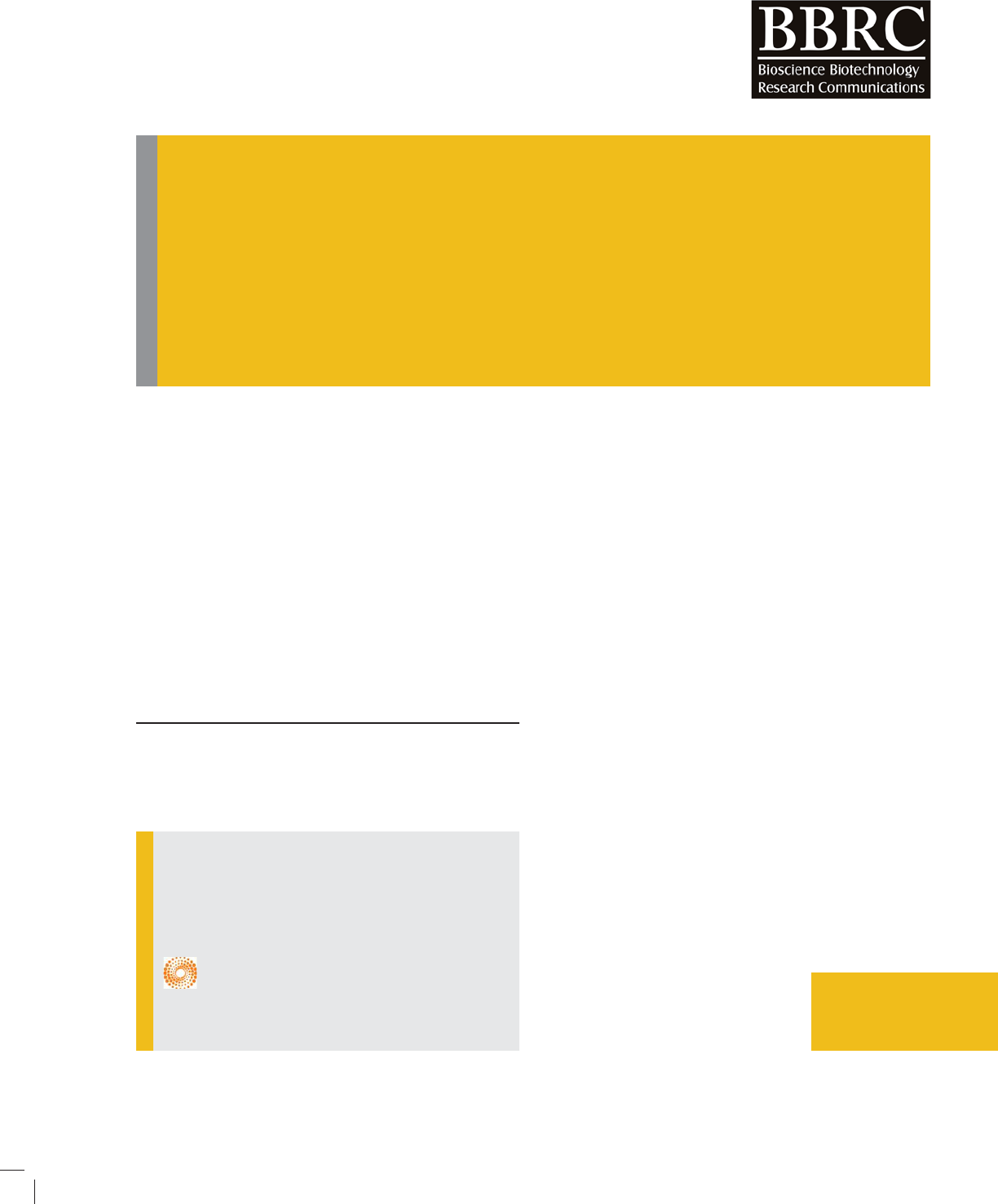
Medical
Communication
Biosci. Biotech. Res. Comm. 10(1): 151-160 (2017)
Preparation methods of nanoliposomes containing
Zataria
multi ora
essential oil: A comparative study
Mohammad Hosein Arabi
1
, Azam Mirzapour
1
, Hora Chabok
1
, Mehdi Sha ee
Ardestani
2
and Mostafa Saffari
3
*
1
Department of Biochemistry, School of Medicine, Kashan Uiversity of Medical Sciences, Kashan, Iran
2
Department of Radiopharmacy, School of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
3
Herbal Medicine Research Center & Department of Pharmaceutics, School of Pharmacy, Branch of
Pharmaceutical Sciences. Islamic Azad Universty, Tehran, Iran
ABSTRACT
Nowadays there is a substantial interest on the biological activities of essential oils. Zataria multi ora essential oil
has a wide spectrum of pharmacological activities. However, essential oils are unstable and susceptible to degradation
by oxygen, light and temperature and also low penetration in transdermal administration. Therefore, nanoliposo-
mal systems can be used as promising strategies to overcome these limitations due to their unique set of properties:
Nanoliposomes were prepared via two different methods based on thin lm hydration method, including extrusion
and sonication methods. The physical properties of nanoliposomes such as particle size, polydispersity index, zeta
potential, encapsulation ef ciency and stability were also determined. In comparison of two methods, sonicated
nanoliposomes had smaller mean particle size and better dispersity while mean encapsulation ef ciency in extruded
ones were higher. It can be deducted by consideration of signi cance level; physicochemical properties of the vesicles
were strongly in uenced by essential oil and cholesterol concentration besides the preparation method.
KEY WORDS: NANOLIPOSOME; ZATARIAMULTIFLORA; ESSENTIAL OIL; THIN FILM HYDRATION; SONICATION
151
ARTICLE INFORMATION:
*Corresponding Author: mostafa.saffary@gmail.com
Received 19
th
Jan, 2017
Accepted after revision 22
nd
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Zataria multi ora is one of the lamiacea family herbs
with remarkable pharmacological properties. It natively
grows in Iran, Pakistan, and Afghanistan (Saedi Dezaki
et al., 2016). It has various therapeutic effects such as
antiseptic, antispasmodic, carminative, expectorant, anti-
in ammatory, antiparasitic, spasmolytic, antiviral, anti-
bacterial, antifungal and antioxidant properties (Sunar et
al., 2009).
Most of these properties are related to the main

152 PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mohammad Hosein Arabi et al.
constituents of its essential oils, which are thymol and
carvacrol and signi cant quantities of phenolic monoter-
penes (Misaghi & Basti., 2007; Ali et al., 2000). Essential
oils are con rmed to possess wide spectrum of pharma-
cological properties, besides their classical roles as natu-
ral food additives, such as the antioxidant, antibacterial,
antifungal and anti-in ammatory activities (Shahsavari
et al., 2008; Edris, 2007). Unfortunately, essential oils are
biologically unstable, poorly soluble in water and are
very sensitive to environment (Martin et al., 2007). All
these obstacles restrict the application of essential oils as
candidates for pharmatherapeutic treatments. Currently,
nanoencapsulation of these oils in drug delivery systems,
among which is the use of liposomal encapsulation,
have
been proposed to improve solubility, stability and ef cacy
of essential oil-based formulations (Belay et al., 2011; Saf-
fari
a
et al., 2016).
Liposomes represent versatile and progressive nano
delivery system for extensive range of biologically active
compounds (Hofheinz et al., 2005). Liposome is a lipoidal
vesicle composed of a bilayer membrane that have been
developed to deliver drug to speci c site in the body for
more than four decades (Chetanachan et al., 2008); El-
Samaligy et al., 2006). Because of biodegradation, non-
toxicity, biocompatibility, non-immunogenicity,
as well
as superior ef cacy, they are excellent carrier systems for
a variety of applications, and in particular for essential
oils (Sinico et al., 2005; Liolios et al., 2009; Saffari
b
et al.,
2016). Encapsulation in liposome protect the essential oil
from light, air and humidity, increases the solubility of
the oil , enhancing the bioavailability of this drug, drug-
targeting and makes the oil easier to handle (Wen et al.,
2010; Valenti et al., 2001). The aim of this study was to
prepare phosphatidyl choline based nanoliposomes via
optimization the concentrations of essential oil, choles-
terol and lipid composition through different methods for
encapsulating zataria multi ora essential oil. Furthermore,
to compare these methods via physicochemical properties
of nanoliposomes including size, PDI index, zeta potential,
encapsulation ef ciency and drug release pro le.
MATERIALS AND METHODS
Lecithin, Egg PC, DOTAP were purchased from LIPOID
(Germany). Cholesterol was from Sigma-Aldrich Co.
The solvents (chloroform, methanol and ethanol) were
purchased from commercial source and were in analyti-
cal grade. The essential oils were obtained from Barij
essence Co. in Kashan, Iran.
PREPARATION OF LIPOSOME
Liposomes can be formulated and processed to vary in
size, composition and charge (Akbarzadeh et al., 2013).
For production of different types of liposomes, many
methods are available (Meure et al., 2008). All Prepa-
ration methods can be simpli ed as to involve three
basic steps: 1) dissolve the lipids in aqueous media in
order to forming liposomes. 2) Puri cation of prepared
liposomes. 3) Analysis of nal product. Liposomes in our
study prepared with three different methods as describe
here:
1. Thin lm hydration
The most conventional and classical method, known
by hydration of dried phospholipid lms (Bangham et
al., 1965). Multilamellar vesicles (MLVs) were prepared
based on the thin lm hydration method. Brie y the
precise amount of lecithin, cholesterol and essential oil,
according to several ratios of essential oil to total lipids,
molar ratio of cholesterol to lecithin and lipid compo-
sition (DOTAP), were dissolved in chloform-methanol
(2:1, v/v) in a 500 ml round-bottomed ask. The organic
solvent was eliminated by rotary evaporator (heidolph
Hei-VAP Germany) under reduced pressure and high
vacuum for 2 hour until a thin lm was formed on the
walls (above the lipid phase transition temperature, Tc).
Then the obtained lipid lm was dispersed in Phosphate
Buffer Saline solution (PBS, pH=7.4). This suspension
was allowed to hydrate for 1.5 hour. Finally milky white
suspension is formed. During the process, the conditions
such as speed (120 rpm) and temperature (above the Tc
of lecithin) for conventional liposomes were maintained
constant.
a. Concentration of essential oil
The total lipid concentration to prepare liposomes was
50 mM. Different ratios of essential oil to total lipid (1/2,
1/3, 1/4) with a constant molar ratio of cholesterol to
phosphatidyl choline (1: 1) were prepared.
b. Concentration of cholesterol
After nding optimum concentration of essential oil
to total lipid, cholesterol concentration was optimized
by various concentrations as 7.1, 6.4, 4.83 mg/ml on a
weight basis and 1:1, 1:2, 1:3 (molar ratio of Cholesterol:
PC) in the overall formulation.
c. Charge-inducing lipids
EO-loaded cationic liposomes were formulated with pc,
1,2-dioleoyl-3-trimethylammonium-propane (DOTAP),
and cholesterol at a 1:1:1 molar ratio of E80: DOTAP:
CH.
2. Sonication method
Sonication is a simple method for reducing the size of
liposomes to the nanoscale and manufacture of nanoli-
posomes. Liposomal suspension of MLVs transferred

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL 153
Mohammad Hosein Arabi et al.
to a tube and placed in ice bath. Then the suspension
was sonicated using prob sonicator (Hielscher UP400S,
Germany).Vertically, the probe of a sonicator was fully
immersed in the middle of the volume. The liposomal
suspension was exposed to ultrasonic irradiation with
an output of 70 Watt amplitude and duration of continu-
ous 20 minute (6 times for 5 min). The energy input into
liposomal dispersion is very high in this method.
3. Extrusion method
To obtain large unilamellar vesicles, the liposomal dis-
persion up to 3 mL was extruded three times through
a polycarbonate membrane (100, 200 nm pore size)
at above the lipid transition temperature by using an
extruder to give a translucent solution.
Different formulation are listed in table 1 based on
their method of preparation and their components and
composition.
Puri cation of Liposomes
Entrapped vesicles were separated from non-entrapped
vesicles using centrifugation method (Shashi et al.,
2012). Vesicular dispersions were centrifuged at 10000
rpm for 10 minutes (laboratory centrifuge Hettich Uni-
versal 320 R). The supernatant was removed and the
liposomes were reconstituted with PBS buffer. Concen-
tration of essential oil in both fractions was determined.
PHYSICOCHEMICAL CHARACTERIZATION OF
LIPOSOMES
Particle Size distribution
Mean vesicle size and polydispersity index (PDI) was
determined by using Zetasizer (nanoZs Malvern ZEN
3600), which the principle is based on the Brownian
motion of particles in medium. Dynamic light scattering
is a simple and rapid method to determine the particle
size and size distribution of liposomes.
Zeta potential () determination
Zeta potential is another important factor that is respon-
sible for a description of the liposome surface charge and
predicts the stability. Charge on loaded vesicles surface
and average zeta potential was determined using Zeta-
sizer ZEN 360 Malvern Instruments (Bhatia et al., 2004).
Imaging of liposomes by Scanning Electron Microscopy
(SEM)
The shape and morphology of drug-loaded liposomal
formulation were observed using a KYKY-EM3200 scan-
ning electron microscopy (SEM, KYKY Instruments,
China). Sample was dispersed on glass slide and gold
paste used as lament and then viewed using an acceler-
ating voltage of 20 kilovolt at different magni cations.
Images of liposomes exhibited the diameter and
size of vesicles and this is in agreement with the
results of Zetasizer
(Figure 4).
Evaluation of the Encapsulation ef ciency
In case of puri ed liposomal suspension, after the cen-
trifugation, by the sediment, the quantity of essential
oil was measured using a UV Visible Spectrophotometer
at = 270 nm. The encapsulation ef ciency (EE %) of
essential oil was calculated using the following formula:
S3/ L3
Encapsulation Ef ciency = ---------- × 100
S2/ L2
Which in this equation; S3= EO amount in sediment &
L3= phospholipid amount in sediment,
S2= EO amount before centrifuged & L2= phospho-
lipid amount before centrifuged
In vitro essential oil release studies
Release studies were performed using dialysis membrane
method. In vitro release was done for selected formula-
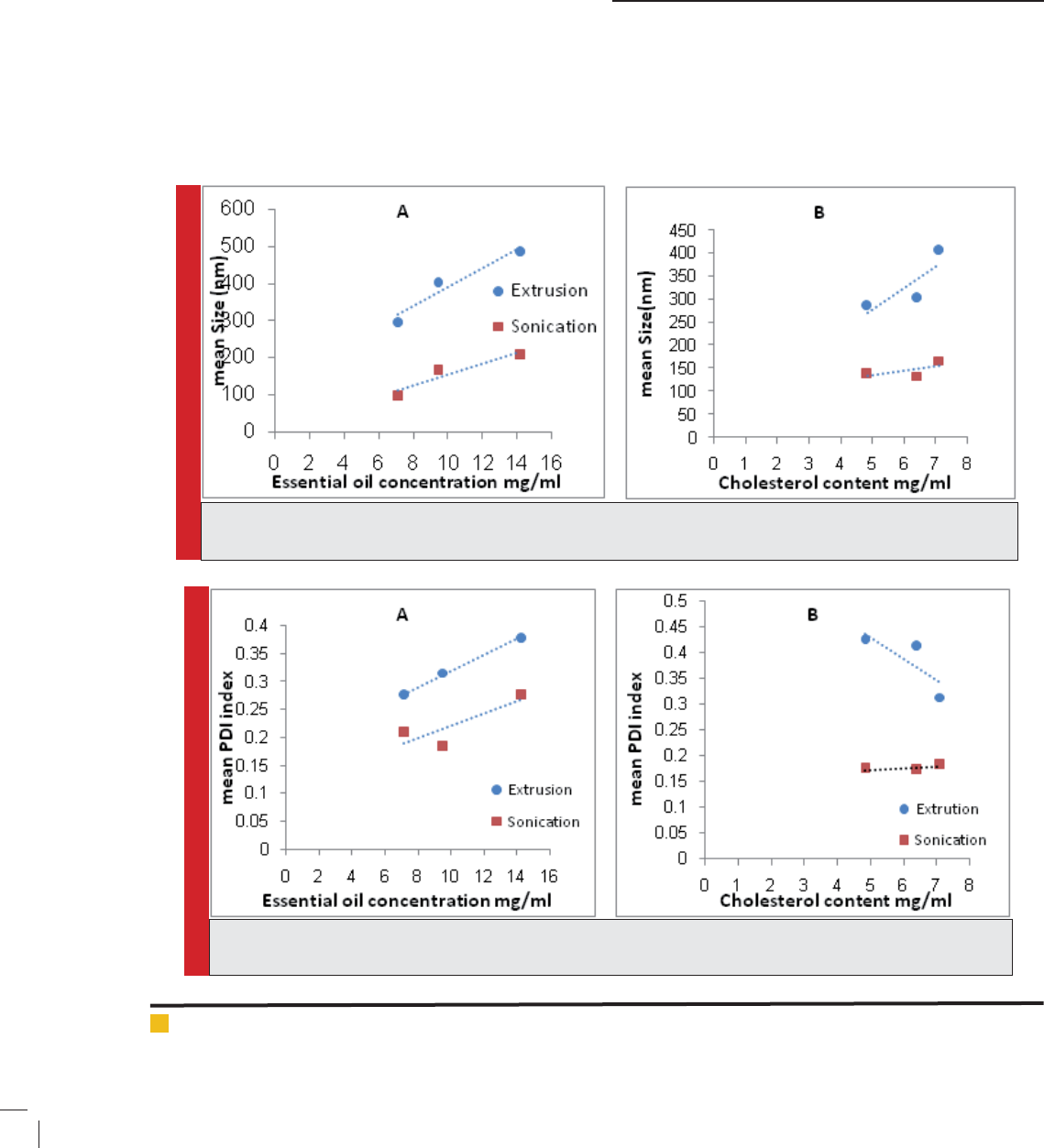
Table 1. formulation based on method of preparation and composition
Method Composition EO /Total lipid CH:PC
Thin lm hydration
PC+CH+EO
PC+CH+EO
0.5, 0.33,0.25
0.33
0.5, 0.33, 0.25
0.33
0.5, 0.33, 0.25
0.33
1:1
1:2,1:3
1:1
1:2,1:3
1:1
1:2,1:3
Sonication
PC+CH+EO
PC+CHO+EO
Extrusion
PC+CH+EO
PC+CH+EO
PC: phosphatidyl choline; CH: cholesterol; EO: essential oil
Mohammad Hosein Arabi et al.
154 PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 1. A is effect of essential oil concentration on Size of nanoliposomes and B is effect of cholesterol content
on Size of nanoliposomes
FIGURE 2. A is effect of essential oil concentration on PDI index and B is effect of cholesterol content on PDI
index of nanoliposomes
tion. In brief, 1000 μL of the 7.1 mg/mL essential oil
encapsulated liposome suspension was added in a dialy-
sis bag (MWCO 12kDa, Thermo Fisher Scienti c). The
dialysis system was suspended in a release volume of 100
mL PBS at 25°C and rotated at 100 rpm (1:100 dilution
relation between donor and acceptor compartments). At
scheduled intervals, 1 ml of the release medium was col-
lected for the UV- spectrophotometric assay. The same
volume of fresh PBS buffer at the same temperature was
added immediately to maintain constant release volume.
The length of the dialysis tubing was kept consistent
for all methods to ensure that the surface area available
for dialysis remained constant. To ensure that dilution
between the donor and acceptor compartments provided
sink conditions, a 1:100 dilution study was conducted
and release volume was set at 100 mL PBS pH 6.5.
STATISTICAL ANALYSIS
Result has been reported as mean ± standard deviation
of three times repetition assays for each method. The
mean values compared by T-Test and one-way analy-
sis of variance (ANOVA), and statistical signi cance
declared at P<0.05.
RESULTS & DISCUSSION
MEAN PARTICLE SIZE
The results showed that the mean size of the vesicles
was strongly affected by EO and CH concentration,
besides of preparation method. Since the mean size
of particles prepared by thin lm method was around
Mohammad Hosein Arabi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL 155
a few microns, 1426.3± 50 nm, particle size reduc-
tion in nano scale by two methods was quite impres-
sive. According to the table 2, the mean size soni-
cated oil loaded vesicles ranged from about 97.8 to
about 210 nm, while extruded liposomes were larger
(256–489 nm). It’s clearly seen that as the amount of
the essential oil increases, size of vesicles increases. Also
with decrease of cholesterol content, size of liposomes
reduced (Figure 1).
Polydispersity index
All MLV liposomes prepared by thin lm method had
high PDI index (>0.4), which re ects the heterogeneity
and dissimilarity of their colloidal system. While the size
distribution of sonicated and extruded nanoliposomes
was relatively narrow. Nanoliposomes prepared by the
sonication method were most homogenous in size dis-
tribution than liposomes prepared by two other methods
(Table 2).
As seen in gure 2 (A) by increasing the concentration
of essential oil in two methods PDI was increased. In the
case of cholesterol, gure 2 (B), in extruded method PDI
decreases with increased cholesterol levels, this trend is
more imperceptibly in sonication method.
Zeta potential
The zeta potential measurement of liposomal sam-
ple containing DOTAP was +14.7 mV. Other liposomes
showed neutral charge that was owing to lack of charge
ingredients.
Encapsulation Ef ciency
The encapsulation ef ciency greatly depends on lipo-
somal content, lipid concentration, method of prepara-
Table 2. Effect of preparation parameters on Zataria multi ora essential oil Loaded
nanoliposomes in two different preparation methods
Essential oil
concentration mg/ml
Size (nm) PDI EE%
Extrusion method
14.2 489.1±21 0.38±0.15 53.66±0.55
9.46 405.66±67.5 0.31±0.03 56.26±1.99
7.1 296.5±127.49 0.27±0.09 83.03±7.42
Sonication method
14.2 208.333±7.63 0.27±0.006 11.91±0.17
9.46 167.333±2.08 0.18±0.007 14.13±0.36
7.1 97.866±0.80 0.21±0.01 16.67±0.30
All data are mean value ± standard deviation
Table 3. Effect of cholesterol concentrations on properties of Zataria multi ora essential oil
loaded nanoliposomes in two different preparation methods
Cholesterol: lecithin Size(nm) PDI EE%
Extrusion method
Sonication method
1:1 (7.1mg/ml)
1:2 (6.4mg/ml)
1:3 (4.83mg/ml)
405.66±67.50
302.66±62.54
285.033±15.59
0.313±0.03
0.412±0.03
0.424±0.12
56.26±1.99
62.39±4.50
70.56±2.58
1:1
1:2
1:3
167.33±2.08
133.66±0.57
138±1
0.183±0.01
0.172±0.001
0.174±0.0005
14.16±0.31
18.59±0.01
18.96±0.05
All data are mean value ± standard deviation
Mohammad Hosein Arabi et al.
156 PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
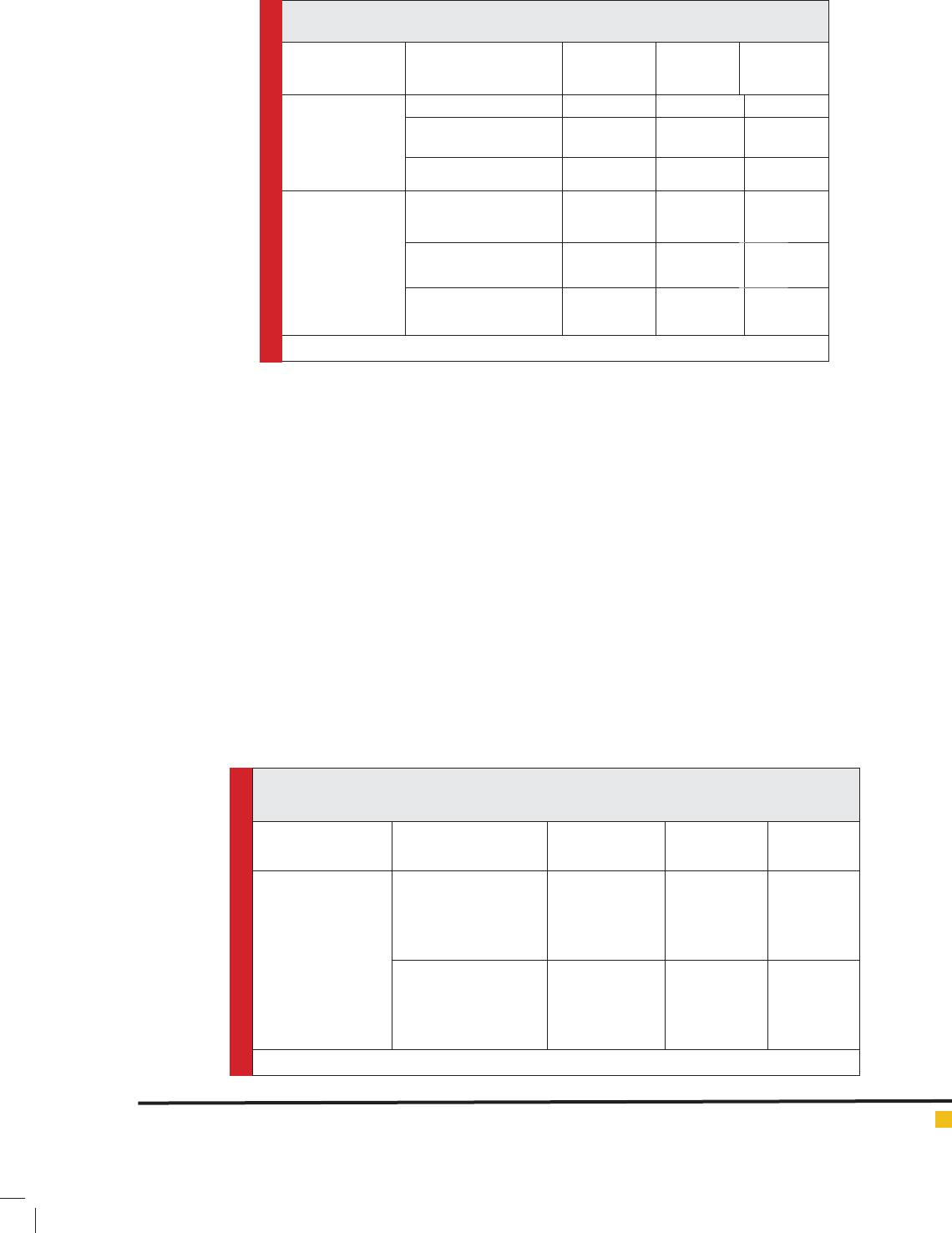
FIGURE 3. A is effect of essential oil concentration on encapsulation ef ciency and B is effect of cholesterol
content on encapsulation ef ciency of nanoliposomes
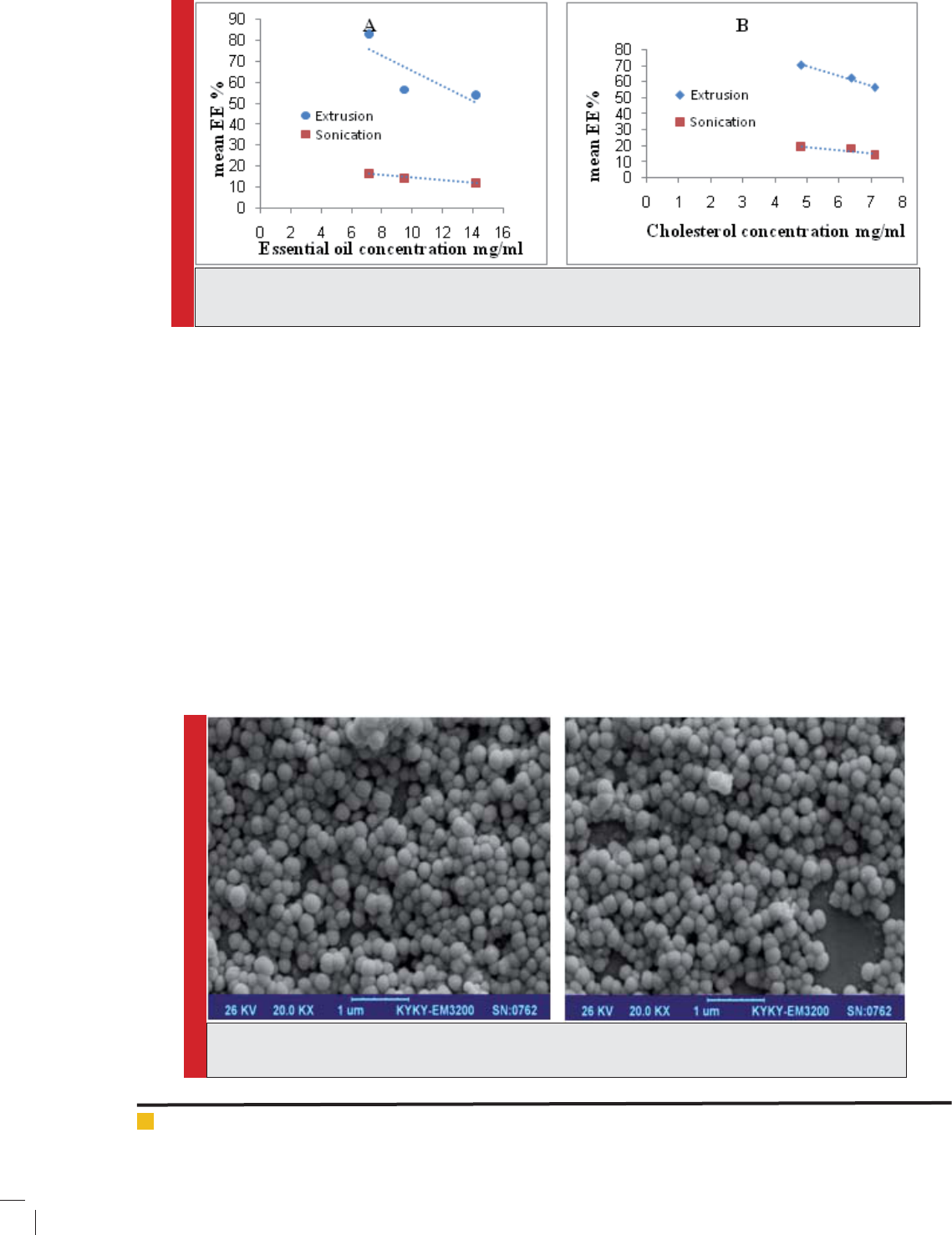
FIGURE 4. SEM image of Zatariamulti oraessential oil loaded liposomes by sonication (right image) and
extrusion (left image) method.
tion and the drug which is used. The results show that
both two methods have acceptable incorporation ef -
ciency. However sonication method gave a lower con-
tent in incorporated essential oil than extrude method
(11-18%). Extruded vesicles showed high encapsulation
ef ciency (53-83%) of the essential oil. The highest
mean entrapment ef ciency was found at 0.25 of EO/
total lipid (Table 2). Moreover, in the case of choles-
terol effect, it was also observed that liposomal formula-
tion PC/Chol (3:1) load the highest amount of the active
ingredient in each method (70.56±2.58 % for extruded
vesicles and 18.96±0.05 % for sonication method) (Table
3). From gure 3(A), we observed a decrease of incorpo-
rated oil with increase of essential oil concentration in
each method. Also, it can be appreciated that decrease
content of cholesterol lead to increase loading of the
essential oil ( gure 3 B). Results were summarized in
Table 2 and 3. On the whole, ANOVA test showed that oil
concentration in uence on physicochemical properties
of each method signi cantly (p <0.05).
From gure 3(A), we observed a decrease of incorpo-
rated oil with increase of essential oil concentration in
each method. Also it can be appreciated that decrease
content of cholesterol lead to increase loading of the
essential oil ( gure 3B).
Image of selected nanoliposome
SEM image of prepared nanoliposomes has been shown
in Figure 4. As it can be seen all nanoliposomal parti-
cles have spherical structure and system is homogenous
in both methods. Both formulations in which used EO:
PC: CH 1:4:1. Liposomes prepared by sonication method
Mohammad Hosein Arabi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL 157
(right image) were smaller than liposomes treated via
extrusion method (left image). It can be due to higher
content of essential oil that uidize liposomal bilayers
and increase their susceptivity for aggregation.
In vitro essential oil release studies
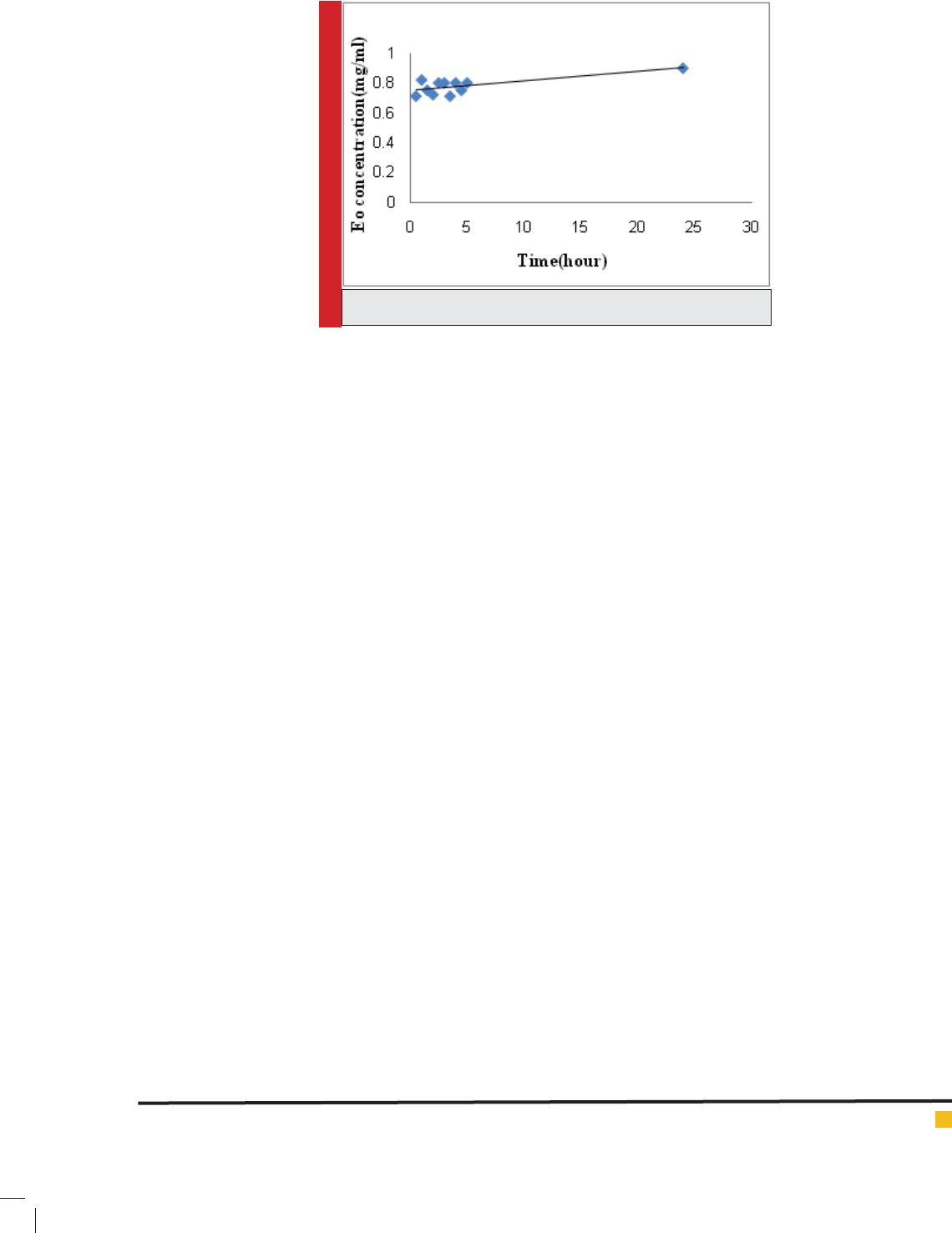
As showed in Figure 5, the release of EO within 24 hours
from nanoliposomes was very inconsiderable and negli-
gible. In the other words, it can be said that EO leakage
from liposomes does not occur. The selected formulation
was EO: PC: CH 1:4:3 nanoliposomes containing Zataria
essential oil.
Liposome stability
The physical and chemical stability of soni-
cated formula were evaluated at 5°C, 25°C and
37°C for two months. The sonicated formula (1/4) loaded
with Zataria essential oil were stable for at least 8 weeks
at room and refrigerator temperatures as the particle size
and the EE% of the liposomes did not change signi -
cantly during this period (data not shown).
Effect of essential oil concentration on liposomes
characteristics
Different methods presented different results in the for-
mation of the nano-sized liposomes. Due to high energy
used in probe sonicator, liposomes prepared by this
method have, smaller mean size and PDI index com-
pared to extruded ones. Sonication generally produces
SUV liposomes with lower size and lower incorporated
essential oil than MLVs that are according to Sinico et
al ndings (Sinico et al., 2005). Comparison the aver-
age size of nanoliposomes in different concentration of
essential oil in two methods showed signi cant differ-
ence (p<0.05). It has been showed that nanoliposomes
size could be affected by EO amount incorporated.
Also entrapment of lipophilic compounds in liposome
membrane depends on size of liposome (Schwendener
& Schott, 2017). Varona et al, also showed an essential
oil amount impact on the liposome size (Varona et al.,
2011). According to our results, by increasing the con-
centration of essential oil to total lipid with the same
ratio of PC: CH, average size of the nanoparticles have
increased. Probably because of higher EO concentration
required greater mechanical force in the reduction size
process of vesicles. In study of Detoni and colleagues, it
was not observed any changes in the size of the nano-
particles containing different ratios of Zanthoxylum tin-
goassubia oil to the phospholipid, which is inconsistent
with our study (Detoni et al., 2009).
PDI (poly dispersity index) usually is considered to
be as an indicator of colloidal particle size distribution.
The smaller index indicates the particle distribution is
more limited and so the system will be homogeneous
and more uniform in size (Ruozi et al., 2005). Two meth-
ods had low value PDI index although the mean PDI
index in sonication method is lower than the extrusion
method. In both methods, the mean PDI index changes
made no signi cant difference, in different concentra-
tion of EO (0.05<P). The liposomes encapsulated atrac-
tylodes macrocephala essential oil that formed by the
modi ed RESS had uniform and narrow size distribution
(Wen et al., 2010). The same results have been obtained
by Valenti et al and Sinico et al which is consist with our
study (Sinico et al., 2005; Valenti et al., 2001). On the
contrary, Yoshida et al reported PDI index higher than
0.7 for their MLV dispersions using Eugenia uni ora EO
(Yoshida et al., 2010). According to the above gure (3),
it can be observed that, in both two methods, the incor-
poration ef ciency decreased when the EO amount was
increased. Ortan et al, showed a slight decrease in the
encapsulation ef ciency of anethum graveolens essential
FIGURE 5. In vitro essential oil release curve over 24 hours

Mohammad Hosein Arabi et al.
158 PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
oil with the increase of its concentration which is consist
with present study (Ortan et al., 2009). Lavandin and
his colleagues obtain varying amounts of encapsulation
ef ciency by loading different ratio of essential oil to
phospholipid (Varona et al., 2011). With increasing of
essential oil concentration the encapsulation ef ciency
increases because of more EO can be encapsulated into
liposomes that is consistent by the studies of Fang et al
and also Varona et al which in contrast with present study
(Varona et al., 2011; Fang et al., 2001). The difference in
the encapsulation ef ciency was explained by Detoni et
al. According to their study, the physical and chemical
properties of essential oils, or changes may be created
in the techniques of liposomes preparation such as con-
centration of phospholipids, cholesterol and the ratio of
EO to total lipid can affect this property (Detoni et al.,
2012). In our study, Signi cance differences in incorpo-
ration ef ciency related to the preparation method was
observed (p<0.05). It has been reported that produced
liposomes by sonication method, may be have lower
encapsulation ef ciency due to the degradation possibil-
ity of phospholipids and encapsulated compounds (Riaz,
1996) . In a similar study, Zataria multi ora essential oil
into the nanoliposomes was encapsulated. The essential
oil encapsulation within liposomes were 18 to 22 % by
providing multiple ways (Khatibi et al., 2015). Celia et
al, have incorporated bergamot essential oil by using
extrude method with an encapsulation ef ciency of 75%
(Ceilia et al., 2015). The result obtained in present study
recon rms approximately their ndings.
Effect of cholesterol content on liposomes
characteristics
By maintaining the ratio of EO to total lipid (1/3) in
the formulations, the effect of different cholesterol
concentration on liposome features was also deter-
mined. Cholesterol as a usual
composition plays an
important role in the structure of the liposome. Cho-
lesterol due to its amphiphilic properties, perch into
liposome so that a hydroxyl group to the core of water
oriented and the hydrophobic tail to the phospholipid
bilayer of the liposomes. In previous studies it has been
widely reported that the formation and stability of the
liposomes is highly dependent on the ratio of cholesterol
to phospholipid, so have great impact on the behavior
of the carrier in vitro and in vivo conditions (Haeri et
al., 2014). Other effects of cholesterol are control and
reduce membrane permeability, making of hardness to
membrane and layer structural stability (Chan et al.,
2004). Results shown in gure 1 part B demonstrated
that liposome size increased with the increasing amount
of cholesterol in both two methods. Varona and col-
leagues observed that with lower cholesterol levels, the
size of the liposomes reduced which it’s in line with the
results of this study (Varona et al., 2011). It is reported
that the insertion of cholesterol in liposomal membrane
causes liposomes more rigid and resistant to size reduc-
tion (Briuglia et al., 2015). Comparison the mean size
of two methods, sonication method showed a signi -
cant size reduction (p <0.05). Also the mean PDI index
in different molar concentrations of cholesterol in two
methods showed that changes are signi cant (p<0.05).
Sonication method gave smaller and more homogene-
ous nanoliposomes than extrude method. Lipophilic
molecules, competes with cholesterol molecules for the
lipophilic space in the lipid bilayer (Jaafar-Maalej et al.,
2010) so, cholesterol might distaste the incorporation of
hydrophobic molecules into the liposome bilayer mem-
brane (Fathi Moghaddam et al., 2008; Rezaee et al, 2015;
Saffari
c
et al, 2013 . Ortan et al in their study showed
that by increasing the amount of Cholesterol, amount
of encapsulated EO (EE%) in liposomes reduced (Ortan
et al., 2009). This result was con rmed by Varona et
al via essential oil of lavandi (Varona et al., 2011). The
same result was obtained in present study with both two
methods and encapsulation ef ciency in both methods
have signi cantly decreased (p < 0.05).
In summary, this study contributes to the understand-
ing effect of preparation method & the different con-
centration of EO, Cholesteol content on Zataria essen-
tial oil loading into liposomes. The study presented here
suggested that the physicoch emical features of loaded
nanoliposomes clearly in uenced by EO concentration,
cholesterol content and liposome preparation method. It
can be deduced by consideration of signi cance level,
sonication method gave smaller nanoliposomes than the
extrude method, while higher EO incorporation obtained
by extruded method.
ACKNOWLEDGEMENTS
The authors thank Ms Zahra Abbasian & Ms Leila Asta-
rakifor their valuable technical assistance. This research
was performed with support from Kashan University of
Medical Sciences.
REFERENCES
A li MS, Saleem M, Ali Z, Ahmad VU.(2000) Chemistry of
Zataria multi ora (Lamiaceae). Phytochemistry. 55(8):933-6.
Ak barzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zar-
ghami N, Hanifehpour Y,(2013) Liposome: classi cation, prep-
aration, and applications. Nanoscale Res Lett. 8(1):102.
B angham A, Standish MM, Watkins J. (1965) Diffusion of uni-
valent ions across the lamellae of swollen phospholipids. Jour-
nal of molecular biology. 13(1):238-IN27.
Belay G, Tariku Y, Kebede T, Hymete A, Mekonnen Y.(2011)
Ethnopharmacological investigations of essential oils isolated

Mohammad Hosein Arabi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL 159
from ve Ethiopian medicinal plants against eleven patho-
genic bacterial strains. Phytopharmacology. 1(5):133-43.
Bh atia A, Kumar R, Katare OP.(2004) Tamoxifen in topical
liposomes: development, characterization and in-vitro evalua-
tion. J Pharm Pharm Sci. 7(2):252-9.
Briuglia M-L, Rotella C, McFarlane A, Lamprou DA.(2015)
In uence of cholesterol on liposome stability and on in
vitro drug release. Drug delivery and translational research.
5(3):231-42.
Ce lia C, Trapasso E, Locatelli M, Navarra M, Ventura CA, Wolf-
ram J.(2013) Anticancer activity of liposomal bergamot essen-
tial oil (BEO) on human neuroblastoma cells. Colloids and Sur-
faces B: Biointerfaces. 112:548-53.
Chan YH, Chen BH, Chiu CP, Lu YF.(2004) The in uence of
phytosterols on the encapsulation ef ciency of cholesterol
liposomes. International journal of food science & technology.
39(9):985-95.
C hetanachan P, Akarachalanon P, Worawirunwong D, Dararu-
tana P, Bangtrakulnonth A, Bunjop M. (2008) Ultrastructural
characterization of liposomes using transmission electron
microscope. Advanced Materials Research. 55:709-11.
Detoni C, Cabral-Albuquerque E, Hohlemweger S, Sampaio C,
Barros T, Velozo E. (2009) Essential oil from Zanthoxylum tin-
goassuiba loaded into multilamellar liposomes useful as anti-
microbial agents. Journal of microencapsulation. 26(8):684-91.
De toni CB, de Oliveira DM, Santo IE, Pedro AS, El-Bacha R, da
Silva Velozo E.(2012) Evaluation of thermal-oxidative stability
and antiglioma activity of Zanthoxylum tingoassuiba essential
oil entrapped into multi-and unilamellar liposomes. Journal of
liposome research.22(1):1-7.
Ed ris AE. Pharmaceutical and therapeutic potentials of essen-
tial oils and their individual volatile constituents: a review.
Phytotherapy Research. 21(4):308-23.
El -Samaligy M, A N, Mahmoud E. (2006) Increasing bio-
availability of silymarin using a buccal liposomal delivery sys-
tem: preparation and experimental design investigation. Inter-
national journal of pharmaceutics. 308(1):140-8.
Fa ng J-Y, Hong C-T, Chiu W-T, Wang Y-Y. (2001) Effect of
liposomes and niosomes on skin permeation of enoxacin.
International Journal of Pharmaceutics. 219(1):61-72.
Fathi Moghaddam H, Sha ee Ardestani M, Saffari M, Navid-
pour L, Sha ee A, Rahmim A. (2008) Dopaminergic but not
glutamatergic neurotransmission is increased in the striatum
after selective cyclooxygenase-2 inhibition in normal and
hemiparkinsonian rats. Basic & clinical pharmacology & toxi-
cology; 103(4):293-296.
Ha eri A, Alinaghian B, Daeihamed M, Dadashzadeh S.(2014)
Preparation and characterization of stable nanoliposomal
formulation of uoxetine as a potential adjuvant therapy
for drug-resistant tumors. Iranian Journal of Pharmaceutical
Research. 13:3-14.
Ho fheinz R-D, Gnad-Vogt SU, Beyer U, Hochhaus A.(2005)
Liposomal encapsulated anti-cancer drugs. Anti-cancer
drugs.;16(7):691-707.
Li olios C, Gortzi O, Lalas S, Tsaknis J, Chinou I.(2009) Liposo-
mal incorporation of carvacrol and thymol isolated from the
essential oil of Origanum dictamnus L. and in vitro antimicro-
bial activity. Food chemistry. 112(1):77-83.
Ja afar-Maalej C, Diab R, Andrieu V, Elaissari A, Fessi H. (2010)
Ethanol injection method for hydrophilic and lipophilic drug-
loaded liposome preparation. Journal of liposome research
20(3):228-43.
Kh atibi SA, Misaghi A, Moosavy M-H, Amoabediny G, Basti
AA.(2015) Effect of Preparation Methods on the Properties of
Zataria multi ora Boiss. Essential Oil Loaded Nanoliposomes:
Characterization of Size, Encapsulation Ef ciency and Stabil-
ity. Pharmaceutical Sciences. 20:141.
Martin A, Varona S, Navarrete A, Cocero MJ. (2010)Encapsula-
tion and co-precipitation processes with supercritical uids:
applications with essential oils. Open Chemical Engineering
Journal. 4(1):31-41.
Me ure LA, Foster NR, Dehghani F. (2008)Conventional and
dense gas techniques for the production of liposomes: a review.
Aaps Pharmscitech. 9(3):798-809.
Mi saghi A, Basti AA. (2007)Effects of Zataria multi ora Boiss.
essential oil and nisin on Bacillus cereus ATCC 11778. Food
control. 18(9):1043-9.
Or tan A, Câmpeanu G, Dinu-Pirvu C, Popescu L.(2009) Studies
concerning the entrapment of Anethum graveolens essential
oil in liposomes. Roum Biotechnol Lett. 14:4411-7.
Rezaee S, Khalaj A, Adibpour N, Saffary M.(2015) Correla-
tion between lipophilicity and antimicrobial activity of some
2-(4-substituted phenyl)-3 (2H)-isothiazolones. DARU Journal
of Pharmaceutical Sciences.;17(4):256-263.
Ri az M. (1996) Liposomes preparation methods. Pak J Pharm
Sci. 9(1):65-77.
Saedi Dezaki E, Mahmoudvand H, Shari far F, Fallahi S, Mon-
zote L, Ezatkhah F. (2016) Chemical composition along with
anti-leishmanial and cytotoxic activity of Zataria multi ora.
Pharm Biol. 2016;54(5):752-8.
Saffari Ma, Hoseini Shirazi F, Moghimi HR(2016) Terpene-
loaded Liposomes and Isopropyl Myristate as Chemical Permea-
tion Enhancers Toward Liposomal Gene Delivery in Lung Cancer
cells; A Comparative Study. Iran J Pharm Res. 15(3):261-267.
Saffari M, Moghimi HR, Dass CR. (2016) Barriers to Liposo-
mal Gene Delivery: from Application Site to the Target. Iran J
Pharm Res. 15(Suppl):3-17.
Saffaric M, Tamaddon AM, Shirazi FH, Oghabian MA, Moghimi
HR.(2013) Improving cellular uptake and in vivo tumor sup-
pression ef cacy of liposomal oligonucleotides by urea as a
chemical penetration enhancer.; The journal of gene medicine.
15(1):12-19.
Sc hwendener RA, Schott H. (2017) Li posome Formulations of
Hydrophobic Drugs. Methods Mol Biol.;1522:73-8.
Shahsavari N, Barzegar M, Sahari MA, Naghdibadi H. (2008)
Antioxidant activity and chemical characterization of essen-
tial oil of Bunium persicum. Plant foods for human nutrition.
63(4):183-8.
Mohammad Hosein Arabi et al.
160 PREPARATION METHODS OF NANOLIPOSOMES CONTAINING
ZATARIA MULTIFLORA BOISS
ESSENTIAL OIL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Sh ashi K, Satinder K, Bharat P. (2012) A complete review
on: liposomes. International Research Journal of Phar-
macy.;3(7):10-16.
Si nico C, De Logu A, Lai F, Valenti D, Manconi M, Loy G.(2005)
Liposomal incorporation of Artemisia arborescens L. essential
oil and in vitro antiviral activity. European Journal of Pharma-
ceutics and Biopharmaceutics.;59(1):161-8.
Sunar S, Aksakal O, Yildirim N, Agar G, Gulluce M, Sahin F.
(2009) Genetic diversity and relationships detected by FAME
and RAPD analysis among Thymus species growing in eastern
Anatolia region of Turkey. Romanian Biotechnological Let-
ters.;14(2):4313-8.
Ru ozi B, Tosi G, Forni F, Fresta M, Vandelli MA.(2005) Atomic
force microscopy and photon correlation spectroscopy: two
techniques for rapid characterization of liposomes. European
Journal of Pharmaceutical Sciences. 25(1):81-9.
Va lenti D, De Logu A, Loy G, Sinico C, Bonsignore L, Cottiglia
F.(2001) Liposome-incorporated Santolina insularis essential
oil: preparation, characterization and in vitro antiviral activ-
ity. Journal of liposome research. 11(1):73-90.
Varona S, Martín Á, Cocero MaJ.(2011) Liposomal incorpora-
tion of lavandin essential oil by a thin- lm hydration method
and by particles from gas-saturated solutions. Industrial &
Engineering Chemistry Research.;50(4):2088-97.
We n Z, Liu B, Zheng Z, You X, Pu Y, Li Q. (2010)Preparation
of liposomes entrapping essential oil from Atractylodes mac-
rocephala Koidz by modi ed RESS technique. Chemical Engi-
neering Research and Design. 88(8):1102-7.
Yo shida P, Yokota D, Foglio M, Rodrigues RF, Pinho S. (2010)
Liposomes incorporating essential oil of Brazilian cherry (Eugenia
uni ora L.): characterization of aqueous dispersions and lyophi-
lized formulations. Journal of microencapsulation.;27(5):416-25.