Medical
Communication
Biosci. Biotech. Res. Comm. 10(1): 11-21 (2017)
Serotypes of dengue viruses circulating in Jazan
region, Saudi Arabia
A.A. Alsheikh
1
, O.M. Daffalla
1
, E.M. Noureldin
1
, W.S. Mohammed
1
, K.J. Shrwani
1
,
Y.A. Hobani
1
, A.D. Abkar
2
, F.A. Alsheikh
3
and A.M. Assiri
4
1
National Center for Vector-Borne Diseases, MoH-Jazan, Saudi Arabia
2
Department of Medical Parasitology, Faculty of Medical Laboratory Sciences, University of Gezira, Sudan
3
Faculty of Public Health and Tropical Medicine, Jazan University, Saudi Arabia
4
Minister Deputy Assistant for Preventive Health, MoH-Riyadh, Saudi Arabia
ABSTRACT
Dengue fever is considered to be the most important mosquito-borne disease and considered as endemic disease in
Jazan region, Saudi Arabia. The present study aimed to analyze the prevailing dengue virus serotypes for the rst
time in the region. Serum samples of 220 suspected dengue cases were collected throughout 2016 and tested by
one step Reverse Transcription Polymerase Chain Reaction (RT-PCR) with a set of speci c primers for detection of
four dengue virus serotypes followed by sequencing the PCR products to con rm the results. Out of the 220 serum
samples, 124 were found positive for dengue infection (56.4%). Three dengue virus serotypes were detected; DEN-1,
DEN-2 and DEN-3. DEN-2 is the most common and predominant type in the region rating 83.9% (104/124), followed
by DEN-1 8.9% (11/124), and then DEN-3 7.2% (9/124). The high seroprevalence of dengue virus infections in Jazan
region indicates its endemicity. The present study highlights the importance of tracking the spread of dengue virus
types and its implication for analyzing changes in dengue endemicity in speci ed areas over time. Complete genome
sequencing is required for the three detected dengue virus serotypes circulating in the region (DEN-1, DEN-2, and
DEN-3) to serve as references for any future epidemiological researches and/or outbreaks.
KEY WORDS: DENGUE FEVER, SEROTYPES, 1, 2, AND 3, JAZAN REGION, SAUDI ARABIA
11
ARTICLE INFORMATION:
*Corresponding Author: adelalsheikh@gmail.com
Received 1
st
Feb, 2017
Accepted after revision 26
th
March, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/

12 SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Alsheikh et al.
INTRODUCTION
Dengue fever is considered to be the most important
re-emerging vector-borne disease worldwide and is
endemic in more than 125 countries (Murray et al.,
2013). Four hundred million of cases are estimated to
occur annually (CDC, 2016). Dengue is a viral disease
transmitted to humans by the bite of infected females of
the main vector Aedes aegypti and to a lesser extent by
Aedes albopictus mosquitoes (WHO, 2009).
There are ve genetically related but antigenically
distinct single-stranded RNA serotypes belonging to
Flaviviridae family and genus F lavivirus; DEN-1, DEN-
2, DEN-3, DEN-4 (WHO, 1997), and DEN-5 (Mustafa
et al., 2015).
However, no cross protection occurred between the
dengue serotypes, the immunity is serotype speci c.
According to disease severity, the World Health
Organization has classi ed dengue into three categories;
Dengue Fever (DF), Dengue Hemorrhagic Fever (DHF),
and Dengue Shock Syndrome (DSS) (WHO, 1997).
Unplanned urbanization and climatic factors, includ-
ing high temperatures and rainfall, might contribute to
epidemics of dengue (Mackenzie et al., 2004; Crowell
et al., 2011; Banu et al., 2011).Aedes mosquito is found
in the urban settings, especially in tropical areas, where
it maintains a sustainable relationship with humans
leading to reemergence of dengue infections and creat-
ing a public health threat (Glenn and Sia, 2008).Spa-
tial patterns in the recent and sequential circulation of
DEN1-5, along with the host and virus genetics, should
be regarded as potential population risk factors for sever
forms of dengue fever (Guilarde et al., 2008; Chaturvedi
2006) because most secondary infection bearing heter-
ologous dengue virus type may lead to severe disease
complications (Green and Rothman, 2006; Vaughn et al.,
2000; Gibbons and Vaughn, 2002; Rico-Hesse, 2003).
In Saudi Arabia, the rst dengue outbreak has been
reported in 1994 in Jeddah with 289 con rmed cases,
and DEN-1 and DEN-2 were circulating dengue virus
serotypes (Fakeeh and Zaki, 2001). Since then, several
dengue fever outbreaks have been recorded in Saudi
Arabia (Fakeeh and Zaki, 2003; Ahmed, 2010; Khan et
al, 2008; Ayyub et al., 2006; El-Badry et al., 2013; Zaki
et al., 2008) and Yemen (Madani et al., 2013). The case
fatality rate was 4.6 per thousand in 2007 (Saudi Min-
istry of health, 2007).The incidence of dengue fever has
increased in Saudi Arabia during the past few years;
6512 cases in 2013; 2081 cases in 2014; and 4312 cases
in 2015 (Saudi Ministry of health, 2016).
The emergence of DEN-3 in Jeddah was in 1997
(Fakeeh and Zaki, 2001), and since then all the 3 dengue
serotypes (DEN 1–3) were being circulated in the city
(Azhar et al., 2015). Recently, Organji et al (2017) have
reported DEN-1, DEN-2, and DEN-3 to be circulated in
Makkah city. In Jazan region, there were 1790 con rmed
dengue cases between 2005 and 2016 with highest out-
breaks in 2016 (555 cases), followed by 2010 (290 cases),
and 2012 (289 cases) (Dengue control program in Jazan).
Al-Arzaqi et al (2013) reported dengue prevalence of up
to 26.5% in Jazan region, while Gamil et al (2014) noted
47.74% dengue positivity rate in the area.To the best of
our knowledge, no data has been published on the cir-
culation of dengue virus serotypes in Jazan region, thus
our present study is the rst of its own and aimed to
analyze the prevalence of dengue virus serotypes circu-
lating in Jazan region, southwest of Saudi Arabia.
MATERIAL AND METHODS
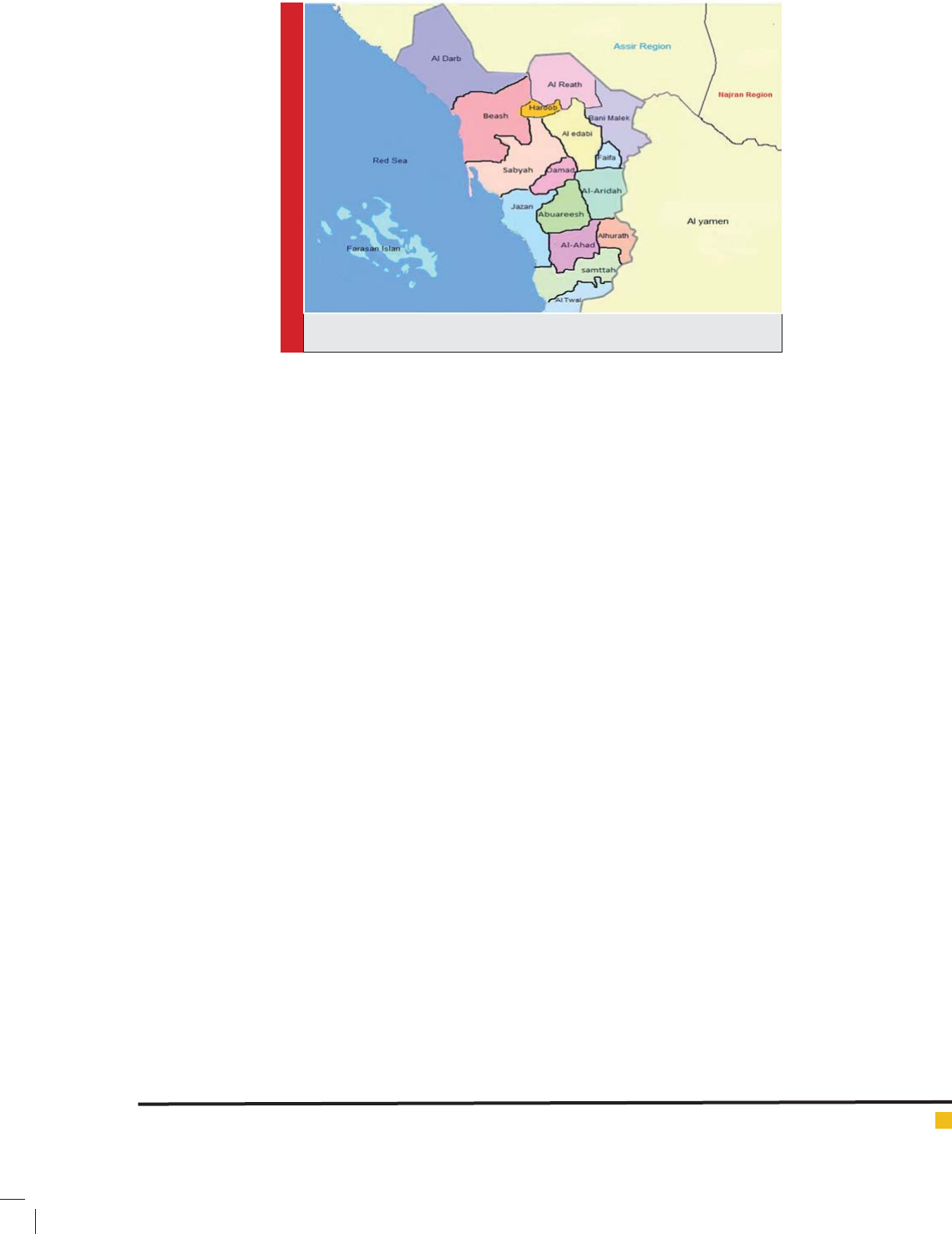
Study area: Jazan Region in Southwest Saudi Arabia lies
between 16°-12, and 18°-25, latitude north. It is bordered
in the South by Arabic republic of Yemen with total area
of about 22,000 km² and 1.3 million populations (cen-
sus 2011). Thirty percent of the population concentrated
in six major cities, and the remainders living in over
3500 villages (Alsheikh, 2011). Jazan region is situated
in the subtropical zone and has average monthly tem-
peratures ranging between 25.8°C in January to 33.4°C
in July. The average relative humidity ranges between
55% and 72.5%. The rainy season is started at August
through October with a monthly average of 77 and 56.7
mm, respectively (Alsheikh, 2011). Jazan is divided into
eleven small Governates (Al-Aridah, Damad, Twal, Al-
Ahad, Jazan , Al-Khobah, Samttah, Abuareesh, Sabyah,
Beash and Al-Darb), these locations (Fig.1) although
with different altitudes and geographical Characteristics,
they are almost share the same demographical, agricul-
tural, educational, cultural, housing, health system, and
environmental characteristics.
Sampling: During 2016 about 220 suspected dengue
fever patients serum samples included in this study were
collected from ve different hospitals in Jazan region
and stored at -80° till further use.
RNA isolation: High Pure Viral Nucleic Acid Kit
from Roche applied science (Germany) used for extrac-
tion of RNA follow the manufacture procedure; 200 µl
of binding buffer supplemented with poly (A) and 50
µl Proteinase K added to 200 µl of serum sample then
mixed immediately and incubated for 10 minutes at
72°C. Addition of 100 µl Proteinase K was mixed with
sample and transferred to High Filter Tube inserted into
Collection Tube. After centrifugation for 1 minute at
10000 rpm, the collection tube was discarded. The l-
ter tube combined with new collection tube and 500 µl
of inhibitor removal buffer was added and centrifuged
for 1 minute at 10000 rpm. After changing collection
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA 13
Alsheikh et al.
tube, the high lter tube washed twice by adding 450
µl of wash buffer at the same condition of centrifuga-
tion, followed by centrifugation for 15 seconds at 13000
rpm to remove any residual wash buffer. Then the high
lter tube was inserted into nuclease free, sterile 1.5 ml
centrifuge tube and 50 µl of elution buffer was added to
elute the viral nucleic acid by centrifugation at 10000
rpm for 1 minute.
Reverse Transcriptase Polymerase Chain
Reaction (RT-PCR)
One step RT- PCR is a rapid, sensitive, and simple for
dengue serotype-speci c diagnosis method. The test was
performed according to the protocol of Lanciotti et al
(1992) with some modi cation; DEN consensus prim-
ers and serotype-speci c primers (Table 1) were used to
amplify the viral genome in this study and synthesized
in Integrated DNA Technology (Belgium). The one step
RT-PCR reactions were performed according to access
RT-PCR–system protocol (Promega-USA) in total vol-
ume of 50 µl containing 10 µl of AMV/T 5X Reaction
Buffer, 1 µl of dNTP Mix (10mM each dNTP, nal con-
centration 0.2mM), 2 µl of 25mM MgSO4 ( nal concen-
tration 1mM), 1 µl of AMV Reverse Transcriptase 5u/
l ( nal concentration 0.1u/l), 1 µl of T DNA Poly-
merase 5u/l ( nal concentration 0.1u/l), 50pmol ( nal
concentration 1M) of each forward (D1) and reverse
(D2) primers, 5 µl of RNA virus and nuclease free water
to total volume 50 µl. The thermal cycling incubations
temperatures programmed as follows: incubation for 1
hour at 42°C (to convert the RNA to cDNA) then initial
denaturation for 3 minutes at 94°C followed by 35 cycle
of denaturation (94°C, for 30 second), primers annealing
(55°C for 1 minute), primer extension (72°C for 2 min-
utes) and nal extension for 5 minutes.
Nested-PCR
Nested PCR was performed in 2 tubes for each sample
in 50 l reaction mixture containing 25 µl GoTag®G2
green master mix ready to use from Promega, 10 µl of
the diluted (1:100) RT-PCR product, 50 pmol ( nal con-
centration 1M) of each forward primer D1 and TS1,
TS3 as reverse primers for the rst tube and TS2, TS4
as reveres primers for another tube. The samples were
subjected to initial denaturation at 94°C for 3 minutes,
30 cycles of denaturation (94°C, 30 s), primer annealing
(55°C, 1 min), primer extension (72°C, 2 min) and nal
extension for 5 minutes. In each run negative and posi-
tive controls were included. The PCR products of nested
ampli cation were analyzed by gel electrophoresis (1.5
agarose in Tris-Acetate EDTA buffer) staining with eth-
idium promide. The visualization was carried out using
Gel Doc XR Imaging System (Bio-Rad).
Sequencing and bioinformatics analysis
Puri cation and standard sequencing for RT- PCR prod-
ucts were performed by Macrogen Company (Seoul,
Korea). Sequencing reactions were performed in a MJ
Research PTC-225 Peltier Thermal Cycler using a ABI
PRISM® BigDyeTM Terminator Cycle Sequencing Kits
with AmpliTaq® DNA polymerase (FS enzyme) (Applied
Biosystems), following the protocols supplied by the
manufacturer. Single-pass sequencing was performed
on each template using D1 (forward) primer. The
uorescent-labeled fragments were puri ed from the
unincorporated terminators with Big Dye®X Terminator™
puri cation protocol.The samples were resuspended in
distilled water and subjected to electrophoresis in an ABI
3730xl sequencer (Applied Biosystems). The sequences
were searched for sequence similarity through BLAST
FIGURE 1. Map of Jazan region showing the different Governates.

Alsheikh et al.
14 SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
(www.ncbi.nlm.nih.gov/BLAST/) (Atschul et al., 1997)
and compared to reference sequences of Dengue sero-
types detected in BLAST and downloaded from GenBank
(www.ncbi.nlm.nih.gov/genbank/).
Similarity tree was obtained from database online by
phylogeny.fr (http://www.phylogeny.fr/).
RESULTS
RT-PCR and Nested-PCR: One hundred twenty four
samples out of 220 (56.4%) suspected patient serum
samples tested by RT-PCR were con rmed positive for
dengue virus when using D1 and D2 primers (511bp)
for all serotypes, and the RT-PCR product was used as
a sample for the nested-PCR using a set of serotype-
speci c primers pair as described in the methodology.
Three dengue virus types (DEN-1, DEN-2 and DEN-3)
were detected and the results showed that DEN-2 is the
most common and predominant type in Jazan region
rating one hundred four out of one hundred twenty four
(83.9%), followed by DEN-1 (eleven out of one hundred
twenty four, 8.9%), and then DEN-3 (nine of one hun-
dred twenty four, 7.2%) and serotype 4 was not detected
(Table 2 and Fig. 2).
Sequencing: To con rm the serotype-speci c results,
the partial sequencing was done for nineteen RT-PCR
product samples represent the three serotypes (DEN-1,
DEN-2, and DEN-3). The Blast search showed that the
sequences of our samples aligned along with many pub-
lished sequences of dengue virus serotypes as shown in
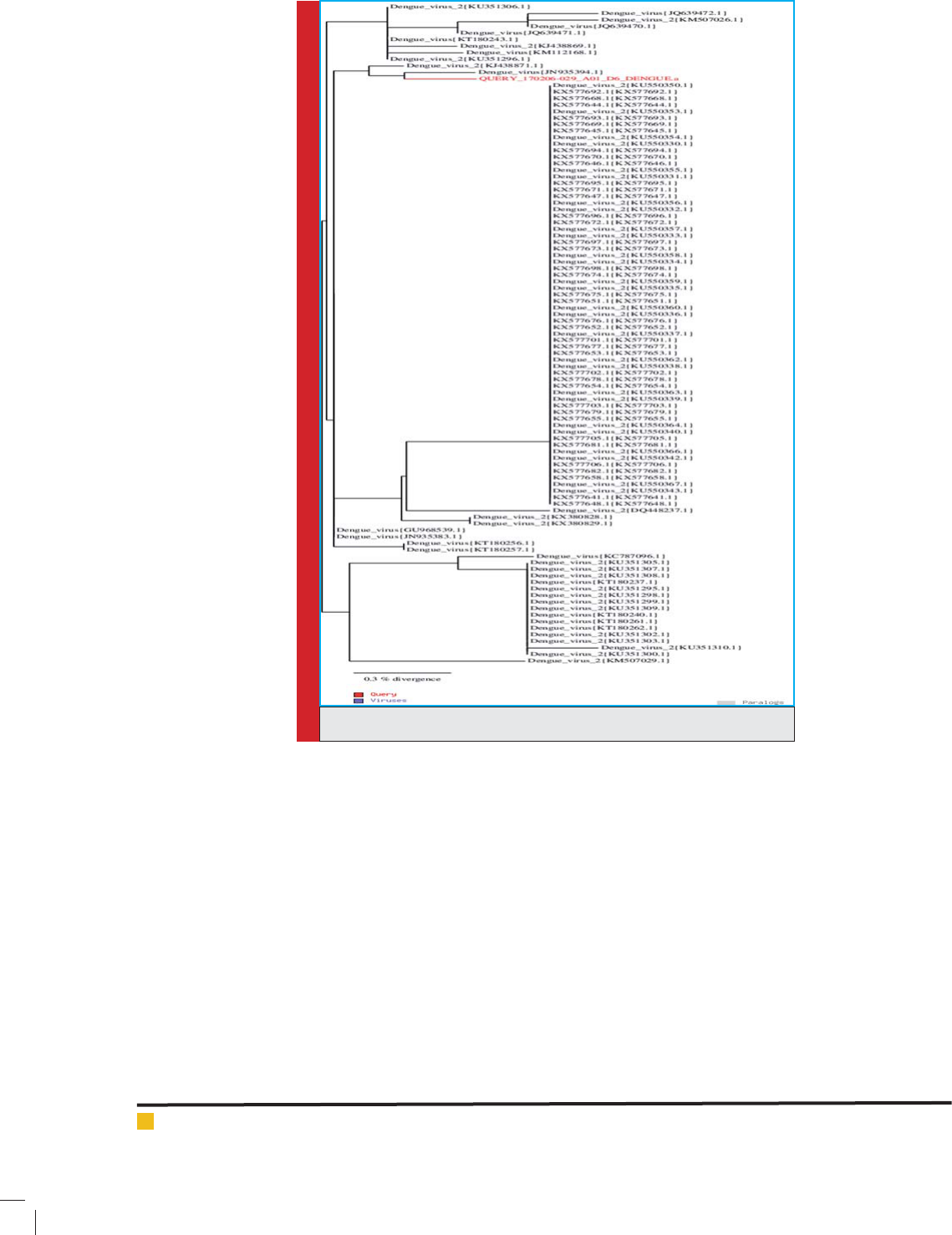
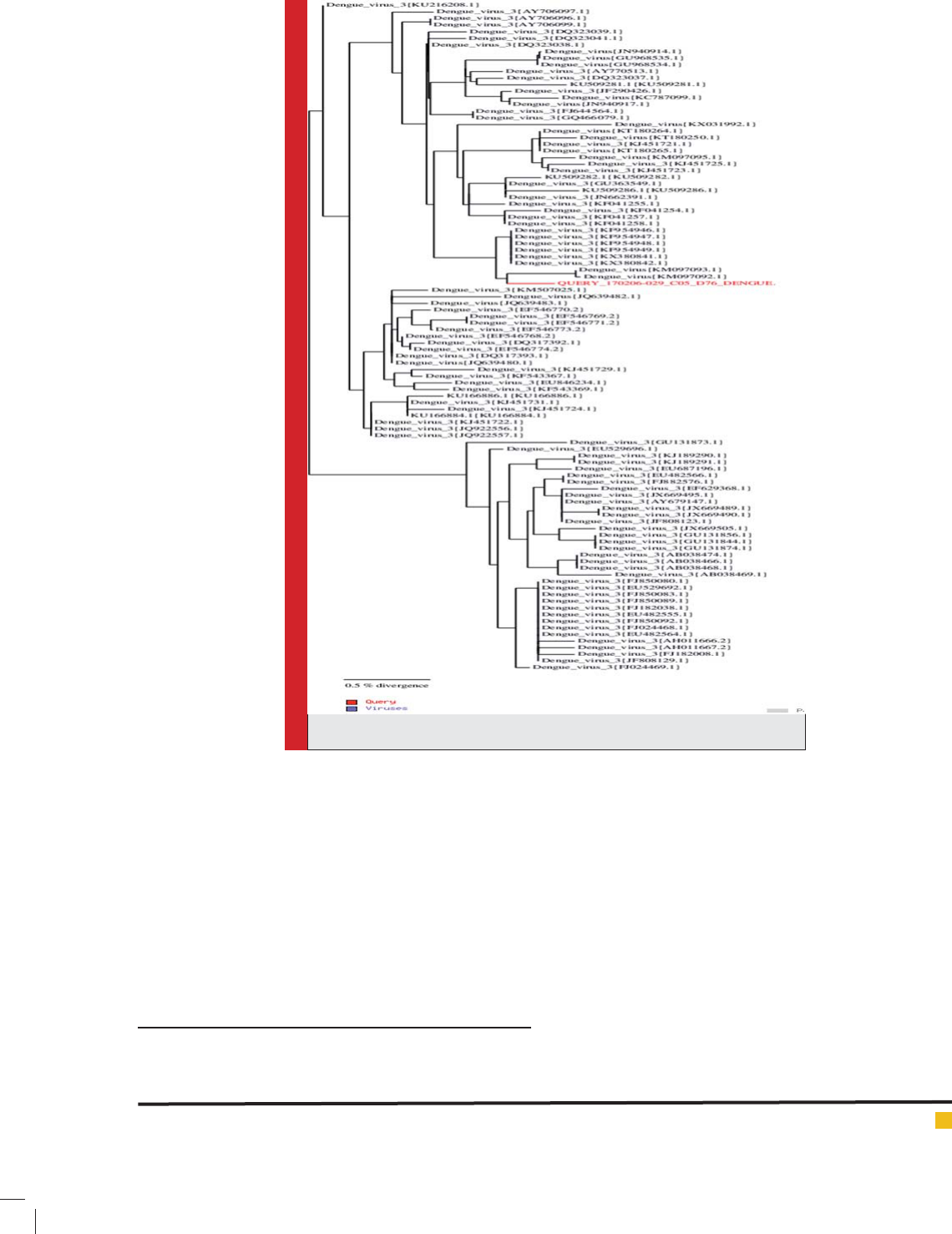
Table 3 and Fig.3, Fig.4 and Fig.5, and similarity tree
(Fig.6, Fig.7 and Fig.8) which illustrates the Gen bank
accession numbers and the country of isolates.
Table 1. oligonucleotide primers used in RT-PCR and Nested-PCR
primer Sequence 5 - 3
Genome
position
Size in bp
D1 TCAATATGCTGAAACGCGCGAGAAACCG 134-161 511
D2 TTGCACCAACAGTCAATGTCTTCAGGTTC 616-644 511
TS1 CGTCTCAGTGATCCGGGGG 568-586 482 (Dl and TS1)
TS2 CGCCACAAGGGCCATGAACAG 232-252 119 (Dl and TS2)
TS3 TAACATCATCATGAGACAGAGC 400-421 290 (Dl and TS3)
TS4 CTCTGTTGTCTTAAACAAGAGA 506-527 392 (Dl and TS4)
Table 2. Results of dengue virus serotyping using RT-PCR and nested-PCR
No of samples +ve DEN +ve DEN-1 +ve DEN-2 +ve DEN-3 +ve DEN-4
220 124 (56.4%) 11 (8.9%) 104 (83.9% ) 9 (7.2 %) 0 (0%)
FIGURE 2. Agarose gel electrophoresis of RT-PCR (D1, D2 primers) and
nested-PCR by the speci c primers. Lane 1 and 8 DNA 100bp marker,
lane (2) negative control, lane (3) positive sample DEN-1, lane (4,5)
positive samples DEN-2, lane (6) positive sample DEN-3 and lane (7)
positive RT-PCR product sample (D1 and D2 primers for all serotypes).
Alsheikh et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA 15
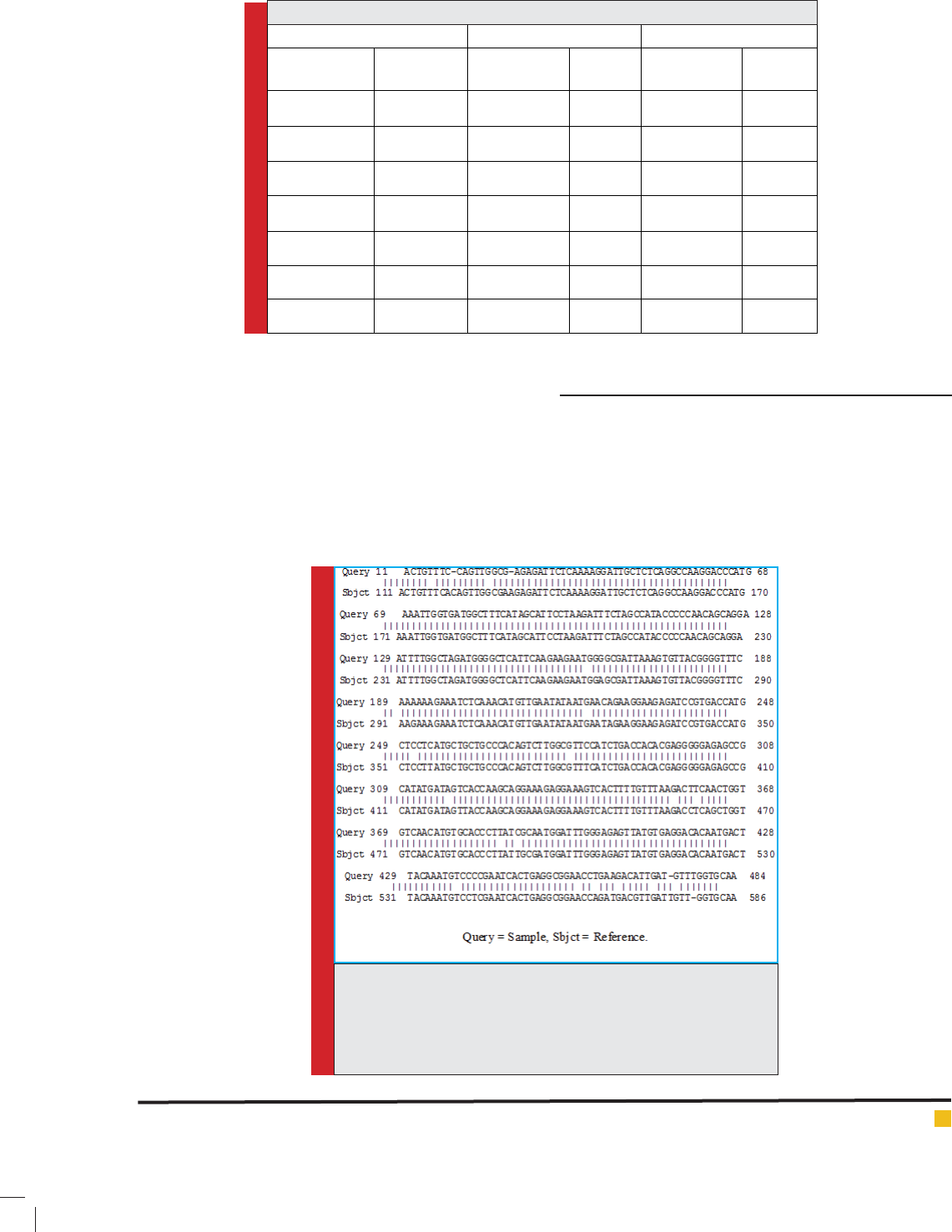
Sequencing of DEN-1 in this study revealed that it is
in close similarity to some Asian (Taiwan, Saudi Arabia,
Thailand, Vietnam, and Cambodia) and African (Djibouti
and Eritrea) types (Table 3, Fig.3, and Fig.6). DEN-2 on
other hand, is similar to varies Indian types (Table 3,
Fig.4, and Fig.7), while DEN-3 is in similarity to some
Asian types including India, China, and Singapore (Table
3, Fig.5, and Fig.8).
DISCUSSION
Jazan region has witnessed several outbreaks during the
recent decade (290 cases in 2010, 289 cases in 2012, and
555 cases in 2016 – Dengue control program in Jazan).
The current available data on dengue in Jazan has con-
centrated mainly on serological surveys (Al-Arzaqi
et al., 2013; Gamil et al., 2014) and has not analyzed the
circulating serotypes in the region.
Table 3. Results of RT-PCR and nested-PCR
DEN-1 DEN-2 DEN-3
Gen bank
accession No
Country
Gen bank
accession No
Country
Gen bank
accession No
Country
AB608788 Taiwan JN935383 India KM097092 India
KJ649286 Saudi Arabia KU351296 India KM097092 Singapore
JN638338 Thailand GU968539 India KF954949 China
AF298808 Djibouti KX577706 China GQ466079 India
Z74047 Vietnam KT180256 India FJ644564 India
AF538024 Cambodia KU351306 India DQ317393 India
KU509258 Eritrea JQ639472 India KU216208 India
FIGURE 3. Identities between DEN-1 from Jazan and DEN-1 of Tai-
wan (dbj|AB608788.1) Dengue virus 1 gene for polyprotein, com-
plete cds, strain: 832, Length=10693, Score = 778 bits (421), Expect
= 0.0, Identities = 459/477 (96%), Gaps = 4/477 (1%), Strand= Plus/
Plus.

Alsheikh et al.
16 SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Our results indicated that dengue fever is becoming
highly prevalent in Jazan region (56.4 %) compared to
the previous reports of Al-Arzaqi et al (2013) and Gamil
et el (2014) who reported dengue prevalence of 26.5%
and 47.74% , respectively, in the region. In this study,
three dengue virus types (DEN-1,DEN-2 and DEN-3)
were found circulating in Jazan region with the pre-
dominance of DEN-2 scoring 104 out of 124 dengue
FIGURE 4. Identities between DEN-2 from Jazan and DEN-2 of
India(gb|JN935383.1) Dengue virus strain VCRC/DENV2/03/10
polyprotein gene, partial cds Length=508, Score = 848 bits (459),
Expect = 0.0, Identities = 471/476 (99%), Gaps = 3/476 (1%),
Strand=Plus/Plus
FIGURE 5. Identities between DEN-3 from Jazan and DEN-3 of India
(gb|KF954949.1) Dengue virus 3 isolates 13GDZDVS30E, complete
genomeLength=10677 Score = 861 bits (466), Expect = 0.0, Identi-
ties = 473/476 (99%), Gaps = 1/476 (0%, Strand=Plus/Plus

Alsheikh et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA 17
FIGURE 6. DEN-1 serotype similarity tree.
positive samples (83.9%), followed by DEN-1 (11 out of
124 - 8.9%), and then DEN-3 (9 of 124 - 7.2%), how-
ever serotype 4 was not detected in any of the 124 den-
gue cases. This nding is in complete accordance with
the work of Fakeeh and Zaki (2001) who reported that
DEN-2 was the predominant serotype, followed by DEN-
1, and DEN-3 in Jeddah, Saudi Arabia. Whereas Organji
et al (2017) in Makkah city, showed that DEN-1 was the
predominant dengue virus type, followed by DEN-2 and
then DEN-3, although the positive blood samples they
used were only six.
The results also coincide partially with the ndings of
Khan et al (2008) who reported high prevalence of the
DEN-2 in contrast to the prevalence of DEN-1 found by
Organji et al (2017) in Makkah city.In Jeddah, Zaki et al
(2008) revealed that DEN-1 and DEN-2 caused the major
outbreak in 1994, while DEN-3 emerged in 1997. More-
over, they indicated two genotypes for DEN-1 (America-
Africa genotype, and Asia-2 genotype), DEN-2 genotype
clustered within Cosmopolitan genotype, and DEN-3
clustered within genotype III.
In the present study, we found DEN-2 to be the pre-
dominant dengue virus type, a result which is in line
with the reports of Fakeeh and Zaki (2001, 2003) and
Zaki et al. (2008) who stated that DENV-2 virus is the
predominant serotype in Saudi Arabia particularly in
western Saudi Arabia since 1992.El-Kafrawy et al. (2016)
showed that DEN-2 isolate from Jeddah belongs to the
Cosmopolitan genotype was most genetically related to
isolates from Pakistan circulating from 2008 to 2013.The
Alsheikh et al.
18 SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 7. DEN-2 serotype similarity tree.
three dengue virus serotypes DEN-1, DEN-2, and DEN-3
are thought to be predominant in the Middle East, espe-
cially in Yemen and Saudi Arabia (Nedjadi et al., 2015).
Dengue viruses circulating locally in Saudi Arabia are
likely to have been imported into Saudi Arabia by Saudi
traveling abroad to dengue endemic countries, or during
Hajj and Umrrah seasons, or by migrant labour (Zaki
et al., 2008).The introduction of the three dengue virus
types in Jazan region may be resulted from several fac-
tors; traveling of the Jazan citizens for Hajj and Umrrah
or for trade or other purposes, or by traveling abroad to
dengue endemic countries, or by migrant labour, or due
to the proximity of Jazan region to Yemen where the
disease is endemic and the three dengue virus serotypes
DEN-1, DEN-2, and DEN-3 are circulating.
The close similarity of DEN-1 in this study to some
Asian (Taiwan, Thailand, Vietnam, and Cambodia) and
African (Djibouti and Eritrea) types, the similarity of
DEN-2 to varies Indian types, in addition to, the similar-
ity of DEN-3 to some Asian types including India, China,
and Singapore suggested the likelihood of introduction
of theses serotypes to Jazan region either by travel-
ling from and to those countries especially the migrant
labours (DEN-1,DEN-2, DEN3,), or through direct intro-
duction from Jeddah (DEN-1 Jeddah genotype) and
Yemen which is closet to Djibouti and Eritrea (DEN-1
African origin).
It is stated that shifts in circulating dengue virus type
or introduction of new dengue virus type in endemic
areas have shown to be related with incidence of severe
Alsheikh et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA 19
FIGURE 8. DEN-3 serotype similarity tree.e
dengue infections; DHF and DSS (Messer et al., 2003;
Rico-Hesse et al., 1997). Moreover, it is worthy to note
that the primary infections by DEN-1 and DEN-3 are
related with more dengue sever infections, whereas
infections with DEN-2 and DEN-4 are associated with
increased dengue severity when they present as second-
ary infections (Balmaseda et al, 2006). Results of our
study have analysed multiple dengue serotypes which
would help in providing clear evidence of current active
dengue transmission and endemicity in Jazan region.
CONCLUSION
The results of this study reported for the rst time the
dengue virus types DEN-1, DEN-2, and DEN-3 circu-
lating in Jazan region with the DEN-2 being the pre-
dominant one. The high seroprevalence of dengue virus
infection in Jazan region indicates its endemicity. The
present study highlights the importance of tracking the
spread of dengue virus types and its implication for ana-
lyzing changes in dengue endemicity in speci ed areas
over time. Continuous surveillance of dengue virus sero-
types in the region to detect as earlier the local origin
circulating serotypes from the imported ones especially
new types DEN-4 and DEN-5, for which continued sur-
veillance is imperative. Complete genome sequencing is
required for the three detected dengue virus serotypes
circulating in the region (DEN-1, DEN-2, and DEN-3)
to serve as references for any future epidemiological
researches and outbreaks.
Alsheikh et al.
20 SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
REFERENCES
Ahmed MM. (2010): Clinical pro le of dengue fever infection
in King Abdul Aziz University Hospital Saudi Arabia. J Infect
Dev Ctries. 4:503–10.
Alsheikh, A.A. (2011): Larval Habitat, Ecology, Seasonal Abun-
dance and Vectorial Role in Malaria Transmission of Anoph-
eles Arabiensis in Jazan Region of Saudi Arabia. Journal of
Egyptian society of parasitology, Vol.41. No. 3. December
2001.
Al-Azraqi, T.A., El Mekki, A.A. and Mahfouz, A.A., (2013):
Seroprevalence of dengue virus infection in Aseer and Jizan
regions, Southwestern Saudi Arabia.Transactions of the Royal
Society of Tropical Medicine and Hygiene, p.trt022.
Atschul SF, Madden TL, Schaffer AA (1997): Gapped BLAST
and PSI-BLAST. A new generation of protein database search
programs. Nucleic Acid Res,25 : 3389-3402.
Ayyub M, Khazindar AM, Lubbad EH, Barlas S, Al AY, Al-
Ukayli S. (2006): Characteristics of dengue fever in a large
public hospital, Jeddah, Saudi Arabia. J Ayub Med Coll Abbot-
tabad. 18:9–13.
Azhar, E.I., Hashem, A.M., El-Kafrawy, S.A., Abol-Ela, S.,
Abd-Alla, A.M., Sohrab, S.S., Farraj, S.A., Othman, N.A., Ben-
Helaby, H.G., Ashshi, A. and Madani, T.A., (2015): Complete
genome sequencing and phylogenetic analysis of dengue type
1 virus isolated from Jeddah, Saudi Arabia. Virology jour-
nal,12(1), p.1.
Balmaseda A, Hammond SN, Perez L, Tellez Y, Saborio SI, Mer-
cado JC ( 2006) Serotype-speci c differences in clinical mani-
festations of dengue. Am J Trop Med Hyg. 74:449–56.
Banu S, Hu W, Hurst C, Tong S. (2011)Dengue transmission in
the Asia-Paci c region: impact of climate change and socio-
environmental factors. Tropical Medicine & International
Health 16: 598–607. doi: 10.1111/j.1365-3156.2011.02734.
CDC (2016): Dengue fact sheet. Available at: https://www.cdc.
gov/dengue/ (Accessed 10/03/2017).
Chaturvedi, U. (2006): Dengue and dengue haemorrhagic fever:
implications of host genetics. FEMS Immunol. Med. Microbiol.
47, 155–166
Crowell G, Cazelles B, Broutin H, Munayco CV. (2011): The
in uence of geographic and climate factors on the timing of
dengue epidemics in Peru, 1994–2008. BMC Infectious Dis-
eases 11: 164. doi: 10.1186/1471-2334-11-164.
El-Badry AA, El-Beshbishy HA, Al-Ali KH, Al-Hejin AM, El-
Sayed WSM. (2013): Molecular and seroprevalence of imported
dengue virus infection in Al-Madinah, Saudi Arabia. Comp
Clin Pathol. doi.org/10.1007/s00580- 013-1704-x.
El-Kafrawy SA, Sohrab SS, Ela SA, Abd-Alla AM, Alhabbab
R, Farraj SA, Othman NA, Hassan AM, Bergoin M, Klitting R.
(2016): Multiple Introductions of Dengue 2 Virus Strains into
Saudi Arabia from 1992 to 2014. Vector Borne Zoonotic Dis.
16(6):391-9. Epub 2016 May 2.
Gibbons, R.V. and Vaughn, D.W. (2002): Dengue: an escalating
problem. BMJ 324, 1563–1566
Gmail, M.A., Eisa, Z.M., Eifan, S.A. and Al-Sum, B.A. (2014).
Prevalence of dengue fever in Jizan area, Saudi Arabia.Jour-
nal of Pure and Applied Microbiology,8(1), pp.225-31.
Glenn L. Sia Su. (2008): Correlation of Climatic Factors and
Dengue Incidence in Metro Manila, Philippines. AMBIO: A
Journal of the Human Environment: June 2008, Vol. 37, No.
4, pp. 292-294.
Green, S. and Rothman, A. (2006_: Immunopathological
mechanisms in dengue and dengue hemorrhagic fever. Curr.
Opin. Infect. Dis. 19, 429– 436 .
Guilarde, A.O.,Turchi MD, Siqueira JB Jr, Feres VC, Rocha B,
Levi JE (2008): Dengue and dengue hemorrhagic fever among
adults: clinical outcomes related to viremia, serotypes, and
antibody response. J. Infect. Dis. 197, 817–824.
Fakeeh M, Zaki AM. (2001): Virologic and serologic surveil-
lance for dengue fever in Jeddah, Saudi Arabia, 1994–1999.
Am J Trop Med Hyg 65:764–
Fakeeh M, Zaki AM. (2003): Dengue in Jeddah Saudi Arabia.
Dengue Bull 27:13–8.
Khan NA, Azhar EI, El-Fiky S, Madani HH, Abuljadial MA,
Ashshi AM. ( 2008): Clinical pro le and outcome of hospital-
ized patients during rst outbreak of dengue in Makkah, Saudi
Arabia. Acta Trop. 2008;105:39–44.
Konongoi, L., Ofula, V., Nyunja, A., Owaka, S., Koka, H., Makio,
A., Sang, R. (2016): Detection of dengue virus serotypes 1, 2
and 3 in selected regions of Kenya: 2011–2014.Virology Jour-
nal,13, 182. http://doi.org/10.1186/s12985-016-0641-0.
Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and
A. V. Vorndam. (1992): Rapid detection and typing of dengue
viruses from clinical samples by using reverse transcriptase-
polymerase chain reaction. J. Clin. Microbiol. 30:545–
551.
Mackenzie JS, Gubler DJ, Petersen LR. (2004): Emerging a-
viviruses: the spread and resurgence of Japanese encephalitis,
West Nile and dengue viruses. Nature Medicine 10: S98–109.
doi: 10.1038/nm1144.
Madani TA, Abuelzein e-TM, Al-Bar HM, Azhar EI, Kao M,
Alshoeb HO, ( 2013): Outbreak of viral hemorrhagic fever
caused by dengue virus type 3 in Al-Mukalla, Yemen. BMC
Infect Dis. 13:136.
Messer WB, Gubler DJ, Harris E, Sivananthan K, de Silva AM.
(2003): Emergence and global spread of a dengue serotype 3,
subtype III virus. Emerg Infect Dis. 9:800–9.
Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA,
Bhatt S, Katzelnick L, Howes RE, Battle KE, Simmons CP,
Hay SI. (2014): Global spread of dengue virus types: map-
ping the 70-year history.Trends in Microbiology. 2014 March;
22(3):138-146. doi:10.1016/j.tim.2013.12.011.
Ministry of Health. (2007): Ministry of Health Report 2006.
Riyadh: Saudi Arabia; ISSN: 1319–3229.
Ministry of Health; Department of Statistics, (2016): Health
Statistical Year Book 2016, Saudi Ministry of Health, Riyadh,
Saudi Arabia.
Alsheikh et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS SEROTYPES OF DENGUE VIRUSES CIRCULATING IN JAZAN REGION, SAUDI ARABIA 21
Murray NE, Quam MB, Wilder-Smith A. (2013): Epidemiology
of dengue: past, present and future prospects. Clin Epidemiol.;
5:299–309.
Mustafa MS,Rasotgi V, Jain S, Gupta V. (2015): Discovery of
fth serotype of dengue virus (DENV-5): A new public health
dilemma in denguecontrol. Med J Armed Forces India. 71(1):67-
70. doi: 10.1016/j.mja .2014.09.011. Epub 2014 Nov 24.
Nedjadi T., S. El-Kafrawy, S. S. Sohrab, P. Despr`es, G. Daman-
houri, and E. Azhar (2015): Tackling dengue fever: current sta-
tus and challenges,Virology Journal, vol. 12, 2015.
Organji Sameer R., Hussein H. Abulreesh, and Gamal E. H.
Osman (2017): Circulation of Dengue Virus Serotypes in the City
of Makkah, Saudi Arabia, as Determined by Reverse Transcrip-
tion Polymerase Chain Reaction. Canadian Journal of Infectious
Diseases and Medical Microbiology Volume 2017 (2017), Article
ID 1646701, 5 pages. https://doi.org/10.1155/2017/1646701
Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A,
Ramos C. (1997): Origins of dengue type 2 viruses associ-
ated with increased pathogenicity in the Americas. Virology.
230:244–51.
Rico-Hesse R. (2003) Microevolution and virulence of dengue
viruses. Adv. Virus Res. 59, 315–341.
Vaughn, D.W. (2000): Dengue viremia titer, antibody response
pattern, and virus serotype correlate with disease severity. J.
Infect. Dis. 181, 2–9
World Health Organization (1997): Dengue haemorrhagic
fever: diagnosis, treatment, prevention and control. 2nd ed.
Geneva: WHO: 48-59.
Zaki A, Perera D, Jahan SS, Cardosa MJ. (2008): Phylogeny of
dengue viruses circulating in Jeddah, Saudi Arabia: 1994 to
2006. Trop Med Int Health. 13:584–92.