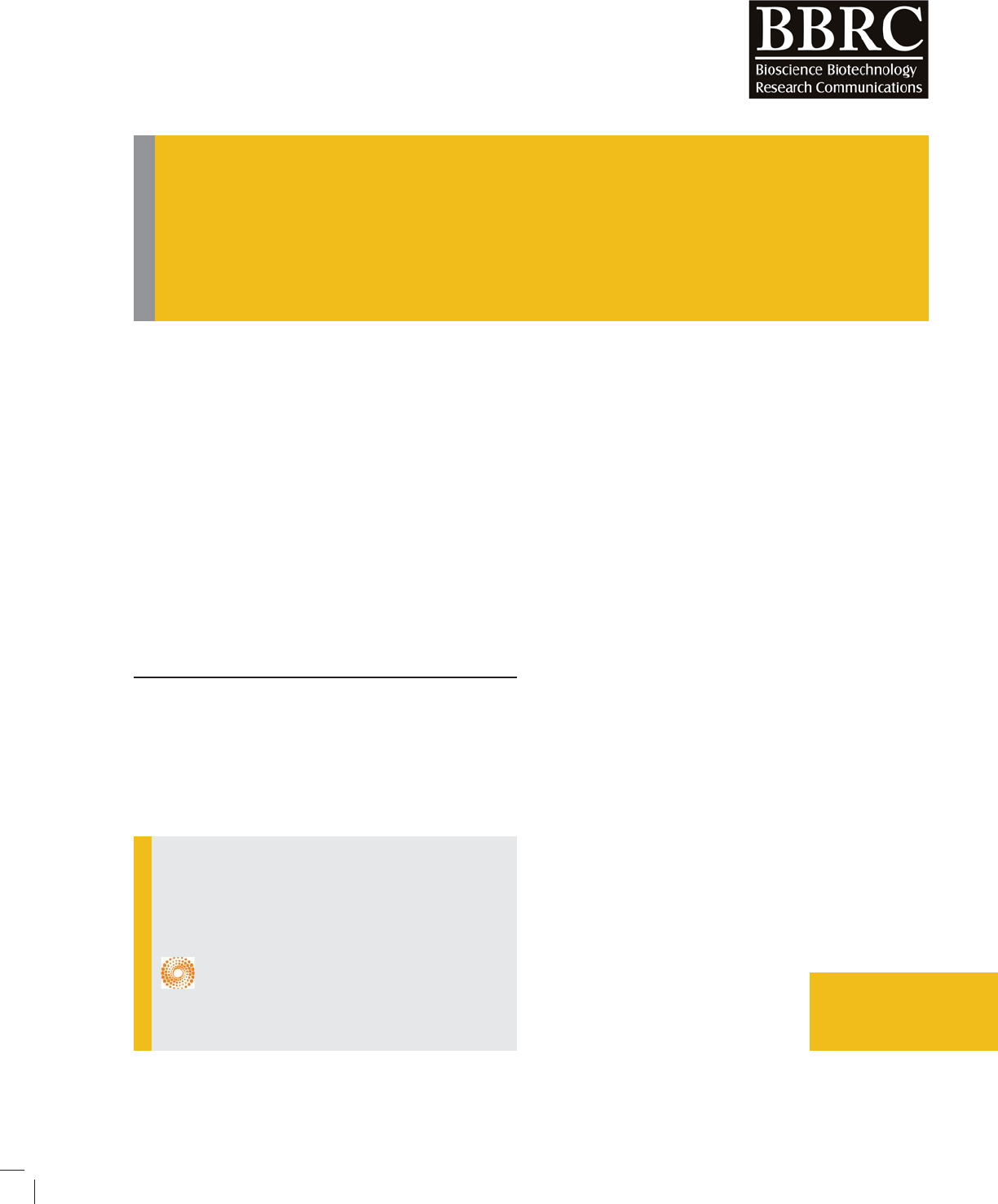
Pharmaceutical
Communication
Biosci. Biotech. Res. Comm. 10(1): 44-50 (2017)
Evaluation of antimicrobial and synergistic effects of
selected medicinal plants of Hail area with antibiotics
Abdel Moneim E. Sulieman, Sherif M. Shaarawy, Ahmed A. Alghamdi, Vajid N. Veettil*,
Mohanad Abdelgadir and Nasir A. Ibrahim
Department of Biology, Faculty of Science, University of Hail, Hail, Kingdom of Saudi Arabia
ABSTRACT
In this study, the antibacterial activity of selected medicinal plants and their synergistic effect with antibiotics were
investigated. Different antibiotic resistant bacterial species were employed including: Escherichia coli (E. coli), Kleb-
siella pneumoniae (K. pneumonia), Acinetobacter baumannii (A.baumannii), Pseudomonas aeruginosa (P. aeruginosa),
Staphylococcus aureus (S. aureus) and Salmonella spp. The results indicated that methanol extracts of most of the
tested plants inhibited growth of the tested bacteria, however, the highest inhibition was by Senna followed by Har-
mal, saf ower and Guaada, where the inhibition zone diameter was 8 mm, 4 mm, 3 mm, 2 mm and 2 mm, respec-
tively. The synergism between plants extract and antibiotics resulted in inhibition of the tested organisms; generally,
the synergism with Harmal extract was more effective in inhibition of antibiotic-resistant bacteria, followed by Senna
and nally Saf ower. The outcomes additionally demonstrated that methanolic concentrate of the plant has more
inhibitory impact than that of the water extract. Further investigation of the plants extract to seclude and recognize
the dynamic xings is prescribed.
KEY WORDS: ANTIBIOTIC-RESISTANT BACTERIA, INHIBITION ZONE, SAFFLOWER, METHANOL.
44
ARTICLE INFORMATION:
*Corresponding Author: vajidnv@gmail.com
Received 12
th
Jan, 2017
Accepted after revision 22
nd
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Plants as a source of medicinal compounds have kept on
assuming to play a dominant role in the maintenance
of human health since ancient times. As indicated by
the World Health Organization plant extracts or their
dynamic constituents are utilized as people prescription
in customary treatments of 80% of the total population.
More than half of all modern clinical drugs are of regu-
lar item source (Kirbag et al., 2009). Medicinal plants
possess immunomodulatory and antioxidant properties,
prompting to antibacterial activities. They are known
to have adaptable immunomodulatory activity by
stimulating both non-particular and particular immu-
nity (Pandey and Chowdhry, 2006). In the most recent
couple of years, various reviews have been directed in

Abdel Moneim E. Sulieman et al.
various countries to demonstrate such effectiveness.
Many plants have been utilized in light of their antimi-
crobial traits, which are due to compounds synthesized
in the secondary metabolism of the plants, (Nascimento
et al., 2000 Amenu 2014, Wink 2015 and Egamberdieva
et al., 2017).
Antimicrobial screening of plant extracts and phy-
tochemicals speaks to a beginning stage for antimicro-
bial medication disclosure. Phytochemical studies have
pulled in the consideration of plant researchers because
of the advancement of new and complex strategies.
These systems assumed a critical part in the scan for
extra assets of crude material for pharmaceutical indus-
try. (Shakeri et. al., 2012).
Microbiologists differentiate two groups of antimicro-
bial agents used in the treatment of infectious disease:
antibiotics, which are natural substances produced by
certain groups of microorganisms, and chemotherapeu-
tic agents, which are chemically synthesized. A hybrid
substance is a semisynthetic antibiotic, wherein a molec-
ular version produced by the organism is in this way
adjusted by the scienti c expert to accomplish desired
properties. Furthermore, some antimicrobial substances,
originally discovered as microbial products, can be man-
ufactured entirely by chemical methods. In the medi-
cal and pharmaceutical worlds, all these antimicrobial
agents used in the treatment of disease are referred to
as antibiotics. Different antibiotics practice their inhibi-
tory action on various pathogenic organisms (Chanda
and Rakholiya, 2011). Multiple drug resistance in human
pathogenic microorganisms has been developed due to
unpredictable utilization of commercial antimicrobial
ordinarily utilized as a part of the treatment of infectious
diseases.
In general, bacteria have the genetic ability to trans-
mit and acquire resistance to drugs used as therapeutic
agents (Nascimento et. al. 2000). The improvement of
antimicrobial resistance is multifactorial, including the
particular way of the relationship of bacteria to antibi-
otics, the utilization of antibacterial agent, host charac-
teristics and environmental factors. This situation has
constrained researchers to scan for new antimicrobial
substances from different sources as novel antimicro-
bial chemotherapeutic agents, yet the cost generation
of manufactured medications is high and they create
antagonistic impacts contrasted with plant determined
medications (Abiramasundari et. al., 2011).
Due to development of bacterial resistance to many
of the extensively utilized antibiotics has necessitated
the scan for new antibacterial agents or a mix of drugs
to have the capacity to combat new resistant patho-
genic bacteria. Therefore, in this study, we evaluated
the antimicrobial activity of selected medicinal plants
in Hail area and the possible synergism between water
and methanol extracts of these plants and certain known
antibiotic drugs.
MATERIAL AND METHODS
COLLECTION OF THE SELECTED PLANT
SAMPLES
A total of 7 owering plants growing wildly were col-
lected from various geographical sites in Hail area, in
addition to commercial samples. The plant materials
included: Pergularia tomentosa L. (Umlebena), Peganum
harmala L. (Harmal), Senna italica Mill (Senna), Aspho-
delus stulosus (Bargog), Teucrium polium (Guaada)
and Carthamus tinctorius (Saf ower) were collected
from various sites in Hail area. The plants were identi-
ed through consultation of the ora of Saudi Arabia
(Chaudhary, 2008), The plants samples were representa-
tive of medicinal plants as a whole. Repeated sampling
was implemented. The collected plant materials were
rinsed thoroughly with distilled water to remove extra-
neous contaminants and then cut into small pieces,
oven-dried at 50°C until the dry weight stabilized, and
ground into a powder with an electric-grinder.
Tested microorganisms
The tested bacteria used in the study which were anti-
biotic resistance, included: Escherichia coli (E. coli),
Klebsiella pneumoniae (K. pneumonia), Acinetobacter
baumannii (A.baumannii), Pseudomonas aeruginosa (P.
aeruginosa), Staphylococcus aureus (S. aureus) and Sal-
monella spp. These microorganisms were obtained from
King Khalid Hospital, Hail. The microorganism were
maintained on Brain Heart Infusion (BHI) agar bauman-
nii medium (HiMedia) at 4 ºC for further experiments.
Preparation of plant extracts standard concentrations
For aqueous extraction, 20 g of air-dried plant powder
were added to150 ml of distilled water and boiled on
slow heat for 2 hours. Then it was ltered through 8
layers of muslin cloth and centrifuged at 5000g for 10
min and the supernatant was collected. This procedure
was repeated twice; after 6 hours, the supernatant was
collected at an interval of 2 hours, pooled together and
concentrated to make the nal volume one-fourth of the
original volume
One g of each aqueous extract and alcohol pre-
prepared (each separately) were taken and the aqueous
extract was dissolved in 5 grams sterile distilled water,
while alcoholic extracts was dissolved in 5 ml of DiMe-
thyl Sulphoxide (DMSO). Thus 200 mg / ml of stock
were obtained as a standard concentration of aqueous
and alcoholic extracts. Aqueous extracts were sterilized
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF ANTIMICROBIAL AND SYNERGISTIC EFFECTS 45
Abdel Moneim E. Sulieman et al.
using 0.22 μm membrane lters and alcoholic extracts
was pasteurized for 15 minutes at temperature 62 ºC.
Preparation of culture media, inoculums, reagents and
antibiotics
Types of media which was used include: Brain Heart
Infusion broth, Nutrient agar (biolife) and Mueller-
Hinton agar (HiMedia). Also methanol and water were
used for extraction process. These media and the solvent
were purchased from some company in Jeddah city.The
Antibiotics mixture used in the study included: Van-
comycin, Cefotaxime, Tetracyclines, Chloramphenicol
and Ampicillin, these were purchased from pharmacies
in Hail city. For preparation of inocula, stock cultures
were maintained at 4°C on nutrient agar slants for bac-
teria. Active cultures for experiments will be prepared by
transferring a loopful of culture to 5 ml of Brain Heart
Infusion broth and incubated at 37 °C for 24 hours.
Plant extracts activity assay
A suspension of testing microorganisms was spread on
Muller Hinton Agar (MHA) medium. The lter paper
discs (5mm in diameter) will be placed on the agar plates
which were inoculated with the tested microorganisms
and then impregnating with 20μl of plant extract (con-
centration 200 mg/ml). The plates were subsequently
incubated at 37°C for 24 Hrs. After incubation the
growth inhibition zone was quanti ed by measuring the
diameter of the zone of inhibition in mm (Kumar et al.,
2009).
Synergism between plant extract, antibiotics and Non-
antibiotics
The bacterial cultures were grown in BHI broth at 37º C.
After 4 h of growth, each bacterium was inoculated on
the surface of Mueller-Hinton agar plates. Subsequently,
the antibiotic disk (diameter =5mm) were placed on the
surface of each inoculated plate and then added 20 μl
of plant extract, to identify synergies effect between the
plant extract at a concentration of 200mg/ml) and anti-
biotics. The plates were incubated at 37º C for 24 h. The
diameters of clearing zones will be measured.
REULTS AND DISCUSSION
Medicinal plants used in the study
Plants are the greatest medication stores ever known on
Earth, interminable bioactive invention blends which
in uence animal and human health (Abdallah et. al.,
2012). They are rich in secondary - metabolites and are
potential source of drugs which can serve to increase the
income of the producers. Detailed information regard-
ing the plant families, the scienti c and common names,
the families of tested plant species, the plant parts used,
the solvents used for extraction, have been tabulated in
Table (1).
The antibacterial properties
The present study investigated the effect of water and
methanol extracts of the tested plants which included:
Pergularia tomentosa (Umlubena), Peganum harmala
(Harmal), Senna italica (Senna), Carthamus tinctorius
(Saf ower) and Teucrium polium (Guuda) on growth of
the tested bacteria which included: P. aeruginosa, E. coli,
A. baumannii and K. pneumonia. From the results it is
clear that water extracts of most of the tested plants did
not inhibited growth of most of the tested bacteria with
exception to P. aeruginosa which was inhibited by Senna,
Umlubena and Harmal where the inhibition zone diame-
ter was 2 mm, 6 mm and 3 mm, respectively. On the other
hand, E. coli bacterial growth was inhibited by all metha-
nolic extract of the tested medicinal plants, however, the
highest inhibition was by Senna followed by Harmal, saf-
ower and Guaada , where the inhibition zone diameter
was 8 mm, 4 mm, 3 mm, 2 mm and 2 mm, respectively.
Table 1. Medicinal plants used in the study
Pl ant scienti c name Plant common name Family Plant part Solvent used
Pergularia tomentosa L Umlubena Asclepiadaceae Leaves 1- Water (W)
2- Methanol (M)
Peganum harmala L Harmal Zygophyllaceae Leaves 1- Water (W)
2- Methanol (M)
Cassis italic Mill Senna (Senamaka) Fabaceae Leaves 1- Water (W)
2- Methanol (M)
Asphodelus stulosus . Bargog Asphodelaceae Leaves 1- Water (W)
2- Methanol (M)
Carthamus tinctorius Saf ower Asteraceae Leaves 21- Water (W)
2- Methanol (M
Teucrium polium Guaada Lamiaceae Leaves 1- Water (W)
2- Methanol (M
46 EVALUATION OF ANTIMICROBIAL AND SYNERGISTIC EFFECTS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Abdel Moneim E. Sulieman et al.
FIGURE 1. Inhibition zone diameters of the tested plants against
the tested bacteria
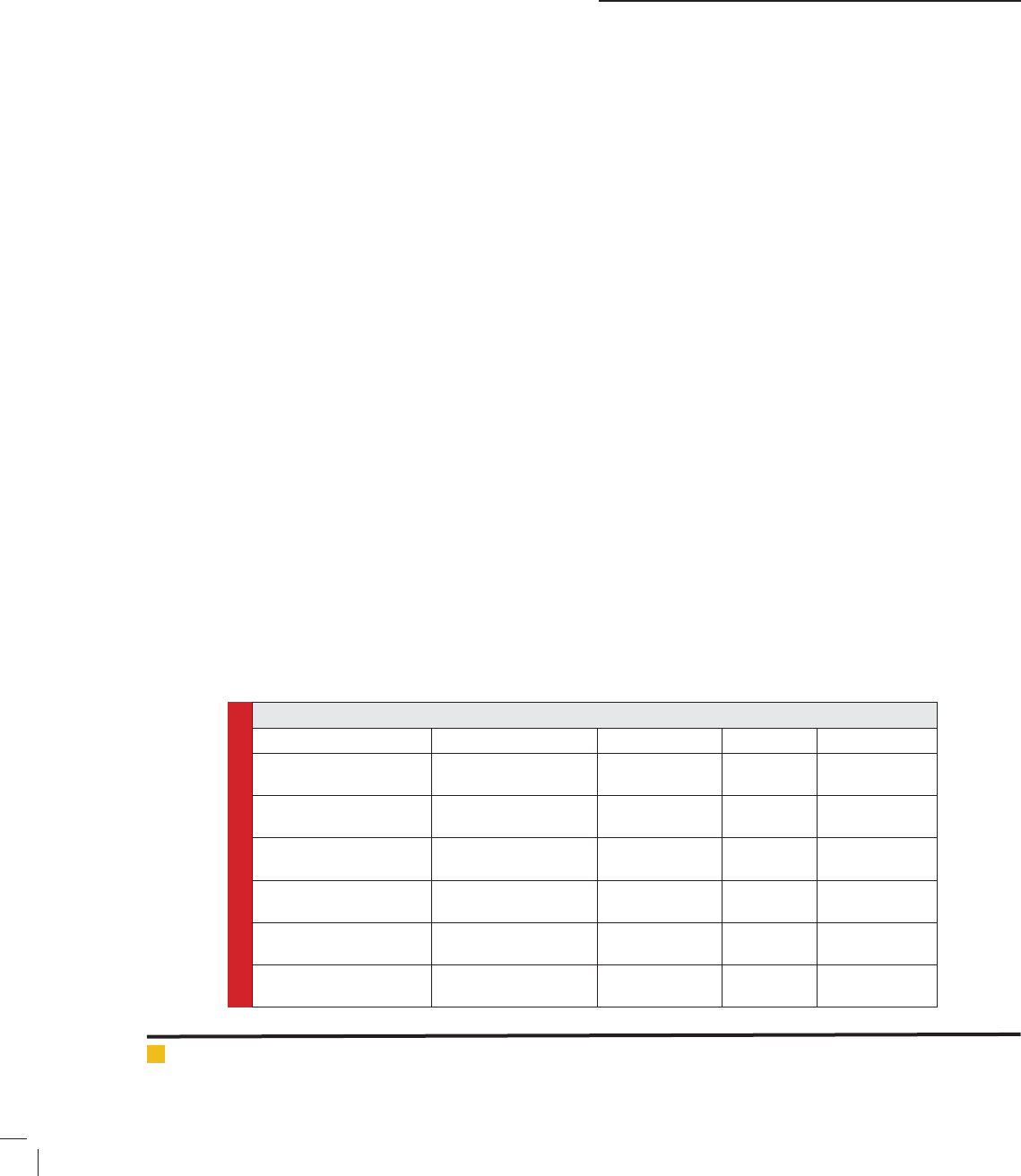
FIGURE 2. Inhibition of S.aureus by the tested plants , antibiotic
and synergetic effect between plant extract and antibiotic
A. baumannii was highly inhibited by Harmal fol-
lowed by Saf ower, then Senna, however, and the inhi-
bition zone diameter was, 13 mm, 5 mm and 3 mm,
respectively. However, the microbe was not inhibited
by either Bargog or Guaada plant extract. Methanolic
extract of all tested medicinal plants did not inhibit
growth of P. aeruginosa although the water extract of
some of these plants inhibited its growth.K. pneumo-
nia bacteria was only inhibited by methanolic extract of
Harmal and Senna where the inhibition zone diameter
was 3 mm and 2 mm, respectively.
In general, the mathanolic extract of the tested medic-
inal plants has more inhibitory effect against the tested
bacteria compared to the water extract of these plants.
Moreover, both Harmal and Senna plants exhibited the
highest antibacterial effects compared to the other tested
plants.
Synergism between plant extract and antibiotics
The synergism between plant extract and antibiotics is
presented in Fig. (2-5). It is clearly seen that all tested
plants inhibited the tested microorganism by using anti-
biotics and the inhibition zones increased upon adding
the antibiotics to the plant extracts. This means the syn-
ergism between plant extracts and antibiotics resulted
in inhibition of tested microorganisms with varying
degrees. However, Harmal plant exhibited the highest
inhibition, followed by Senna and nally Saf ower.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF ANTIMICROBIAL AND SYNERGISTIC EFFECTS 47
Abdel Moneim E. Sulieman et al.
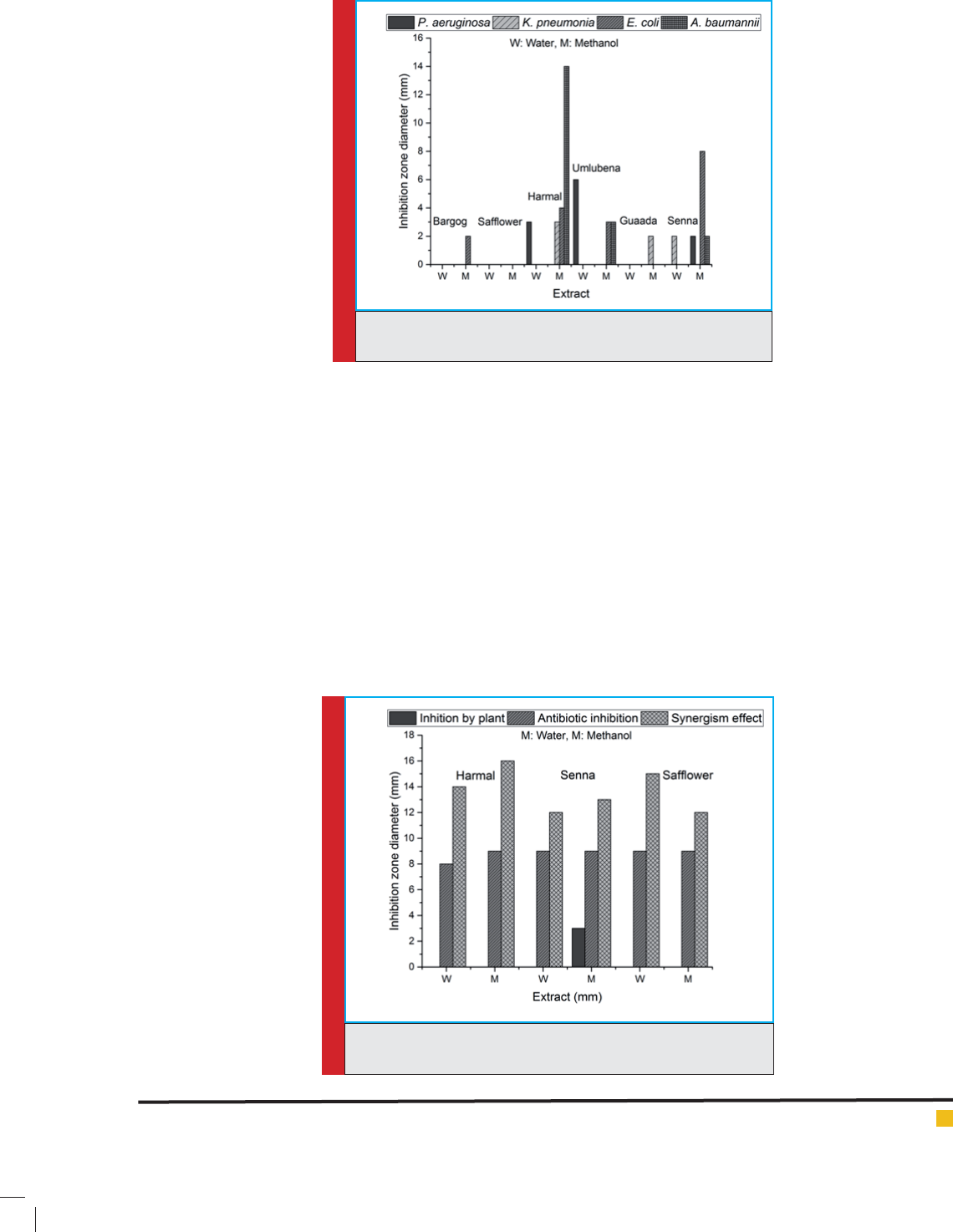
FIGURE 3. Inhibition of K. pneumonia by the tested plants , antibiotic
and synergetic effect between plant extract and antibiotic
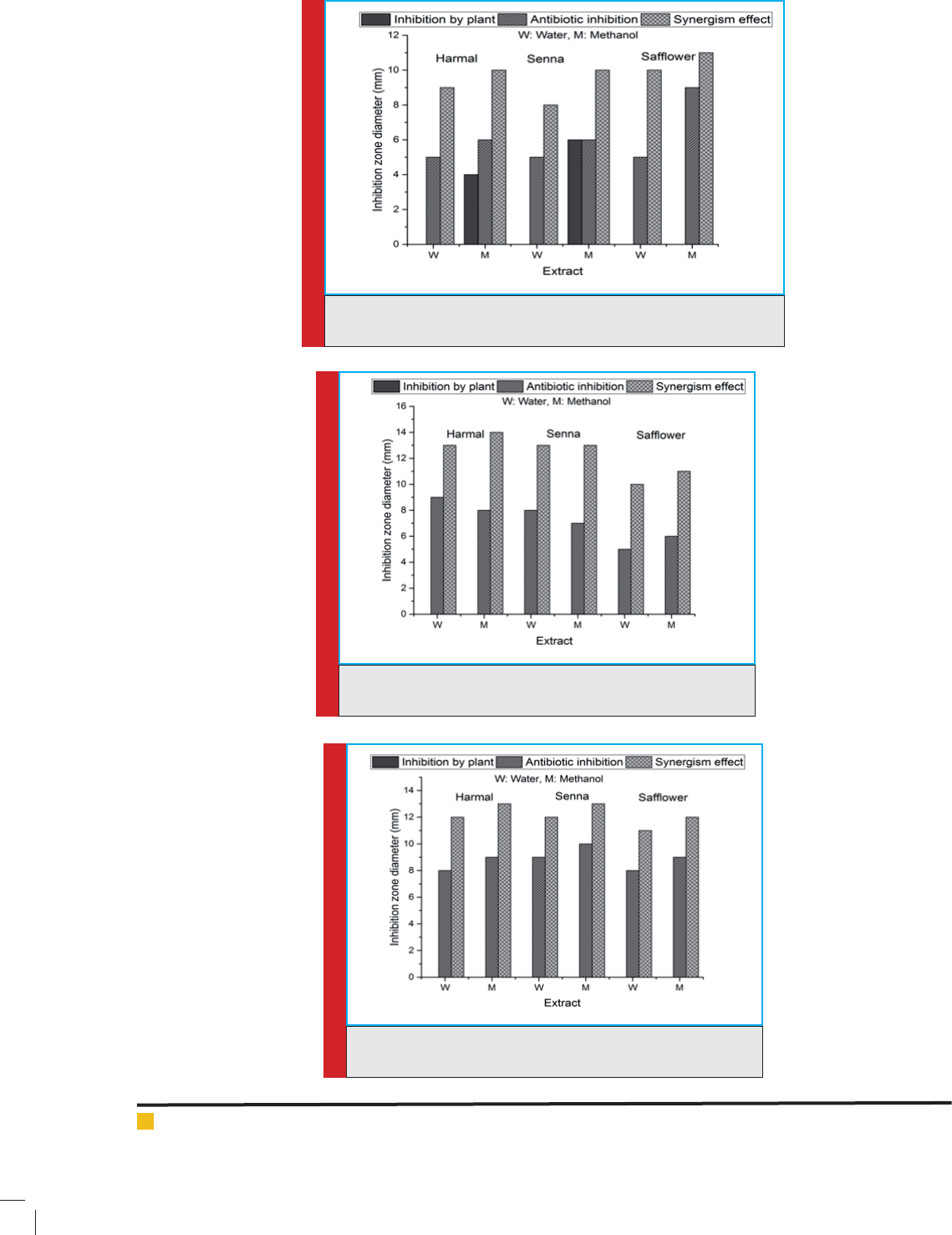
FIGURE 4. Inhibition of E.coli by the tested plants , antibiotic
and synergetic effect between plant extract and antibiotic
FIGURE 5. Inhibition of A. baumannii by the tested plants, anti-
biotic and synergetic effect between plant extract and antibiotic
48 EVALUATION OF ANTIMICROBIAL AND SYNERGISTIC EFFECTS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Abdel Moneim E. Sulieman et al.
Moreover, the methanol extract was more effective than
water extracts for all tested plants.
Generally, the synergism between Harmal extract
was more effective in inhibition of antibiotic-resistant
bacteria, followed by Senna and nally Saf ower. The
most inhibited microorganism was Staphylococci aureus
where the inhibition zone diameter was 16 mm and 9
mm for the combination of Harmal extract plus antibi-
otic and for antibiotic only, respectively.
Eschrichia coli bacteria were highly inhibited by Har-
mal, Senna and Saf ower water and methanolic extract
combined with antibiotics. However, Harmal-antibiotic
combination has the highest inhibitory effect followed
by Senna-antibiotic and saf ower-antibiotic, where the
inhibition zone diameter was 14 mm, 13 mm and 12 mm
, respectively. Moreover, the methanolic extracts of the
tested plants with added antibiotic was more effective
compared to the water extracts of the tested plants with
added antibiotic. Acinetobacter baumannii was inhibited
by Harmal-antibiotic and Senna-antibiotics combinations
at the same extent and the inhibition zone diameter of the
methanolic and water extract combined with antibiotics
was 13 mm and 10 mm, respectively. Saf ower water and
methanolic extract also inhibited the Acinetobacter bau-
mannii, however, combination of the methanolic plant
extract with antibiotic was more effective.
In the present study, the plant extracts had different
synergistic ability to inhibit the growth of microorgan-
isms. However the zones of inhibition of all tested micro-
organisms resulted from plant extract-antibiotic combi-
nations were greater than those resulted from antibiotics
merely. Therefore, increased plants antimicrobials have
been found to be synergistic enhancers in that though
they may not have any antimicrobial properties alone,
but when they are taken concurrently with antibiotics
they enhance the effect of these drugs.
Drug synergism between antimicrobial agents and
plant extracts has been reported (Nascimento et. al.,
2000; Aburjai et. al., 2001; Aqil et. al., 2005; Esimon et.
al., 2006; Ibezim et. al., 2006).
Staphylococcus aureus is recognized as one of the
major causes of infections in humans occurring in both
the community and the hospital. Methicillin-resistant and
multidrug resistant staphylococci have become a major
nosocomial pathogen (NNIS System, 2004). Therefore, it
is of vital importance to identify new effective antimicro-
bial agents. Among the potential sources of new agents,
medicinal plants have long been investigated.
CONCLUSION
In c onclusion, the medicinal plants which included: Per-
gularia tomentosa L. (Umlabina), Peganum harmala L.
(Harmal), Cassis italic Mill (Senna), Asphodelus stulo-
sus (Bargog), Teucrium polium (Guaada) and Carthamus
tinctorius (Saf ower) collected from various sites in Hail
area have antimicrobial properties against the tested
antibiotic-resistant bacteria with various degrees which
may re ect the antibacterial activity of plant active
ingredients that inhibit bacterial growth. However, both
Harmal and Senna plants exhibited the highest anti-
bacterial effects against the antibiotic-resistant bacteria
compared to other tested plants. The results will also
reveal the importance of plant extracts when associated
with antibiotic drugs in control of pathogenic and spoil-
age bacteria. Future investigation of the plants extract
to isolate and recognize the active ingredients is highly
recommended.
ACKNOWLEDGEMENTS
The authors express their gratitude to the Deanship of
Scienti c Research of University of Hail, Saudi Arabia
for nancing this research (Project No. 01150009).
REFRENCES
Abiramasundari.P, Priya .V, Jeyanthi.G.P, and Gayathri Devi.
S (2011). Evaluation of the Antibacterial activity of Cocculus
hirsutus. Hygeia. Journal for Drugs and Medicines vol.3 (2),
26-31.
Aburjal T, Darwish RM, Al-Khalil S, Mahgzah A, Al-Abbdi A
2001. Screening of antibiotic resistant inhibitors from local
plant materials against two different strains ofPseudomonas
aeruginosa.J Ethnopharmacol 76: 39-44.
Amenu D (2014) Antimicrobial Activity of Medicinal Plant
Extracts and Their Synergistic Effect on Some Selected Patho-
gens American Journal of Ethnomedicine, Vol. 1, No. 1, 018-
029
Aqil F, Khan MSA, Owais M, Ahmad I 2005. Effect of cer-
tain bioactive plant extracts on clinical isolates of ß-lactamase
producing methicilin resistantStaphylococcus aureus. J Basic
Microbiol 45: 106-114.
Chanda. S and Rakholiya. K (2011). Combination therapy: Syn-
ergism between natural plant extracts and antibiotics against
infectious diseases. Science against microbial pathogens: com-
municating current research and technological advances A.
Méndez-Vilas (Ed.).
Chang, Y.L., King, B., Lin, S.C., Kennison, J.A., Huang, D.H.
(2007). A double-bromo domain protein, FSH-S, activates the
homeotic gene Ultrabithorax through a critical promoter-prox-
imal region. Mol. Cell. Biol.27(15)
: 5486--5498.
Choudhary. K., Singh. M and Pillai. U (2008). Ethnobotanical
Survey of Rajasthan - An Update. American-Eurasian Journal
of Botany, Vol.1 (2): 38-45
Egamberdieva D. S. Wirth U Behrendt P Ahmad G Berg (2017)
Antimicrobial Activity of Medicinal Plants Correlates with the
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF ANTIMICROBIAL AND SYNERGISTIC EFFECTS 49
Abdel Moneim E. Sulieman et al.
Proportion of Antagonistic Endophytes Front. Microbiol., 09
February 2017 |https://doi.org/10.3389/fmicb.2017.00199
Esimone CO, Iroha IR, Ibezim EC, Okeh CO, Okpana EM (2006).
An in vitro Evaluation of the interaction between tea extracts
and penicillin G against Staphylococcus aureus by the decimal
assay for additivity (DAA) method. Afr. J. Biotechnol. 5(11):
1082 –1086
Ibezim EC, Esimore CO, Obodo CE, Nnamani PO Brown SA,
Onyishi IV (2006). A study of the in vitro interaction of cotri-
moxazole and ampicillin using the checker Board method. Afr.
J. Biotechnol. 5(13): 1284-1288
Kirbag. S, Zengin. F and Kursat. M (2009). Antimicrobial
Activities of Extracts of some Plants. Pakistan Journal of Bot-
any Vol.41(4): 2067-2070.
Kumar, V., Alla, S.R., Krishnan, K.S., Ramaswami, M. (2009).
Syndapin is dispensable for synaptic vesicle endocytosis at the
Drosophila larval neuromuscular junction. Mol. Cell. Neuro-
sci.40(2): 234-241.(Export to RIS)
Nascimento GGF, Locatelli J, Freitas PC, Silva GL (2000). Anti-
bacterial activity of plant extracts and phytochemicals on
antibiotic-resistant bacteria. Braz. J. Microbiol. 31: 247-256.
NNIS System. (2004). National Nosocomial Infections Surveil-
lance (NNIS) System Report, data summary from January 1992
through pJune 2004, issued October 2004. Am. J. Infect. Con-
trol 32: 470-485.
Pandey, A.K. and Chowdhry, P.K (2006) Propagation tech-
niques and harvesting time on productivity and root quality
of Withania somnifera. Journal of Tropical Medicinal Plants
Vol.7:79-81.
Shakeri. A, Hazeri.N, Vlizadeh. J, Ghasemi. A and Tavallaei. F
(2012). Photochemical Screening, Antimicrobial and Antioxi-
dant Activity of Anabasis aphylla L. Extracts. Kragujevac J.
Sci. 34: 71-78.
Wink M. (2015) Modes of Action of Herbal Medicines and Plant
Secondary Metabolites Medicines 2, 251-286; doi:10.3390/
medicines2030251
50 EVALUATION OF ANTIMICROBIAL AND SYNERGISTIC EFFECTS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS