Microbiological
Communication
Biosci. Biotech. Res. Comm. 10(1): 137-142 (2017)
Chemical composition and antimicrobial effects of
Thymus daenensis
on
Helicobacter pylori
Parisa Moradi
1*
, Tahereh Falsa
1
, Neda Saffari
2
, Ebrahim Rahimi
3
,
Hassan Momtaz
4
and Behzad Hanedi
5
1
Department of Biology, Faculty of Biological Sciences, Alzahra University, Tehran, Iran
2
Department of Biotechnology, Faculty of Biological Sciences, Alzahra University, Tehran, Iran
ABSTRACT
High occurrence of resistance in the Helicobacter pylori strains of human clinical samples caused medical practition-
ers to found a good alternative therapeutic choice. High phenolic contents of the Thymus daenensis essential oil cov-
ered its high antimicrobial effects. The present investigation was done to study the chemical composition and anti-H.
pylori effects of T. daenensis essential oil. Aerial parts of the T. daenensis were collected from the Yasuj city and
transferred to the laboratory. Essential oil was extracted using the Clevenger apparatus. Chemical components of T.
daenensis was identi ed using the GC-mass analyzer. Anti-H. pylori effects of T. daenensis was determined using the
disk diffusion method. Twelve (99.24%) chemical components were totally identi ed in the essential oil of the T. dae-
nensis. Thymol (42.81%), gamma-Terpinene (20.39%) and para-Cymene (9.72%) were the most commonly identi ed
chemical components. Distribution of beta-Myrcene, D-Limonene, beta-Pinene and Terpinen-4-ol were moderate. H.
pylori strains harbored the highest levels of sensitivity against tetracycline and ampicillin antibiotics, while those of
resistance were seen for the erythromycin, clarithromycin and metronidazole antibiotics. Inhibition zone diameter
increased from 0 to 18.6±0.5 mm which represented dose depended anti-H. pylori effects of T. daenensis essential oil.
Extensive production of T. daenensis full from thymol, gamma-Terpinene and para-Cymene will help researchers to
formulate an effective antibiotic agent for treatment of the cases of H. pylori-infection.
KEY WORDS:
THYMUS DAENENSIS
, CHEMICAL COMPONENTS, ANTIMICROBIAL EFFECTS,
HELICOBACTER PYLORI
137
ARTICLE INFORMATION:
*Corresponding Author:
Received 21
st
Dec, 2016
Accepted after revision 25
th
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/

138 ANTI-HELICOBACTER PYLORI EFFECTS OF
THYMUS DAENENSIS
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Parisa Moradi and Neda Saffari
INTRODUCTION
Helicobacter pylori (H. pylori) is an extracellular gram-
negative, microaerophilic and spiral bacterium which
is known as the causative agent of various types of
gastrointestinal diseases and disorders such as gastric
adenocarcinoma, gastric ulcer, gastritis, and lymphoma
(Shrestha et al., 2012). It has been estimated that 30 to 90
percent of hospitalized patients with such gastrointesti-
nal diseases and disorders were infected with H. pylori
(Shrestha et al., 2012; Mastromarino et al., 2005; Vu and
Ng, 2000). Prevalence of H. pylori in in developed and
developing countries is about 40% and 90%, respec-
tively (Suerbaum and Michetti, 2002). Gastrointestinal
diseases caused by H. pylori often treated with antimi-
crobial agents. Prescription of ampicillin, amoxicillin,
clarithromycin, metronidazole and tetracycline antibiot-
ics is the most common way for treatment of the cases of
H. pylori (Bytzer et al., 2011; Rimbara et al., 2011; Yang
et al., 2014). Nevertheless, occurrence of extreme levels
of antibiotic resistance in the H. pylori strains caused
several concerns regarding their treatment (Bytzer et al.,
2011; Rimbara et al., 2011; Yang et al., 2014; Ghotaslou
et al., 2015).
Therefore, medical practitioners and also pharmaco-
logical companies have a tendency to found a suitable
alternative treatment for H. pylori. Medicinal plants may
be a powerful sources of antimicrobial agents which can
be used for treatment of infectious diseases. The genus
Thymus L. goes to the major family (Lamiaceae), and
included approximately 215 species in the world. Totally,
14 species of Thymus have been described in the zone
of Iran (Stahl-Biskup and Saez, 2002; Rechinger, 1982;
Mojab et al., 2008; Fachini-Queiro et al., 2012). T. daen-
ensis, T. carmanicus, T. daenensis, T. persicus, T. vulga-
ricus and T. trautvetteri are the most commonly endemic
species in Iran (Stahl-Biskup and Saez, 2002; Rechinger,
1982; Mojab et al., 2008; Fachini-Queiro et al., 2012). T.
daenensis subsp. daenensis is an endemic subspecies of
Iran and especially high altitudes of Zagros mountains
(Stahl-Biskup and Saez, 2002; Rechinger, 1982; Mojab
et al., 2008; Fachini-Queiro et al., 2012). Areal parts and
volatile constituents of T. daenensis are used in Iranian
traditional medicine.
Thymus species and especially T. daenensis are usu-
ally applied as tasty and avoring agents in tea and
also spices and medicinal drives including antimicro-
bial, anti-fungal, antispasmodic, and anti-in amma-
tory agent (Stahl-Biskup and Saez, 2002; Rechinger,
1982; Mojab et al., 2008; Fachini-Queiro et al., 2012;
Gautam et al., 2014). Previous investigations showed
high anti-parasites, antibacterial, antiviral, antifungal,
spasmolytic and antioxidant effects of Thymus species
(Stahl-Biskup and Saez, 2002; Rechinger, 1982; Mojab
et al., 2008; Fachini-Queiro et al., 2012; Gautam et al.,
2014).
There were no previously published data on the anti-
microbial effects of Thymus daenensis on the H. pylori.
Therefore, the present investigation was carried out
to study the chemical composition and antimicrobial
effects of the essential oil extracted from the Thymus
daenensis on ten clinical isolates of H. pylori.
MATERIAL AND METHODS
PLANT AND ESSENTIAL OIL EXTRACTION
From July to August 2013, aerial parts of T. daenensis
at the owering stage were collected from the plains of
the Dena mountain, Yasuj, South-west of Iran. The sam-
ples of the plant were identi ed and a voucher specimen
was deposited at the Herbarium of Research Center of
Agricultural of Shahrekord city, Iran. Essential oil of the
collected
T. daenensis was extracted by water distilla-
tion using the Clevenger apparatus. The essential oil was
dried over anhydrous sodium sulfate and stored at 17°C
until further analysis.
IDENTIFICATION OF THE CHEMICAL
COMPONENTS
Samples were studied by the Gas Chromatography (GC,
Agilent 7890 A) and Mass Spectrometry (MS, Agilent
5975 C). Identi cation of the chemical components was
done according to the method described previously by
Borug˘
a
et al. (2014) (Borug˘
a
et al., 2014). Conditions of
the GC stage were as follow: temperature range of 50 to
250°C at 40°C/min with a solvent delay of 5 min. Injec-
tor of the device was kept at 250°C. Helium at a ow of
1.0 mL/min was the inert gas, and the injected volume in
the splitless mode was 1 L. The MS conditions were as
follow: ionization energy of 70 eV, quadrupole tempera-
ture of 100°C, scanning velocity of 1.6 scan/s and weight
range of 40-500 amu.
BACTERIAL STRAINS
Ten rough H. pylori strains were obtained from the
clinical cases of gastrointestinal disorders of Alzahra
Hospital, Tehran, Iran. H. pylori strains were regularly
cultured on Brucella agar (Merck, Germany) supple-
mented with 5–7% sheep blood, amphotericin (2mg/l),
polymixin-B (8mg/l), and vancomycin (6mg/l) and were
incubated at 37°C for 3–7 days on the microaerophilic
conditions (10% CO2 and 95% humidity). Then, H.
pylori strains were approved using the morphological,
Gram staining and biochemical tests (rapid urease, oxi-
dase and nitrate) and PCR ampli cation of 16S rRNA
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTI-HELICOBACTER PYLORI EFFECTS OF
THYMUS DAENENSIS
139
Parisa Moradi and Neda Saffari
gene (Forward: 5’-CTGGAGAGACTAAGCCCTCC-3’ and
Reverse: 5’-ATTACTGACGCTGATTGTGC-3’) (110 bp) (Ho
et al., 1991).
ANTIMICROBIAL SUSCEPTIBILITY TESTING
Suspensions of the fresh cultures were made in saline
and turbidity was adjusted to 1×10
8
bacteria/ml (corre-
sponding to turbidity with OD 0.8 at 600 nm). Two-hun-
dred microliters of microbial suspension were placed on
50-ml Mueller Hinton agar plates containing 10% fetal
calf serum (Sigma, UK) and incubated for 2–5 days at
37°C under microaerophilic conditions. H. pylori ATCC
26695 were used as quality control. Tests were done
three times. Table 1 shows the standard values for sus-
ceptibility determination (CLSI, 2012). The antimicrobial
effects of T. daenensis essential oil were performed by
the disc diffusion method. For this purpose, sterile blank
disks (6 mm) were inoculated with concentrations of 0.6,
1.25, 2.5, 5 and 10 (%v/v) essential oil and were placed
on dense cultures of bacteria and incubated for 2–5 days
at 37°C under microaerophilic conditions. Susceptibility
of H. pylori strains were also determined against ampi-
cillin (10 µg), metronidazole (5 µg), erythromycin (5 µg),
clarithromycin (2 µg) and tetracycline (30 µg) antibiotic
agents (Oxoid, UK) (CLSI, 2012).
STATISTICAL ANALYSIS
SPSS 20.0 software (SPSS Inc., Chicago, IL, USA) and
one-way analysis of variance (ANOVA) test were used
for statistical analysis. P <0.05 was considered as sig-
ni cant difference.
RESULTS AND DISCUSSION
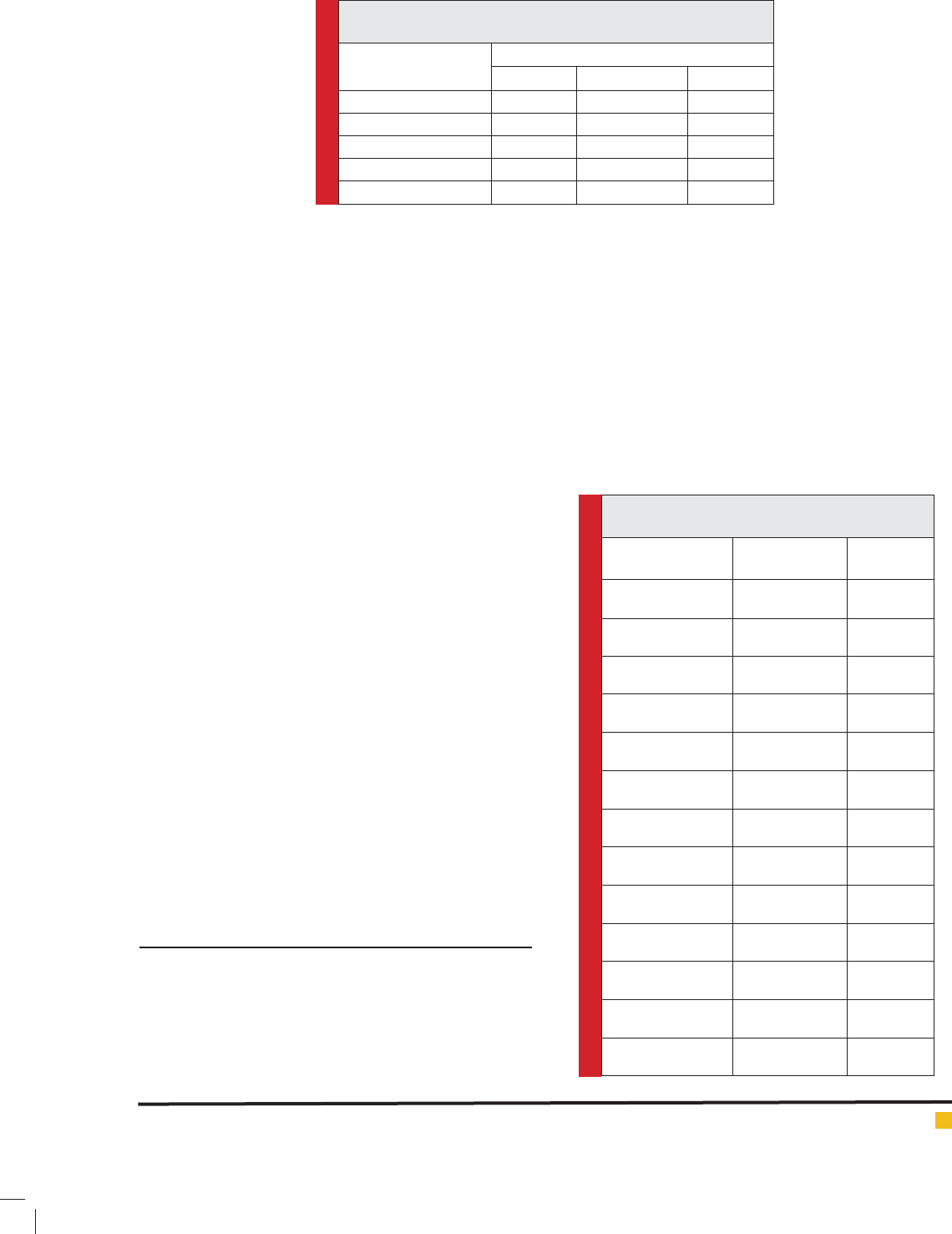
Table 2 represents the chemical components of the
essential oil of the T. daenensis. Totally, 12 chemical
components were identi ed in the essential oil of the
T. daenensis (99.24%). Thymol (42.81%), gamma-Ter-
pinene (20.39%) and para-Cymene (9.72%) were the
most commonly identi ed chemical components in the
essential oil of the T. daenensis. Frequency of beta-
Myrcene, D-Limonene, beta-Pinene and Terpinen-4-ol
were 3.71%, 3.56%, 3.82% and 3.45%, respectively.
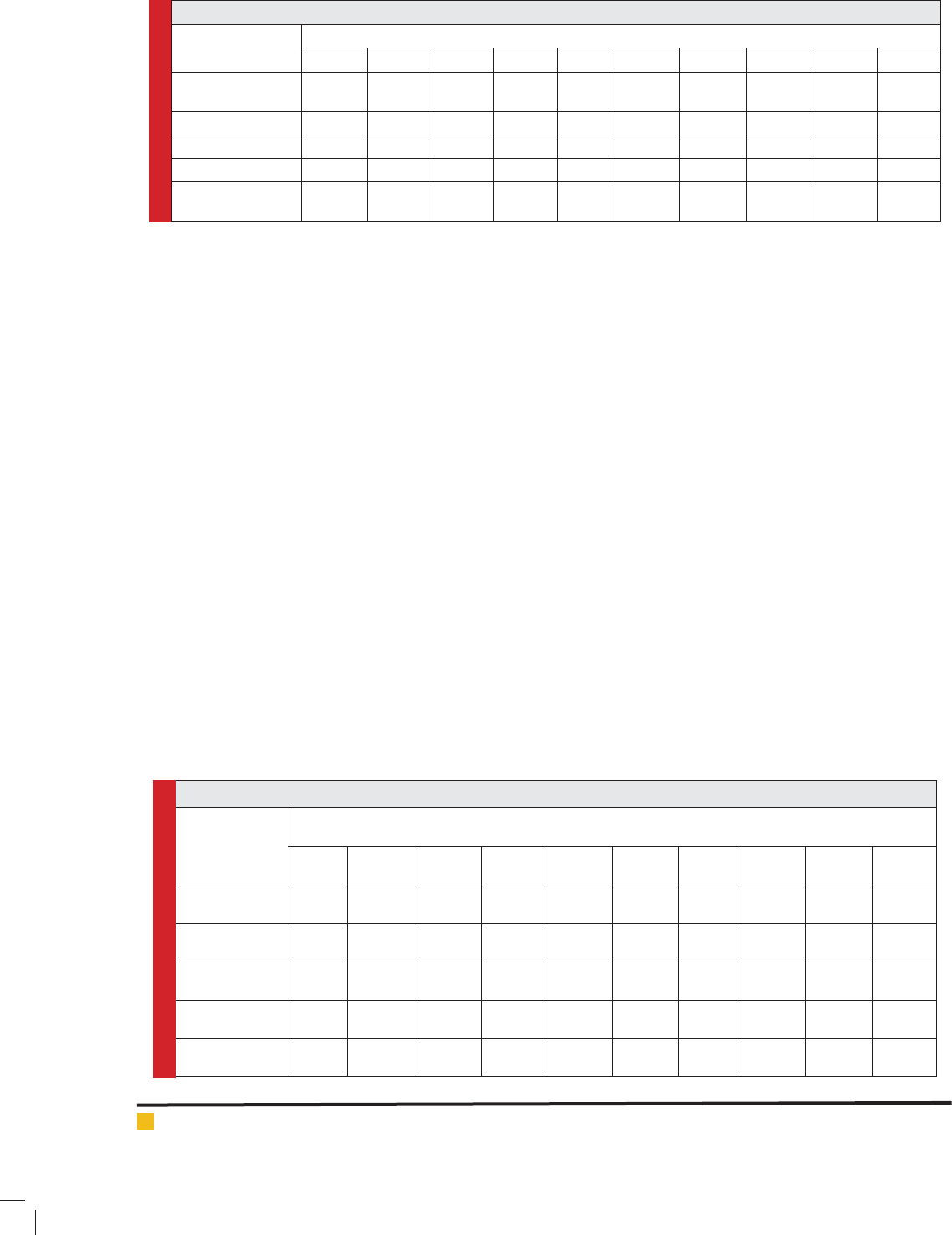
Table 3 represents the inhibition zone diameter for
each of the ten clinical isolates of H. pylori against anti-
biotic agents. Results showed that H. pylori strains had
the highest levels of sensitivity against tetracycline and
ampicillin antibiotics. Most resistance was seen for the
erythromycin, clarithromycin and metronidazole anti-
biotics. Statistically signi cant differences (P <0.05)
were seen between the zone of inhibition and types of
antibiotics.
Table 1. Standard values for determination of sensitivity and
resistance of bacteria against antibiotic agents.
Antibiotic agents
Diameter of inhibition (mm)
Sensitive Intermediate Resistant
Ampicillin (10 µg) ≥17 14-17 ≤13
Metronidazole (5 µg) ≥15 12-15 <12
Erythromycin (5 µg) ≥18 14-17 ≤12
Clarithromycin (2 µg) ≥18 14-17 ≤13
Tetracycline (30 µg) ≥19 15-18 ≤14
Table 2. Chemical components of the essential oil
of the T. daenensis.
Chemical
components
Retention Time
(RT) (min)
Frequency
(%)
beta-Phellandrene 8.26 1.92
beta-Myrcene 6.97 3.71
alpha-Thujene 5.39 2.94
alpha-Phellandrene 7.53 2.07
D-Limonene 8.04 3.56
alpha-Pinene 5.63 2.12
beta-Pinene 6.89 3.82
Terpinen-4-ol 12.55 3.45
Caryophyllene 17.32 2.71
para-Cymene 8.46 9.74
gamma-Terpinene 8.96 20.39
Thymol 16.17 42.81
Total 99.24
Parisa Moradi and Neda Saffari
140 ANTI-HELICOBACTER PYLORI EFFECTS OF
THYMUS DAENENSIS
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Table 3. Inhibition zone diameter for each of the ten clinical isolates of H. pylori against antibiotic agents.
Antibiotic agents
Diameter of the zone of inhibition for H. pylori strains (mm)
HP1 HP2 HP3 HP4 HP5 HP6 HP7 HP8 HP9 HP10
Metronidazole (5
µg)
0 0 0 0 0 0 16±0.4 15.4±0.2 0 0
Tetracycline (30 µg) 15.7±0.5 17.5±0.1 17±0.6 17.9±0.5 16±0.6 16.8±0.7 16.5±0.3 14.2±0.1 0 0
Ampicillin (10 µg) 12.1±0.6 0 11.3±0.8 9.1±0.7 0 19.8±0.5 16.2±0.8 0 34.8±0.8 11.7±0.6
Erythromycin (5 µg) 0 0 0 0 0 0 18.3±0.5 0 0 0
Clarithromycin (2
µg)
0 0 0 0 0 0 23.2±0.4 0 0 0
Table 4. Inhibition zone diameter of the various concentrations of the T. daenensis essential oil against H. pylori.
Essential oil
concentrations
Diameter of the zone of inhibition for H. pylori strains (mm)
HP1 HP2 HP3 HP4 HP5 HP6 HP7 HP8 HP9 HP10
0.6% 0 5.8±0.4 6±0.5 0 5.3±0.8 0 0 4.1±0.2 11.2±0.6 5.5±0.2
1.25% 0 5.3±0.9 7.8±0.1 7±0.0 6.7±0.3 0 0 5.2±0.8 14.3±0.4 5.2±0.5
2.5% 4.6±0.3 8.3±0.0 9.7±0.4 10.2±0.5 9.1±0.1 11.4±0.5 8.8±0.2 7.5±0.5 15.5±0.1 8.4±0.3
5% 5.3±0.5 13.1±0.5 13.2±0.1 12.8±0.6 11.6±0.2 12±0.9 14.1±0.1 10.2±0.4 16.7±0.2 11.6±0.4
10% 6.2±0.7 15.7±0.2 13.6±0.2 14.3±0.4 14±0.5 17.1±0.2 16±0.3 12.3±0.4 18.6±0.5 13.2±0.8
Table 4 represents the inhibition zone diameter of
the various concentrations of the T. daenensis essential
oil against H. pylori strains. We found that increase in
the concentration of essential oil cause its higher anti-
microbial effects on the H. pylori strains isolated from
the clinical cases of gastrointestinal disorders (P <0.05).
Inhibition zone diameter increased from 0 to 18.6±0.5
mm which showed that the anti-H. pylori effects of T.
daenensis essential oil is dose depended.
Failure of majority of therapeutic options and espe-
cially ampicillin, amoxicillin, clarithromycin, metroni-
dazole and tetracycline antibiotics caused us to study
the antimicrobial effects of H. pylori strains against T.
daenensis essential oil. The results of the present inves-
tigation showed that the T. daenensis essential oil had
a high antimicrobial effects on the H. pylori strains iso-
lated from clinical cases of gastrointestinal disorders.
Several studies have been approved the anti-H. pylori
effects of some medicinal plants. Falsa et al. (2014)
(Falsa et al., 2014) reported that Satureja bachtiarica
essential oil showed strong antibacterial activity against
clinical isolates of H. pylori (17.6 ± 1.1 mm and 0.035 ±
0.13 l/ml).They showed that anti-H. pylori effects of S.
bachtiarica is due to its high carvacrol content. Esmaeili
et al. (2012) (Esmaeili et al., 2012) reported that Shoya
powder and essential oils of Thymus vulgaris and Euca-
lyptus globulus had signi cant effects on the H. pylori.
They showed that Minimum Inhibitory Concentration
(MIC) of the T. vulgaris and E. globulus were 10.8 and
46.4 (µg/ml) respectively.
The antimicrobial effects of the T. daenensis essen-
tial oil is due to the presence of some active constitu-
ents with their hydrophobicity which enables them for
rupturing cell membranes and intrastructures. We found
that Thymol (42.81%), gamma-Terpinene (20.39%) and
para-Cymene (9.72%) were the most commonly iden-
ti ed chemical components in the essential oil of the
T. daenensis. Previous study in Romania (Grigore et al.,
2010) showed that the most commonly detected compo-
nents in the essential oil of the Thymus were p-cymene,
-terpinene and thymol which was similar to our nd-
ings, while those of Morocco (Imelouane et al., 2009)
and Spain (Ballester-Costa et al., 2013) are entirely dif-
ferent with us. P-cymene, -terpinene and thymol were
the most commonly detected components in previ-
ous studies conducted on Iran (Pirbalouti et al., 2013),
Poland (Kowalski and Wawrzykowski, 2009), Italy (De
Lisi et al., 2011) and Spain (Rota et al., 2008). Probably
types of samples, method of sampling, genus of plants,
and differences in the climate and weather of various

Parisa Moradi and Neda Saffari
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTI-HELICOBACTER PYLORI EFFECTS OF
THYMUS DAENENSIS
141
geographical regions are the main factors which may
affected on the chemical components of Thymus plants
of different studies.
Antimicrobial effects of T. daenensis essential oil is
depends on its chemical compositions. It seems that high
content of the phenolic compounds (thymol) and ter-
pene hydrocarbons (-terpinene) is the main factor for
the high anti-H. pylori effects of T. daenensis. Previ-
ous investigations showed that para-cymene does not
display any antimicrobial activities alone (Dorman and
Deans, 2000), while it has a strong antibacterial effects
in relation with thymol and -terpinene (Delgado et al.,
2004; Gallucci et al., 2009).
CONCLUSION
In conclusion, we found that T. daenensis essential oil
has a high antimicrobial effects on the H. pylori strains
of human clinical infections. As it showed, Thymol,
gamma-Terpinene and para-Cymene were the most
commonly identi ed chemical components in the essen-
tial oil of the T. daenensis. In compare with the ndings
of other researchers, Preparation of good conditions for
growth of T. daenensis full from these chemical compo-
nents will help us to present an ef cient antibiotic agent
for treatment of the cases of H. pylori-infection.
ACKNOWLEDGEMENTS
Authors would like to thanks from Dr. Tahereh Falsa ,
Dr. Mohaddase Mahboubi, Dr. Behzad Hamedi, Prof.
Ebrahim Rahimi and Prof. Hassan Momtaz for their
important clinically support. This work was supported
by the Alzahra University, Tehran, Iran.
REFERENCES
Ballester-Costa, C., Sendra, E., Fernández-López, J., Pérez-
Álvarez, J.A. and Viuda-Martos, M. (2013). Chemical composi-
tion and in vitro antibacterial properties of essential oils of
four Thymus species from organic growth. Industrial Crops
and Products, Vol. 50, pp. 304–311.
Borug
˘
a
, O., Jianu, C., Mis˛c
˘
a
, C., Gole˛
t
, I., Gruia, A.T. and
Horhat, F.G. (2014). Thymus vulgaris essential oil: chemical
composition and antimicrobial activity. Journal of Medicice
and Life, Vol. 7, No. 3, pp. 56-60.
Bytzer, P., Dahlerup, J.F., Eriksen, J.R., Jarbøl, D.E., Rosen-
stock, S. andWildt, S. (2011). Diagnosis and treatment of Heli-
cobacter pylori infection. Danish Medical Bulletin, Vol. 58, No.
4, pp. C4271.
Clinical and Laboratory Standards Institute (CLSI). (2012).
Performance standards for antimicrobial susceptibility test-
ing. Twenty-second informational supplement M100-S21,
Wayne Pa.
De Lisi, A., Tedone, L., Montesano, V., Sarli, G. and Negro,
D. (2011). Chemical characterization of Thymus populations
belonging from Southern Italy.Food Chemistry, Vol. 125, No.
4, pp. 1284–1286.
Delgado, B., Fernández, P.S., Palop, A. and Periago, P.M.
(2004). Effect of thymol and cymene on Bacillus cereus veg-
etative cells evaluated through the use of frequency distribu-
tions.Food Microbiology, Vol. 21, No. 3, pp. 327–334.
Dorman, H.J.D. and Deans, S.G. (2000). Antimicrobial agents
from plants: antibacterial activity of plant volatile oils.Jour-
nal of Applied Microbiology, Vol. 88, No. 2, pp. 308–316.
Esmaeili, D., Mohabati Mobarez, A. and Tohidpour, A. (2012).
Anti-Helicobacter Pylori Activities of Shoya Powder and
Essential Oils of Thymus vulgaris and Eucalyptus globus. Oepn
Microbiology Journal, Vol. 6, pp. 65-69.
Fachini-Queiroz, F.C., Kummer, R., Estevão-Silva, C.F., Car-
valho, M.D., Cunha, J.M., Grespan, R., Bersani-Amado, C.A.
andCuman, R.K. (2012). Effects of Thymol and Carvacrol, Con-
stituents of Thymus vulgaris L. Essential Oil, on the In amma-
tory Response. Evidence Based Complementary and Alterna-
tive Medicine, Vol. 2012, pp. 657026.
Falsa , T., Moradi, P., Mahboubi, M., Rahimi, E., Momtaz, H.,
Hamedi, B. (2014). Chemical composition and anti-Helicobac-
ter pylori effects of Satureja bachtiarica bunge essential oil.
Phytomedicine, Vol. 22, No. 1, pp. 173–177.
Gallucci, M.N., Oliva, M., Casero, C., Dambolena, J., Luna, A.,
Zygadlo, J. and Demo, M. (2009). Antimicrobial combined action
of terpenes against the food-borne microorganisms Escherichia
coli, Staphylococcus aureus and Bacillus cereus. Flavour and
Fragrance Journal, Vol. 24, No. 6, pp. 348–354.
Gautam, N.,Mantha, A.K. andMittal, S. (2014). Essential oils
and their constituents as anticancer agents: a mechanistic
view. Biomed Research International, Vol. 2014, p. 154106.
Ghotaslou, R.,Leylabadlo, H.E. andAsl, Y.M. (2015). Preva-
lence of antibiotic resistance in Helicobacter pylori: A recent
literature review. World Journal of Methodology, Vol. 5, No. 3,
pp. 164-74.
Grigore, A., Paraschiv, I., Colceru-Mihul, S., Bubueanu, C.,
Draghici, E. and Ichim, M. (2010). Chemical composition and
antioxidant activity of Thymus vulgaris L. volatile oil obtained
by two different methods.Romanian Biotechnological Letters,
Vol. 15, No. 3, pp. 5436–5443.
Ho, S.A.,Hoyle, J.A. andLewis, F.A. (1991). Direct polymer-
ase chain reaction test for detection of Helicobacter pylori in
humans and animals. Journal of Clinical Microbiology, Vol. 29,
No. 11, pp. 2543-2549.
Imelouane, B., Amhamdi, H., Wathelet, J.P., Ankit, M., Khedid,
K. and El Bachiri, A. (2009). Chemical Composition and Anti-
microbial Activity of Essential Oil of Thyme (Thymus vulgaris)
from Eastern Morocco. International Journal of Agricultural
Biology, Vol. 11, No. 2, pp. 205–208.
Kowalski, R. and Wawrzykowski, J. (2009). Essential oils anal-
ysis in dried materials and granulates obtained from Thymus
vulgaris L., Salvia of cinalis L., Mentha piperita L. and Cham-
Parisa Moradi and Neda Saffari
142 ANTI-HELICOBACTER PYLORI EFFECTS OF
THYMUS DAENENSIS
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
omilla recutita L.Flavour and Fragrance Journal, Vol. 24, No.
1, pp. 31–35.
Mastromarino, P.,Conti, C.,Donato, K.,Strappini, P.M.,Cat-
taruzza, M.S. andOrsi, G.B. (2005). Does hospital work consti-
tute a risk factor for Helicobacter pylori infection. Journal of
Hospital Infections, Vol. 60, No. 3, pp. 261-268.
Mojab, F., Poursaeed, M., Mehrgan, H. and Pakdaman, S.
(2008). Antibacterial activity of Thymus daenensis methanolic
extract. Pakistan Journal of Pharmaceutical Science, Vol. 21,
No. 3, pp. 210-3.
Pirbalouti, A.G., Hashemi, M. and Ghahfarokhi, F.T. (2013).
Essential oil and chemical compositions of wild and cultivated
Thymus daenensis Celak and Thymus vulgaris L. Industrial
Crops and Products, Vol. 48, pp. 43–48.
Rechinger, K.H. (1982). Flora Iranica. Eriocaulaceae. Lfg. No.
89, Vol 152. Austria, Graz, Akademische Druck und Verlag-
sanstalt.
Rimbara, E.,Fischbach, L.A. andGraham, D.Y. (2011). Optimal
therapy for Helicobacter pylori infections. Nature Review Gas-
troenterology and Hepatology, Vol. 8, No. 2, pp. 79-88.
Rota, M.C., Herrera, A., Martínez, R.M., Sotomayor, J.A. and
Jordán, M.J. (2008). Antimicrobial activity and chemical
composition of Thymus vulgaris, Thymus zygis and Thymus
hyemalis essential oils.Food Control, Vol. 19, No. 7, pp. 681–
687.
Shrestha, S.,Paudel, P.,Pradhan, G.B.,Shrestha, L. andBhat-
tachan, C.L. (2012). Prevalence study of H. pylori infection in
dyspeptic patients coming to Nepal Medical College Teaching
Hospital, Jorpati, Kathmandu. Nepal Medical Collection Jour-
nal, Vol. 14, No. 3, pp. 229-233.
Stahl-Biskup, E. and Saez, F. (2002). Thyme, The genus Thy-
mus, Taylor and Francis, p. 331
Suerbaum, S. and Michetti, P. (2002). Helicobacter pylori infec-
tion. New England Journal of Medicine, Vol. 347, pp. 1175-86.
Vu, C. and Ng, Y.Y. (2000). Prevalence of Helicobacter pylori in
peptic ulcer disease in a Singapore hospital. Singapore Medical
Journal, Vol. 41, No. 10, pp. 478-481.
Yang, J.C.,Lu, C.W. and Lin, C.J. (2014). Treatment of Heli-
cobacter pylori infection: current status and future concepts.
World J Gastroenterology, Vol. 20, No. 18, pp. 5283-93.