
Dental
Communication
Biosci. Biotech. Res. Comm. 10(3): 525-528 (2017)
Adhesion of
Streptococcus mutans
on glazed IPS e.max
press, glazed feldspatic and dental enamel
Ezzatollah Jalalian
1
, Fahimeh Sarzaeem
2
* and Mahkameh Koochaki Pourchafjiri
3
1
Associate Professor, Department of Fixed Prosthodontics, Member of Dental Material Research Center,
Dental Branch, Islamic Azad University, Tehran, Iran
2
Postgraduate Student, Department of Fixed Prosthodontics, Islamic Azad University, Tehran, Iran
3
Dentist
ABSTRACT
Despite several researches done to determine accuracy of microbial growth in the restoration treatment, the mecha-
nisms for these reports is still unclear.The aim of the study was to comparison adhesion of the Streptococcus mutans
on glazed IPS e.max press, glazed feldspatic and dental enamel. The in vitro study was done on 15 samples: 5 glazed
IPS e.max, 5 glazed feldspatic and 5 dental enamels for vicinity of the bacterial suspension containing Streptococcus
mutans (10 × 10
6
cell/mLit). After 48 hours, Streptococcus mutans colonies were counted with the naked eye. The
mean Streptococcus mutans attached to dental enamel was 24.4±8.44 (P<0.001). The Streptococcus mutans attached
to glazed IPS e.max was 1.8±0.83. The Streptococcus mutans attached to glazed feldspatic was 1.4±0.54. No signi -
cant differences observed between the IPS e.max and feldspatic (P<0.8). The results showed Streptococcus mutans
adhesion to enamel was higher than glazed IPS e.max and glazed feldspatic ceramic material.
KEY WORDS: IPS E.MAX, FELDSPATIC,
STREPTOCOCCUS MUTANS
525
ARTICLE INFORMATION:
*Corresponding Author: sarzaeem@yahoo.com
Received 12
th
June, 2017
Accepted after revision 21
st
Sep, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF: 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/10.3/28
INTRODUCTION
There is a rich ecosystem in the oral cavity, with a count-
less number of microorganisms. Although both peri-
odontal disease and dental caries are considered multi-
factorial diseases, the bacteria in the dental plaque are
the main factor in their onset and progression. Increased
oral microbiota of Streptococcus mutans and Lactoba-
cillus is associated with the onset of tooth deminerali-
zation and periodontal disease. This condition is much
more frequent in orthodontic patients with greater risk
of colonization by these microorganisms ( Brusca et al.
2007, Harikrishnan et al. 2013, Nascimento et al. 2014
Jalalian et al. 2015Duymus et al. 2016).
Fahimeh Sarzaeem et al.
Despite several researches done to determine accu-
racy of microbial growth in the restoration treatment,
the mechanisms for these reports is still unclear However
the saliva composition and secretion rate also in uence
plaque formation (Gameiro et al. 2009).Porcelain has
excellent esthetic properties and biocompatibility, and
major emphasis in research have been directed toward
the enhancement of its strength and aesthetic properties
(Rashid, 2014).
Scarce reports exist on bacterial adhesion to por-
celain restorations (Kamala and Annapurni, 2006). A
previous research stated the best results were obtained
through glazing, since it provided a surface topography
with minimal bacterial af nity (Sarac et al. 2006). Addi-
tionally, it is demonstrated that polished surfaces had
lower bacterial adhesion than glazed surfaces (Kawai
et al. 2000). In a similar study, Jalalian et al. (2015)
reported the adhesion of Streptococcus mutans to the
enamel was higher than that to polished IPS e.max Press
and polished feldspathic porcelain. However, there is no
report comparing effect of porcelains with natural den-
tal enamel. So, the aim of the current study was to deter-
mine adhesion of the Streptococcus mutans on glazed
IPS e.max press, glazed feldspatic and dental enamel
adhesion using in vitro condition.
MATERIAL AND METHODS
The in vitro study was done on 15 samples: 5 glazed
IPS e.max, 5 glazed feldspatic and 5 dental enamels for
vicinity of the bacterial suspension containing Strepto-
coccus mutans (10
9
cell/mLit). The samples had diameter
5× 2mm in laboratory, then phosphate base fabricated.
The feldspatic samples fabricated using feldspatic pow-
ders (lvocular, Germany) based on manufacture instruc-
tions. Ito fabricate IPS e.max press samples, 2 × 5 mm
were blocks produced. The dental enamels obtained from
normal premolar using diamond disks. The samples
glazed at 625ºC beginning temperature and increased
each 20 minutes until nal 920ºC, then cooled in fresh
air. Samples washed using distilled water then autoclave.
STUDY PROTOCOL
To increase hygiene condition, all samples were located
into ultrasonic system for 15 min and then transferred
into 70 % alcohol for 30 min. the being sterile of the
samples was tested using BHI condition for 24 h. Saliva
samples obtained from 2 healthy patients which had
no medication for last 3 months without dental caries
or periodontal disease. The saliva samples sterile using
autoclave (Garcez et al. 2011). Then samples coated
with saliva, put into glass vials and immersed in 2mL
of Streptococcus mutans (PTCCI 683) suspension (×10
9
CFU) and incubated in 37 ºC and 5% CO
2
for 24 h. Sam-
ples washed 3 times with normal saline, immersed into
2 mL of normal saline and shacked for 2 min (Fournier
et al. 1998). Obtained suspension cultured on blood agar
and incubated in 37 ºC with 5% CO
2
for 48h and the
colonies counted (Kantoriski et al. 2006). After 48 hours,
Streptococcus mutans colonies were counted with the
naked eye.
STATISTICAL ANALYSIS
Data for bacteria load was analyzed by one way analy-
sis of variance (ANOVA) using SPSS 16.0 for Windows
and is presented as mean ± Sd. For treatments showing
a main effect by ANOVA, means were compared using
Tukey HSD test. P<0.05 was considered as signi cant
differences between treatments.
RESULTS AND DISCUSSION
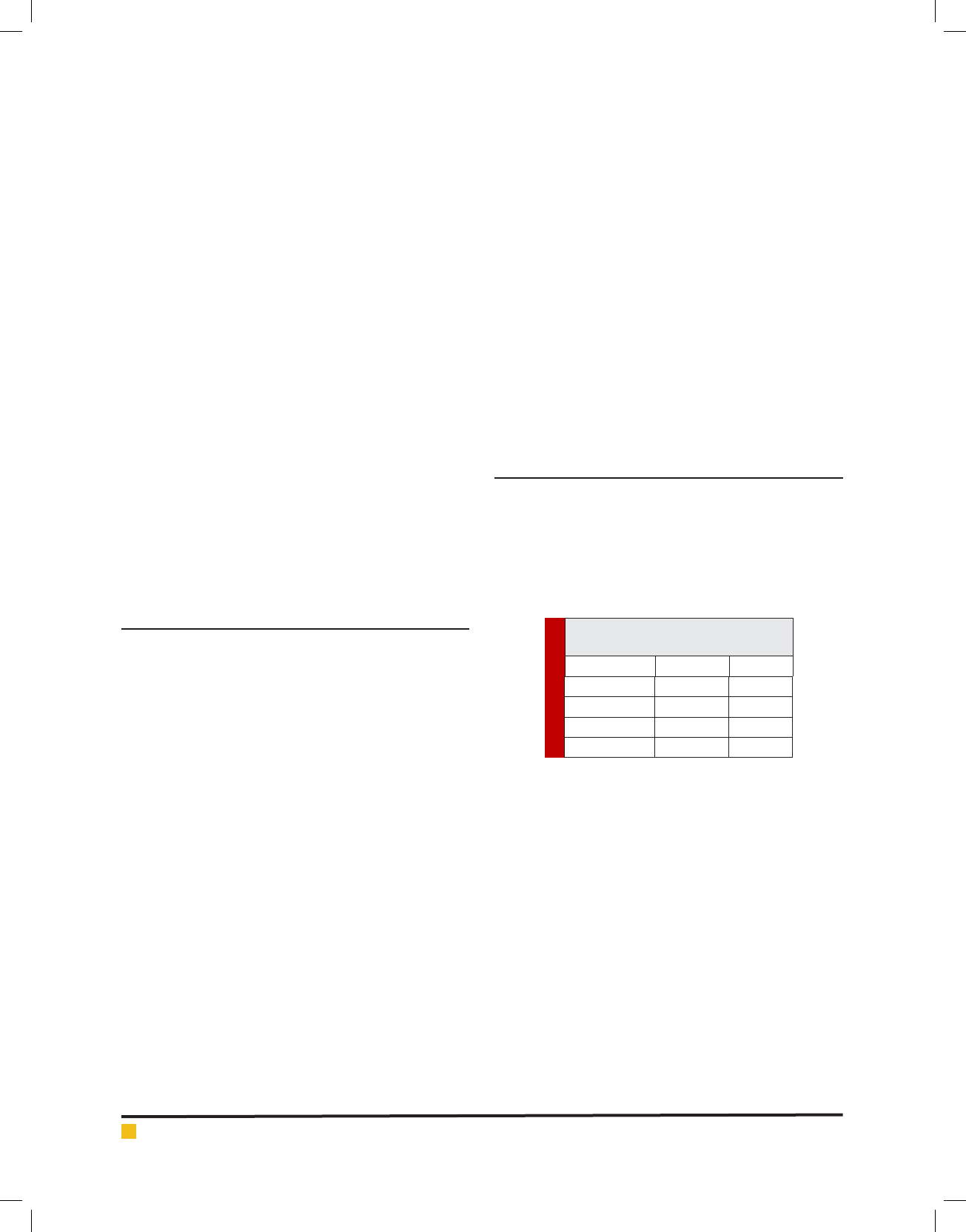
As seen in table, the mean Streptococcus mutans
attached to dental enamel was 24.4±8.44 (P<0.001). The
Streptococcus mutans attached to glazed IPS e.max was
1.8±0.83. The Streptococcus mutans attached to glazed
feldspatic was 1.4±0.54. No signi cant differences
observed between the IPS e.max and feldspatic (P<0.8).
Table 1. The adhesion of streptococcus
mutans on different restoration
adhesion C.V
Feldspatic 1.4±0.54 38.57
IPS e.max 1.8±0.83 46.11
Dental enamel 24.4±8.44 34.59
P value 0.001
As observed in the current study, Streptococcus
mutans attached was lower in glazed feldspatic< IPS
e.max¸ dental enamel. However, no signi cant differ-
ence observed between glazed feldspatic and IPS e.max.
Bacteria-dental interactions typical of enamel or cemen-
tum surfaces, in vivo bio lm formation on restorative
surfaces have physiochemical and biochemical interac-
tions (Hara and Zero, 2010). Pathogenic communities
involving Bi dobacterium dentium, Scardovia wiggsiae,
Bi dobacterium longum, Bi dobacterium adolecentis,
Prevotella spp, Selenomonas spp and Lactobacilli spp
have also been demonstrated to be complicit in the eti-
ology of dental caries (Zhang et al. 2015). Streptococcus
mutans has been demonstrated as the primary etiologic
agent in caries initiation and reductionist approach can
elucidate vital information regarding its interaction with
restoration surfaces; however, additional studies may
also use a more holistic approach (Wessel et al. 2014).
526 ADHESION OF STREPTOCOCCUS MUTANS ON GLAZED IPS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ADHESION OF STREPTOCOCCUS MUTANS ON GLAZED IPS 527
Fahimeh Sarzaeem et al.
The rst stage of colonization by an organism involves
adherence of the organism to a host surface. From this
viewpoint, evaluation of Streptococcus mutans adhesion
and colonization to tooth surfaces and restorative mate-
rials are of most importance for their success (Lassila
et al. 2009). Eick et al. (2004) demonstrated that no cor-
relation found between surface roughness and the num-
ber of Streptococcus mutans. In the oral environment,
the adsorption of salivary proteins to the tooth or restor-
ative surface precedes and promotes bacterial adherence.
They can form an acquired salivary pellicle to which
bacteria and structural substrates may bind (Keulemans
et al. 2009). Plaque accumulation was more in uenced
by the presence of a salivary pellicle than by material
type. Viability, however, was in uenced by material
composition, in this case, differentiated by glass content
(Dittmer et al. 2015).
Feldspathic porcelains are usually used as a veneer-
ing material for metal ceramic restorations and provide
excellent esthetics and compressive strength (Duymus
et al. 2016). Otherwise, the rough porcelain surface is
prone to adhesion and retention of oral microorganisms
causing excessive plaque accumulation, gingival irrita-
tion, increased surface staining and poor esthetics of the
restored teeth and thereby increasing the risk of dental
caries and periodontal disease (Hengtrakool et al. 2011).
The oral cavity is a complex, aqueous environment
where the restorative material is in contact with saliva
(Hengtrakool et al. 2011). Other factors such as low pH
due to acidic foods and drinks may in uence the mate-
rial’s mechanical and physical characteristics (Honorio
et al. 2008). The availability and long-term success of
prosthesis, depends upon the protection of the polished
surface. The degradation of surface nish will cause the
formation of surface cracks and after a while, leaving
the porcelain metal sub-structure. In addition, surface
deterioration will facilitate the involvement of plaque
and microorganisms (Honorio et al. 2008).
Karayazgan et al. (2010) reported that the level of
adhesion of Candida albicans to the polished surface of
feldspathic porcelain was 3.4 ± 0.25 colonies/mm
2
. In a
similar study, enamel used as the control for assessment
of the adhesion of Streptococcus mutans to uncoated
and saliva-coated glass ceramics and composites (Kan-
torski et al. 2008) and their report was consistent with
the ndings of the current study. In a research on the
adhesion to different the ceramics, composites and
amalgam concluded the bacterial af nity was equal in
all groups of ceramics assessed (Kawai et al. 2000). In
conclusion the results showed Streptococcus mutans
adhesion to enamel was higher than glazed IPS e.max
and glazed feldspatic ceramic material. According to the
ndings of the present study, polished IPS e.max Press
and polished feldspathic porcelain exhibit similar char-
acteristics in terms of bacterial adhesion and either one
can be the choice material.
REFERENCES
Brusca MI, Chara O, Sterin-Borda L, Rosa AC.2007 In uence
of different orthodontic brackets on Adherence of microorgan-
isms in vitro. Angle Orthod. 77(2):331-6.
Dittmer MP, Hellemann CF, Grade S, Heuer W, Stiesch M,
Schwestka-Polly R, Demling AP 2015 Comparative three-
dimensional analysis of initial bio lm formation on three
orthodontic bracket materials. Head & Face Medicine 11:10
DOI 10.1186/s13005-015-0062-0
Eick S, Glockmann E, Brandl B, P ster W. 2004 Adherence of
Streptococcus mutans to various restorative materials in a con-
tinuous ow system. J Oral Rehabil 2004; 31: 278-85.
Fournier A, Payant L, Bouclin R. 1998 Adherence of Strepto-
coccus mutans to orthodontic brackets. Am J Orthod Dentofa-
cial Orthop 114(4):414-7.
Gameiro GH, Nouer DF, Cenci MS, Cury JA. 2009 Enamel
demineralization with two forms of archwire ligation investi-
gated using an in situ caries model: a pilot study. Eur J Orthod.
31(5):542-6.
Garcez AS, Suzuki SS, Ribeiro MS, Mada EY, Freitas AZ,
Suzuki H. 2011 Bio lm retention by 3 methods of ligation on
orthodontic brackets: A microbiologic and optical cohernce
tomography analysis. American Journal of Orthodontics and
Dentofacial Orthopedics 140 (4), 193-198.
Hara AT, Zero DT. 2010 The caries environment: saliva, pel-
licle, diet, and hard tissue ultrastructure. Dent Clin North Am
54:455-67.
Harikrishnan P, SakuSubha T, Kavitha V, Gnanamani A. 2013
Microbial Adhesion on Orthodontic Ligating Materials: An in
Vitro Assessment. Advances in Microbiology, 3: p- 108-114.
Hengtrakool C, Kukiattrakoon B, Kedjarune-Leggat U. (2011)
Effect of Naturally Acidic Agents on Microhardness and Sur-
face Micromorphology of Restorative Materials. European
Journal of Dentistry, 5, 89-100.
Honorio, H.M., Rios, D., Francisconi, L.F., et al. (2008) Effect of
Prolonged Erosive pH Cycling on Different Restorative Materi-
als. Journal of Oral Rehabilitation, 35, 947-953.
Jalalian E, Mofrad GH, Rahbar M, Mohseni A, Mohebbi M.
2015 In vitro adhesion of streptococcus mutans to polished
IPS e.max and Feldspathic porcelain. Journal of Islamic Dental
Association of IRAN (JIDAI) 27(4):182-185.
Jalalian E, Mosto SN, Sha ee E, Nourizadeh A, Nargesi RA,
Ayremlou S. 2015 Adhesion of Streptococcus mutans to Zirco-
nia, Titanium Alloy and some other Restorative Materials:” An
in-vitro Study”. Advances in Biosciences & Clinical Medicine.
Jan 1;3(2):13-20.
Kamala KR, Annapurni H. 2006 Evaluation of surface rough-
ness of glazed and polished ceramic surface on exposure to
uoride gel, bleaching agent and aerated drink: An in vitro
study. J of Indian Prosthod Soc. Jul; 6(3):128-32.

Fahimeh Sarzaeem et al.
Kantoriski KZ, Scotti R, Felipe L. 2006 Surface roughness and
Bacterial Adherence.Cience Odontol bras. 9(4):12-17.
Kantorski KZ, Scotti R, Valandro LF, Bottino MA, Koga-Ito
CY, Jorge AO. 2008 Adherence of Streptococcus mutans to
uncoated and salivacoated glass-ceramicsand composites. Gen
Dent. Nov-Dec;56(7):740-7.
Karayazgan B, Atay A, Saracli MA, Gunay Y. 2010 Evaluation
of Candida albicans formation on feldspathic porcelain sub-
jected to four surface treatment methods. Dent Mater J. Mar;
29(2): 147-53.
Kawai K, Urano M, Ebisu S.2000 Effect of surface roughness
of porcelain on adhesion of bacteria and their synthesizing
glucans. J Prosthet Dent. Jun;83(6):664-7.
Keulemans F, Lassila LV, Garoushi S, Vallittu PK, Kleverlaan CJ,
Feilzer AJ. 2009 The in uence of framework design on the load-
bearing capacity of laboratory-made inlay-retained ber rein-
forced composite xed dental prostheses. J Biomech 42: 844-9.
Lassila LVJ, Garoushi S, Tanner J, Vallittu PK, Söderling E.
2009 Adherence of Streptococcus mutans to ber-reinforced
lling composite and conventional restorative materials. The
Open Dentistry Journal, 3, 227-232.
Nascimento LEAG, Souza MMG, Azevedo ARP, Maia LC.2014
Are self-ligating brackets related to less formation of Strepto-
coccus mutans colonies? A systematic review. Dental Press J
Orthod. 19(1):60-8.
Rashid H. 2014 The effect of surface roughness on ceramics
used in dentistry: A review of literature. Eur J Dent 8:571-9.
Sarac D, Sarac YS, Kulunk S, Ural C, Kulunk T.2006 The effect
of polishingtechniques on the surface roughness and color
change of composite resins. J Prosthet Dent. Jul;96(1):33-40.
Wessel SW, Chen Y, Maitra A, van de Heuvel ER, Slomp AM,
Busscher HJ, van der Mei HC. 2014 Adhesion forces and com-
position of planktonic and adhering oral microbiomes. J Dent
Res 93:84-8.
Zhang YF, Zheng J, Zheng L, Zhou ZR.2015 Effect of adsorp-
tion time on the adhesion strength between salivary pelli-
cle and human tooth enamel. J Mech Behav Biomed Mater
42:257-66.
528 ADHESION OF STREPTOCOCCUS MUTANS ON GLAZED IPS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS