Chen, Z. and Chen, L. (2012) Delicious and Healthy Tea: An
Overview. In: L. Chen, Z. Apostolides, and Z. Chen (eds)Global
tea breeding: Achievements, challenges and perspectives.
Berlin Heidelberg, New York: Springer-Verlag, pp. 1-11.
doi:10.1007/978-3-642-31878-8_1
Conesa, A. and Götz, S. (2008) Blast2GO: A Comprehensive Suite
for Functional Analysis in Plant Genomics. International Jour-
nal of Plant Genomics, 2008, 1-12. doi: 10.1155/2008/619832.
Creelman, R. A. and Mullet, J. E. (1995) Jasmonic acid dis-
tribution and action in plants: regulation during develop-
ment and response to biotic and abiotic stress. Proceedings
of the National Academy of Sciences, 92(10), 4114-4119. doi:
10.1073/pnas.92.10.4114.
Cui, M., Lin, Y., Zu, Y., Efferth, T., Li, D. and Tang, Z. (2015)
Ethylene increases accumulation of compatible solutes and
decreases oxidative stress to improve plant tolerance to water
stress in Arabidopsis. J. Plant Biol., 58(3), 193-201. doi:
10.1007/s12374-014-0302-z.
Dinh, S. T., Baldwin, I. T. and Galis, I. (2013) The Herbi-
vore elicitor-regulated1 Gene Enhances Abscisic Acid Lev-
els and Defenses against Herbivores in Nicotiana attenuata
Plants. Plant Physiology, 162(4), 2106-2124. doi: 10.1104/113.
221150.
Eyidogan, F., Oz, M. T., Yucel, M. and Oktem, H. A. (2012) Sig-
nal Transduction of Phytohormones Under Abiotic Stresses. In:
N. A. Khan, R. Nazar, N. Iqbal, and N. A. Anjum(eds)Phytohor-
mones and abiotic stress tolerance in plants. Berlin Heidelberg,
New York: Springer-Verlag, pp. 1-48. doi:
10.1007/978-3-642-
25829-9_1
Fayez, K. A. and Bazaid, S. A. (2014) Improving drought and
salinity tolerance in barley by application of salicylic acid and
potassium nitrate. Journal of the Saudi Society of Agricultural
Sciences, 13(1), 45-55. doi: 10.1016/j.jssas.2013.01.001.
Fraire-Velá zquezS., Sá nchez-Calderó nL. and Rodrí guez-
GuerraR. (2011) Abiotic and Biotic Stress Response Crosstalk
in Plants. In: A. Shanker, and B. Venkateswarlu (eds)Abiotic
Stress Response in Plants—Physiological, Biochemical and
Genetic Perspectives. Rijeka, Croatia: INTECH, pp. 3-26.
doi:
10.5772/23217

Madhurjya Gogoi et al.
206
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Gaille, C., Reimmann, C. and Haas, D. (2003) Isochorismate
Synthase (PchA), the First and Rate-limiting Enzyme in Salicy-
late Biosynthesis of Pseudomonas aeruginosa. J. Biol. Chem.,
278(19), 16893-16898. doi: 10.1074/jbc.m212324200.
Gamalero, E. and Glick, B. R. (2012) Ethylene and abiotic
stress tolerance in plants. In: P. Ahmad and M. N. V. Prasad
(eds) Environmental Adaptations and Stress Tolerance of
Plants in the Era of Climate Change. New York: Springer, pp.
395-412.
doi: 10.1007/978-1-4614-0815-4_18
Gao, Q.-M., Zhu, S., Kachroo, P. and Kachroo, A. (2015) Signal
regulators of systemic acquired resistance. Front. Plant Sci.,
06. doi: 10.3389/fpls.2015.00228.
Hammond-Kosack, K. E. and Jones, J. D. G. (2000) Response to
plant pathogens. In: B., Gruissem, W. Buchannan, and R. Jones
(eds)Biochemistry and molecular biology of plants. Rockville,
MD: American Society of Plant Physiologists, Wiley, pp. 1102-
1157.
Harms, K., Atzorn, R., Brash, A., Kuhn, H., Wasternack, C.,
Willmitzer, L. and Pena-Cortes, H. (1995) Expression of a Flax
Allene Oxide Synthase cDNA Leads to Increased Endogenous
Jasmonic Acid (JA) Levels in Transgenic Potato Plants but Not
to a Corresponding Activation of JA-Responding Genes. The
Plant Cell, 7(10), p. 1645. doi: 10.2307/3870026.
Horváth, E., Szalai, G. and Janda, T. (2007) Induction of Abiotic
Stress Tolerance by Salicylic Acid Signaling. J Plant Growth
Regul, 26(3), 290-300. doi: 10.1007/s00344-007-9017-4.
Huang, D., Wu, W., Abrams, S. R. and Cutler, A. J. (2008) The
relationship of drought-related gene expression in Arabidop-
sis thaliana to hormonal and environmental factors. Journal
of Experimental Botany, 59(11), 2991-3007. doi: 10.1093/jxb/
ern155.
Khan, M. I. R., Fatma, M., Per, T. S., Anjum, N. A. and Khan,
N. A. (2015) Salicylic acid-induced abiotic stress tolerance and
underlying mechanisms in plants. Front. Plant Sci., 6. doi:
10.3389/fpls.2015.00462.
Koornneef, A. and Pieterse, C. M. (2008) Cross Talk in
Defense Signaling. Plant Physiology, 146(3), 839-844. doi:
10.1104/107.112029.
Koornneef, A., Leon-Reyes, A., Ritsema, T., Verhage, A., Den
Otter, F. C., Van Loon, L. and Pieterse, C. M. (2008) Kinetics
of Salicylate-Mediated Suppression of Jasmonate Signaling
Reveal a Role for Redox Modulation. Plant Physiology, 147(3),
1358-1368. doi: 10.1104/108.121392.
Lu, S., Su, W., Li, H. and Guo, Z. (2009) Abscisic acid improves
drought tolerance of triploid Bermuda grass and involves
H2O2- and NO-induced antioxidant enzyme activities. Plant
Physiology and Biochemistry, 47(2), 132-138. doi: 10.1016/j.
plaphy.2008.10.006.
Melotto, M., Underwood, W., Koczan, J., Nomura, K. and He, S.
Y. (2006) Plant Stomata Function in Innate Immunity against
Bacterial Invasion. Cell, 126(5), 969-980. doi: 10.1016/j.
cell.2006.06.054.
Mittler, R. and Blumwald, E. (2010) Genetic Engineering for
Modern Agriculture: Challenges and Perspectives. Annu. Rev.
Plant Biol., 61(1), 443-462. doi: 10.1146/annurev-arplant-
042809-112116.
Mittler, R. and Blumwald, E. (2015) The Roles of ROS and ABA
in Systemic Acquired Acclimation. Plant Cell, 27(1), 64-70.
doi: 10.1105/tpc.114.133090.
Miura, K. and Tada, Y. (2014) Regulation of water, salinity, and
cold stress responses by salicylic acid. Front. Plant Sci., 5. doi:
10.3389/fpls.2014.00004.
Niki, T., Mitsuhara, I., Seo, S., Ohtsubo, N. and Ohashi, Y.
(1998) Antagonistic Effect of Salicylic Acid and Jasmonic Acid
on the Expression of Pathogenesis-Related (PR) Protein Genes
in Wounded Mature Tobacco Leaves. Plant and Cell Physiol-
ogy, 39(5), 500-507. doi: 10.1093/oxfordjournals.pcp.a029397.
O’Donnell, P. J., Calvert, C., Atzorn, R., Wasternack, C., Leyser,
H. M. O. and Bowles, D. J. (1996) Ethylene as a Signal Mediat-
ing the Wound Response of Tomato Plants. Science, 274(5294),
1914-1917. doi: 10.1126/science.274.5294.1914.
Osakabe, Y., Yamaguchi-Shinozaki, K., Shinozaki, K. and Tran,
L.-S. P. (2013) ABA control of plant macro element membrane
transport systems in response to water de cit and high salin-
ity,New Phytol., 202(1), 35-49. doi: 10.1111/nph.12613.
Pal, M., Szalai, G., Kovacs, V., Gondor, O. K. and Janda, T.
(2013) Salicylic acid-mediated abiotic stress tolerance. In: S.
Hayat, A. Ahmed, and M. N. Alyemeni (eds) Salicylic Acid
Plant Growth and Development. Rotterdam, Netherlands:
Springer, pp. 183–247. doi: 10.1007/978-94-007-6428-6_10
Preston, C. A., Lewandowski, C., Enyedi, A. J. and Bald-
win, I. T. (1999) Tobacco mosaic virus inoculation inhibits
wound-induced jasmonic acid-mediated responses within
but not between plants. Planta, 209(1), 87-95. doi: 10.1007/
s004250050609.
Qin, X. and Zeevaart, J. A. D. (1999) The 9-cis-epoxycarot-
enoid cleavage reaction is the key regulatory step of absci-
sic acid biosynthesis in water-stressed bean. Proceedings of
the National Academy of Sciences, 96(26), 15354-15361. doi:
10.1073/pnas.96.26.15354.
Raghavendra, A. S., Gonugunta, V. K., Christmann, A. and
Grill, E. (2010) ABA perception and signaling. Trends in Plant
Science, 15(7), 395-401. doi: 10.1016/j.tplants.2010.04.006.
Rejeb, I., Pastor, V. and Mauch-Mani, B. (2014) Plant Responses
to Simultaneous Biotic and Abiotic Stress: Molecular Mecha-
nisms. Plants, 3(4), 458-475. doi: 10.3390/plants3040458.
Riemann, M., Dhakarey, R., Hazman, M., Miro, B., Kohli, A. and
Nick, P. (2015) Exploring Jasmonates in the Hormonal Network
of Drought and Salinity Responses. Front. Plant Sci., 6. doi:
10.3389/fpls.2015.01077.
Rivas-San Vicente, M. and Plasencia, J. (2011) Salicylic acid
beyond defence: its role in plant growth and development.
Journal of Experimental Botany, 62(10), 3321-3338. doi:
10.1093/jxb/err031.
Rouhier, N. and Jacquot, J.-P. (2008) Getting sick may help
plants overcome abiotic stress. New Phytol, 180(4), 738-741.
doi: 10.1111/j.1469-8137.2008.02673.x.

Madhurjya Gogoi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
IN-SILICO
IDENTIFICATION OF PHYTOHORMONE PATHWAY GENES 207
Serino, L., Reimmann, C., Baur, H., Beyeler, M., Visca, P. and
Haas, D. (1995) Structural genes for salicylate biosynthesis
from chorismate in Pseudomonas aeruginosa. Molec. Gen.
Genet., 249(2), 217-228. doi: 10.1007/bf00290369.
Shinozaki, K. and Yamaguchi-Shinozaki, K. (2007) Gene net-
works involved in drought stress response and tolerance. Jour-
nal of Experimental Botany, 58(2), 221-227. doi: 10.1093/jxb/
erl164.
Sivasankar, S., Sheldrick, B. and Rothstein, S. J. (2000) Expres-
sion of Allene Oxide Synthase Determines Defense Gene Acti-
vation in Tomato. Plant Physiol., 122(4), 1335-1342. doi:
10.1104/122.4.1335.
Suzuki, N., Rivero, R. M., Shulaev, V., Blumwald, E. and Mittler,
R. (2014) Abiotic and biotic stress combinations. New Phytol,
203(1), 32-43. doi: 10.1111/nph.12797.
Tan, B. C., Schwartz, S. H., Zeevaart, J. A. D. and McCarty, D. R.
(1997) Genetic control of abscisic acid biosynthesis in maize.
Proceedings of the National Academy of Sciences, 94(22),
12235-12240. doi: 10.1073/pnas.94.22.12235.
Usha Rani, P. and Jyothsna, Y. (2010) Biochemical and enzy-
matic changes in rice plants as a mechanism of defense. Acta
Physiol Plant, 32(4), 695-701. doi: 10.1007/s11738-009-0449-
2.
Verma, V., Ravindran, P. and Kumar, P. P. (2016) Plant hor-
mone-mediated regulation of stress responses. BMC Plant Biol,
16(1). doi: 10.1186/s12870-016-0771-y.
Vos, I. A., Pieterse, C. M. J. and van Wees, S. C. M. (2013)
Costs and bene ts of hormone-regulated plant defenses. Plant
Pathol, 62, 43-55. doi: 10.1111/ppa.12105.
Wang, K. L.-C., Li, H. and Ecker, J. R. (2002) Ethylene Bio-
synthesis and Signaling Networks. Plant Cell, 14(suppl 1),
S131-S151. doi: 10.1105/tpc.001768.
Wang, X., Wang, Z., Dong, J., Wang, M. and Gao, H. (2009)
Cloning of a 9-cis-epoxycarotenoid dioxygenase gene and
the responses of Caragana korshinskii to a variety of abiotic
stresses. Genes Genet. Syst., 84(6), 397-405. doi: 10.1266/
ggs.84.397.
Wani, S. H., Kumar, V., Shriram, V. and Sah, S. K. (2016) Phy-
tohormones and their metabolic engineering for abiotic stress
tolerance in crop plants. The Crop Journal, 4(3), 162-176. doi:
10.1016/j.cj.2016.01.010.
War, A. R., Paulraj, M. G., Ahmad, T., Buhroo, A. A., Hus-
sain, B., Ignacimuthu, S. and Sharma, H. C. (2012) Mechanisms
of plant defense against insect herbivores. Plant Signaling &
Behavior, 7(10), 1306-1320. doi: 10.4161/psb.21663.
War, A. R., Paulraj, M. G., War, M. Y. and Ignacimuthu, S.
(2011) Herbivore- and Elicitor- Induced Resistance in Ground-
nut to Asian armyworm, Spodoptera litura (Fab.) (Lepidoptera:
Noctuidae). Plant Signaling & Behavior, 6(11), 1769-1777. doi:
10.4161/psb.6.11.17323.
War, A. R., Paulraj, M. G., War, M. Y. and Ignacimuthu, S. (2011)
Jasmonic Acid-Mediated-Induced Resistance in Groundnut
(Arachis hypogaea L.) Against Helicoverpa armigera (Hubner)
(Lepidoptera: Noctuidae). J Plant Growth Regul, 30(4), 512-
523. doi: 10.1007/s00344-011-9213-0.
Wheeler, D. L. (2003) Database resources of the National Center
for Biotechnology.
Nucleic Acids Research, 31(1), 28-33. doi:
10.1093/nar/gkg033.
Wijeratne, M., Anandacoomaraswamy, A., Amarathunga, M.,
Ratnasiri, J., Basnayake, B. and Kalra, N. (2007) Assessment
of impact of climate change on productivity of tea (Camellia
sinensis L.) plantations in Sri Lanka. J. Natn. Sci. Foundation
Sri Lanka, 35(2), p. 119. doi: 10.4038/jnsfsr.v35i2.3676.
Wilmowicz, E., Ku
c
´ko, A., Frankowski, K., Zabrocka-Nowa-
kowska, B., Panek, K. and Kopcewicz, J. (2016) Wounding
stimulates Allene Oxide Synthase gene and increases the level
of jasmonic acid in Ipomoea nil cotyledons. Acta Soc Bot Pol,
85(1). doi: 10.5586/asbp.3491.
Ye, M., Luo, S. M., Xie, J. F., Li, Y. F., Xu, T., Liu, Y., Song, Y.
Y., Zhu-Salzman, K. and Zeng, R. S. (2012) Silencing COI1 in
Rice Increases Susceptibility to Chewing Insects and Impairs
Inducible Defense. PLoS ONE, 7(4), p. e36214. doi: 10.1371/
journal.pone.0036214.
Zaman, A., Gogoi, M., Borchetia, S., Kalita, M. C., Yadav, R.
N. S. and Bandyopadhyay, T. (2016) Comparative assessment
of different protocols for isolation of total RNA from vari-
ous organs of the tea plant (Camellia sinensis), RJLBPCS, 2(3),
95-106. doi: 10.26479/2016.0203.09.
Zhang, F., Zhu, L. and He, G. (2004) Differential gene expres-
sion in response to brown plant hopper feeding in rice. Journal
of Plant Physiology, 161(1), 53-62. doi: 10.1078/0176-1617-
01179.