
Environmental
Communication
Biosci. Biotech. Res. Comm. 10(3): 438-444 (2017)
Composition and quantity of cytotoxic waste
from oncology wards: A survey of environmental
characterization and source management of medical
cytotoxic waste
Y. Ghafuri
1,2
and R. Nabizadeh
3
*
1
Department of Environmental Health Engineering, School of Public Health, International Campus, Tehran
University of Medical Sciences, Tehran, Iran
2
Environmental Research Center, Qom University of Medical Science, Qom, Iran
3
Department of Environmental Health Engineering, School of Public Health. Tehran University of Medical
Sciences, Tehran, Iran
ABSTRACT
Interest in waste drugs as a part of hospital waste in relation to their negative impact on the environment has increased during
the past years. Cytotoxic drugs play an important role in the treatment of various neoplastic conditions and are most often used in
specialized departments such as oncology and radiotherapy units. In this study an initial inventory of pharmaceuticals and unused
pharmaceuticals including hazardous waste drugs and antineoplastic (cytotoxic) chemotherapeutics was provided. By providing
a questionnaire, the rate of cytotoxic consumption, residuals of drugs, vial and syringes, needles, gloves and the other cytotoxic
waste was measured during a 30-day period in two oncology wards of Qom hospitals in Iran. The results determined that mean
production rate of medical waste in two hospital is 435 kg/d and equal to 1.73kg/bed/d, including: 97% infection waste (1.67 kg/
bed/d), 2.5% sharp and syringe waste (43.25 g/bed/d) and 0.5% pharmaceutical waste (8.65 g/bed/d). The rate of cytotoxic waste
in the investigated hospitals was 293.5(gr/d) and equal to 0.07 total medical waste. On the other hand the average rate of cytotoxic
waste in the oncology departments was 21.5 gr/bed and 16.5 gr / patient. The results determined that over 66% of residuals cyto-
toxic drug compounds can be converted in to nontoxic and no genotoxic by chemical degradation. Lack of awareness of health
hazards, insuf cient nancial and human resources and poor control of waste disposal are the most common problems connected
with healthcare wastes.
KEY WORDS: CYTOTOXIC-WASTE-QOM-COMPOSITION
438
ARTICLE INFORMATION:
*Corresponding Author:
Received 12
th
July, 2017
Accepted after revision 28
th
Sep, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF: 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/10.3/17

Ghafuri and Nabizadeh
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COMPOSITION AND QUANTITY OF CYTOTOXIC WASTE FROM ONCOLOGY WARDS 439
INTRODUCTION
Healthcare activities can lead to the generation of
hazardous of wastes that may have adverse effects on
human health and on the environment. Interest in waste
drugs as a part of hospital waste in relation to their neg-
ative impact on the environment has increased during
the past years. Many of the chemicals and pharmaceu-
ticals used in health care are hazardous. They are com-
monly present in small quantities in health-care waste,
whereas larger quantities may be found when unwanted
or outdated chemicals and pharmaceuticals are sent for
disposal, (Drug, 2015).
Considering the classi cation of hazardous health-
care waste by WHO the Categories of hazardous health-
care waste are: sharps, Infectious, Pathological, Pharma-
ceutical and cytotoxic, Chemical waste, and Radioactive
waste. The types and nature of hospital waste depends
upon the nature of the hospital and the service avail-
able in hospital (WHO, 2014). Exposure to genotoxic
substances in health care may also occur during the
preparation of, or treatment with, particular drugs or
chemicals. The main pathways of exposure are inhala-
tion of dust or aerosols, absorption through the skin,
ingestion of food accidentally contaminated with cyto-
toxic drugs, ingestion as a result of bad practice, such
as mouth pipetting, or from waste items. Exposure may
also occur through contact with body uids and secre-
tions of patients undergoing chemotherapy. Genotoxic
waste is highly hazardous and may have mutagenic,
teratogenic, or carcinogenic properties. Genotoxic waste
may include certain cytostatic drugs, vomit, urine, or
faeces from patients treated with cytostatic drugs, chem-
icals, and radioactive material (Ansell, 2015).
Cytotoxic drugs (CDs) are primarily used as anti- can-
cer drugs because they are toxic to cells. These drugs
have been associated with human cancers at high (thera-
peutic) levels of exposure and are carcinogens and ter-
atogens in many animal species. They are most often
used in specialized departments such as oncology units,
whose main role is cancer treatment. Cytostatic drugs
can be categorized as: alkylating agents which cause the
alkylation of DNA nucleotides, leading to the cross-link-
ing and miscoding of the genetic stock; antimetabolites
which inhibit the biosynthesis of nucleic acids in the
cell; and mitotic inhibitors which prevent cell replica-
tion (Antell, 2013). Medical waste is incorrectly managed
throughout the majority of hospitals in Iran. Healthcare
workers are not trained to conceive that a large pro-
portion of medical waste generated in hospitals is Non-
infectious waste. A structured waste management strat-
egy together with clear definitions and staff training will
lead to a decrease in waste volumes, and consequently
to a reduction of costs in healthcare settings. Generated
amounts of the health care waste are not available (for
example Ireland) or the de ned amount of wastes is too
low (for example Bulgaria, Finland) or too high (in case
of Belgium). Among other issues, this might be an indi-
cation of improper waste management practice or poor
data collection in the country; Askarian et al, 2010; Far-
zadkia et al, 2009; Abduli, 2010 Nabizadeh, 2016).
In recent years, the rate of cancer disease and con-
sumption of cytotoxic drugs in oncology wards of Qom
hospital has increased. In this work composition, the
quantity and possibility of chemical degradation of
cytotoxic drug waste have been studied.
MATERIALS AND METHODS
This study was performed in 2015, in the oncology wards
of two hospitals in Qom, Iran including Shahid Beheshti
Hospital with 400 active beds and Hazrat Masoumeh
Hospital with 120 active beds, located in the central part
of Qom Province
. The studied hospitals provide general
medical, surgical, maternity, pediatric, and a range of
specialist services.
Considering the methods of medical waste manage-
ment and the main generation of medical waste in the
two investigated hospitals, the total medical waste was
classi ed in three categories: infection, sharp and Phar-
maceutical waste. Several methods were used to collect
data, namely site visits, interviews, and questionnaires.
An initial inventory of pharmaceuticals and unused
pharmaceuticals including hazardous waste drugs and
antineoplastic (cytotoxic) chemotherapeutics was pro-
vided. Moreover, data
collection consisting of health-
care waste generation, separation, collection, storage,
transportation, and disposal was performed during site
visits to the hospitals.
With the cooperation and coordi-
nation of the personal and management of hospitals and
using the questionnaire, the rate of cytotoxic consump-
tion on, residuals of drugs, vial and syringes, needles,
gloves and the other cytotoxic waste was measured in a
30 day period.
The Chemical degradability of cytotoxic waste was
assessed with exposure to chemical oxidants (WHO,
2014). Specific physico-chemical properties such as:
dissociation constant (pKa), solubility, octanol–water
partition coef cient (Kow) and organic carbon parti-
tion coef cient (Koc), bio-concentration factor (BCF),
atmospheric hydroxyl radical reaction rate and photoly-
sis tendency play critical roles in determining the envi-
ronmental behaviors and fate of cytotoxic waste (Cheng
et al, 2009; Andrew et al, 2008; Toolaram et al, 2014;
Zhang et al, 2013; Besse et al, 2012). The Prediction of
environmental fate and other physico-chemical proper-
ties was carried by a theoretical model (EPI Suite 4.1)
Ghafuri and Nabizadeh
440 COMPOSITION AND QUANTITY OF CYTOTOXIC WASTE FROM ONCOLOGY WARDS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
(EPA, 2014). Two criteria contain octanol–water parti-
tion coefficient (Kow) and solubility was considered. A
logKow<1suggests that the compounds are highly mobile
in an aquatic environment. About solubility, adding or
existance of polar functional groups that increase of
hyrophilicity of drug compound were considered (Zhang
et al, 2013; Besse et al, 2012).
RESULTS AND DISCUSSION
QUANTITIES OF MEDICAL WASTE GENERATION
There were 120 active beds in the Hazrat Masoumeh
Hospital and the rate of medical waste was identi ed
as 220 kg/d. Also there were 400 active beds in Sha-
hid Beheshti Hospital with the medic production rate of
650 kg/d. Table 1 shows the average daily production of
total medical in two hospitals. Medical waste from Haz-
rat Masoumeh hospital equaled 1.83 kg/occupied bed/d,
of which 96.36% was infectious, 3.1% sharp waste and
0.45% Pharmaceutical waste .Shahid Beheshti hospital
medical waste was 1.62 kg/occupied bed/d of which
98% was infectious, 1.7% sharp and 0.3% pharmaceuti-
cal waste.
Category of cytotoxic waste
The rate of cytotoxic waste was assessed in oncology
wards of two hospitals separately. The results are shown
in table 2.
Table 3 shows the mean production rate and category
of cytotoxic drug waste in the hospitals under study.
Characteristics and chemical degradation of
cytotoxic drugs
Twelve chemical structures of conventional cytotoxic
drug compounds used in the oncology wards of hospi-
tals under study were assessed. The results pertaining to
the solubility and degradability of cytotoxic drug when
exposed to chemical oxidants, the results are shown in
Table 4.
Results of the present study determined that mean
production rate of medical waste in the two hospitals
was 435 kg/d and equal to 1.73kg/bed/d, including:
97% infection waste (1.67 kg/bed/d), 2.5% sharp and
syringe waste (43.25 g/bed/d) and 0.5% pharmaceutical
waste (8.65 g/bed/d). The results of study about hospital
waste management status in Iran by farzadkia and at al
showed that the waste generation rate was 2.5 to 3.01 kg
bed(-1) day(-1), which included 85 to 90% of domestic
waste and 10 to 15% of infectious waste. Waste genera-
tion rate in the hospitals varied from 1.25 to 14.8 kg/
bed/d (Zhang et al, 2013; Besse et al, 2012).
Medical waste production depends on factors such
as type of hospital, number of beds, socio-economic
and cultural status of patients and waste management
processes (Cheng et al, 2009). In Thailand, Italy, USA,
India, Peru, Vietnam, and Tanzania 1, 3–5, 5–7, 0.5–2,
0.76–2.6, 1.42, and 0.84 kg/bed/d, of medical waste are
respectively generated, are generated (Dehghani, 2008).
According to a study of the composition and production
rate of pharmaceutical and chemical waste in Greece,
the production rate for total pharmaceutical waste was
7.48 g/bed/d (Voudrias, 2012).
Results of Table 2 and 3 exhibited that the rate of
cytotoxic waste in the investigated hospitals was
293.5(g/d) equal to 0.07 total medical waste. On the
other hand the average rate of cytotoxic waste on the
in the oncology departments was 21.5 g/bed equal to
16.5 g/patient. Moreover, the total amount of genera-
tion waste from cytotoxic drug residuals was 120.2 mg/d
(mean 4.92mg/d and standard deviation ±8.88mg/d
for any cytotoxic drug) and the total amount other
Table 1. Estimated medical waste generation rate of hospital waste for two hospitals investigated
Name of
hospitals
Number of
active beds
Rate of total medical
waste (kg/d)
Separation of medical waste
Infection
waste (kg/d)
Sharp waste
(kg/d)
Pharmaceutical
waste(kg/d)
Hazratmasoumeh 120 220 212 7 1
Shahidbeheshti 400 650 637 11 2
Table 2. Rate of cytotoxic waste in the hospitals investigated
Name of
hospitals
number of active beds in
oncology department
rate of total
cytotoxic waste
rate of cytotoxic
waste
rate of cytotoxic
waste
(gr/d) (gr/bed/d) (gr/patient/d)
Hazrat masoumh 12 337 22.5 18
Shahid beheshti 15 250 20 15

Ghafuri and Nabizadeh
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COMPOSITION AND QUANTITY OF CYTOTOXIC WASTE FROM ONCOLOGY WARDS 441
Table 3. Average production rate and category of cytotoxic drug waste in hospitals investigate
Rate of other cytotoxic waste
that produced from expose to
cytotoxic drug (gr/d)
Waste from residual
of cytotoxic drug
(mg/d)
Daily average
dosage used
(mg/d)
Drug compoundNumber
258120Cytarabine1
186100Etoposide2
248170Vincristine3
28970Carboplatin4
62.727oxaliplatin5
121.515Fluorouracil (5-FU)6
83120Cisplatin7
1618170Cyclophosphamide8
20760bleomycin9
2613130Erinotekan10
3015200mesna11
10850Dacarbazine (DTIC)12
2510130Methotrexate13
10880L-asparaginase14
2517170Ifosfamide (Ifex)15
282120.21512Sum
Table 4. Characterization of solubility and degradability of conventional cytotoxic drug
compounds in oncology departments
Hydrophilicity of
cytotoxic drug with
consider to(Kow)
Solubility of cytotoxic
drugs with consider to polar
functional group
Chemical FormulaCytotoxic drug
compound
++C
15
H
18
N
4
O
3
Carboplatin
++C
46
H
56
N
4
O
1
Vincristine
-+C
7
H
15
Cl
2
N
2
O
2
PCyclophosphamide
++H
6
Cl
2
N
2
PtCisplatin
_+C
55
H
84
N
17
O
21
S
3
Bleomycin
++C
29
H
32
O
13
Etoposide
++C
9
H
13
N
3
O
5
Cytarabine
++C
33
H
38
N
4
O
6
Erinotekan
__C
2
H
5
NaO
3
S
2
Mesna
+_C
6
H
10
N
6
ODacarbazine (DTIC)
+_C
4
H
3
FN
2
O
2
Fluorouracil (5-FU)
++C
20
H
22
N
8
O
5
Methotrexate
Notes: + sign exhibit that cytotoxic drug with log K
ow
<1 or cytotoxic drug has polar functional group
cytotoxic waste generated from contact was 282 g/d
(mean 7.92g/d and standard deviation ±18.8g/d for
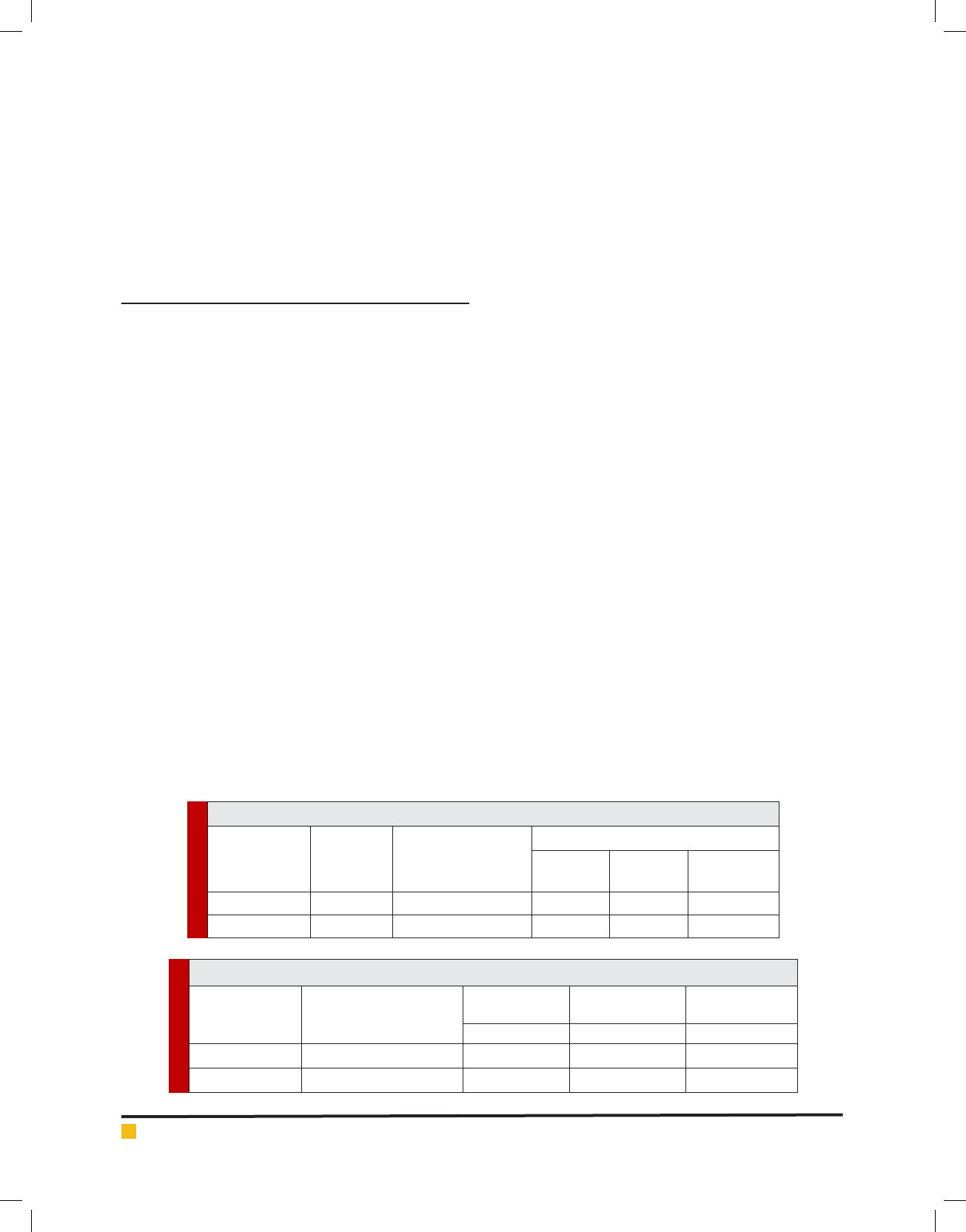
any cytotoxic drug). Figure1 indicated that maximum
production rate of daily average administrated dosage
of cytotoxic drugs is related to Mesna (200 mg/d) and
maximum production rate for waste from residuals is
related to Cyclophosphamide (18mg/d)
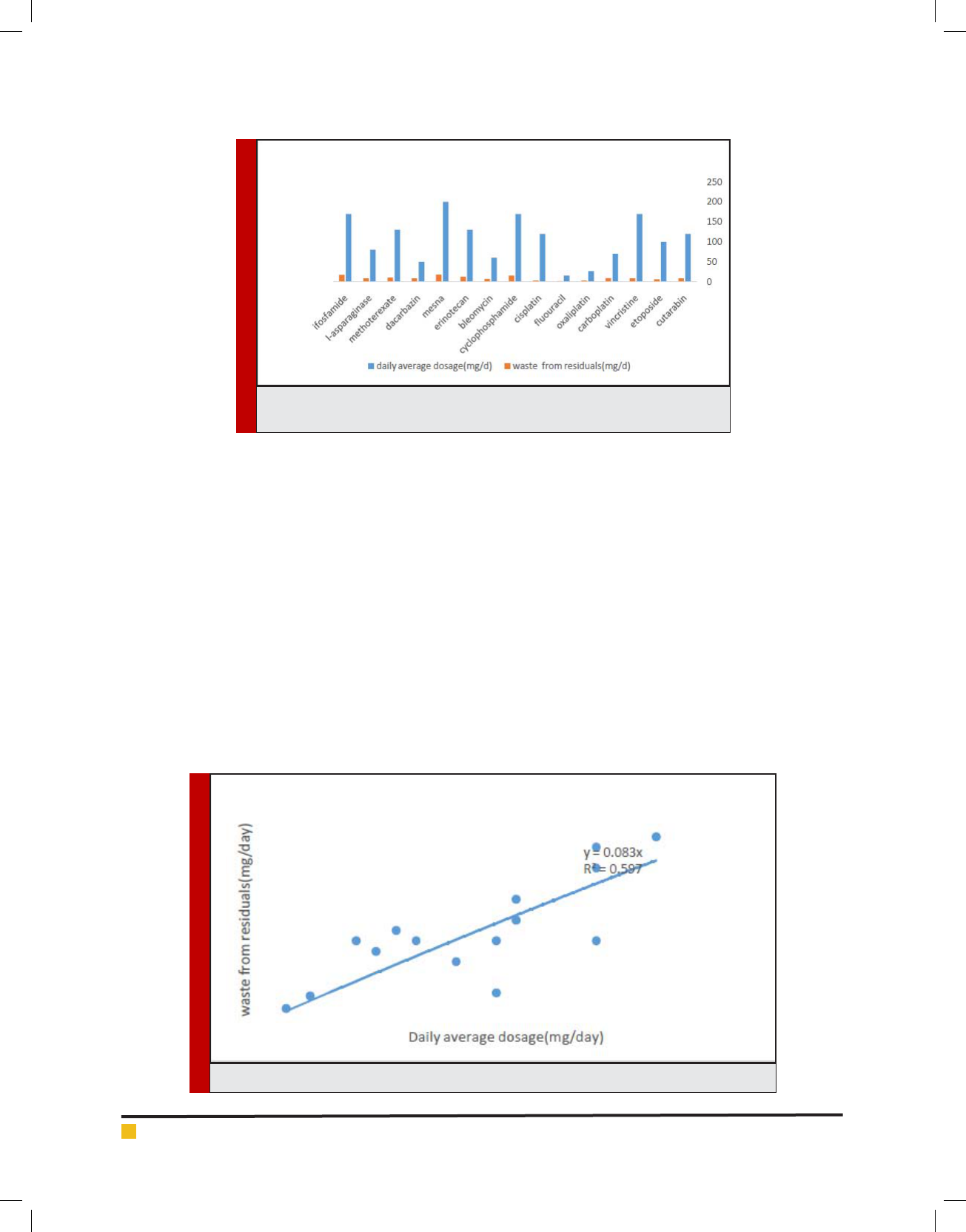
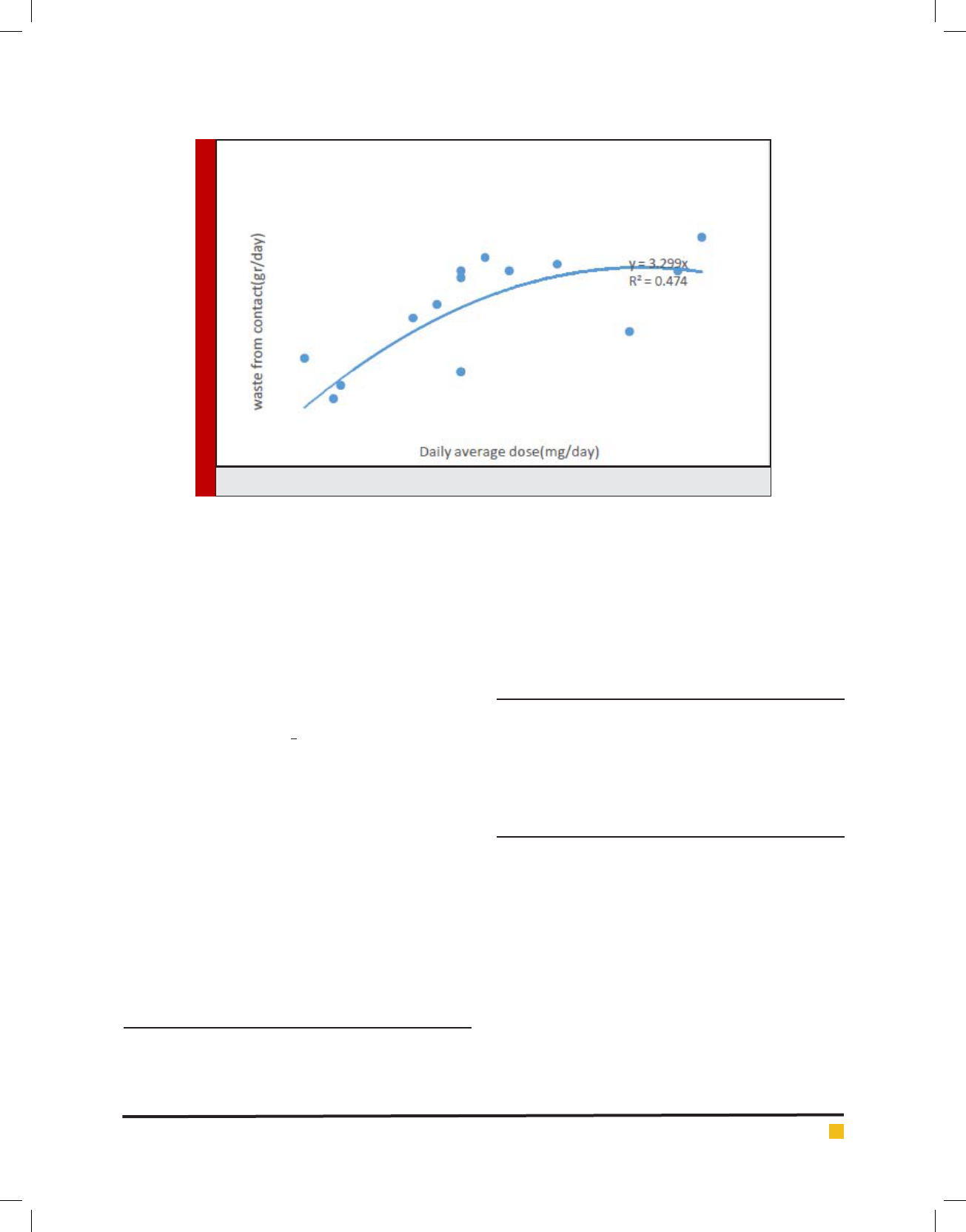
Figure 2 with R
2
= 0.59 and Figure 3 with R
2
=
0.47exhibited a small correlation between daily average
dosage and waste from residuals of cytotoxic drug and
a smaller one daily average dosage and rate of other
cytotoxic waste generated from contact.
The results of the by Tasakona showed that the rate
of cytotoxic waste was 120 l/day and 0.03 from total
Ghafuri and Nabizadeh
442 COMPOSITION AND QUANTITY OF CYTOTOXIC WASTE FROM ONCOLOGY WARDS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 1. Comparison between daily average dosage and waste from residuals of
cytotoxic drug waste
FIGURE 2. Daily average dosage versus waste from residuals of cytotoxic drug
amount of medical waste, was different (medical waste
density in Iran is 170 kg/m
3
) but approve the Bill Brewer,
Andrew Antel study that rate of cytotoxic waste from
chemutraputic drug such as Cyclophosphamide (CP),
Mitomycine, Mycophenolate was 0.08 ib/bed/day (Tsa-
kona, 2010). Results of a survey by Evanglos Vondari-
als, suggested that rate of pharmaceutical’s waste was
3.9% of hazardous waste in agreement with our results
(Voudrias, 2012).
Cytotoxic waste residue as a source of cytotoxic drugs
in the Environmental
With survey of medical waste management in two hos-
pitals, direct disposal as the municipal solid waste is
still a common way for the unused pharmaceuticals.
Four primary sources (hospital ef uent, household
wastewater, and production discharge and drug waste
disposal) of cytostatic residues. Hospitals produce large
quantities of chemically- and which carry high poten-
tial ecotoxicity, and should not be considered as pos-
sessing the same pollutant nature as urban wastewa-
ter. The measured cytostatic levels in hospital sewage
indeed correlated with the daily consumption and the
pharmacokinetic excretion pattern (Zhang et al, 2013;
Besse et al, 2012). At present study, results of predic-
tion model (EPI Suite 4.1) was shown that, cytotoxic
drug waste residue including: Carboplatin, Vincristine,
Cyclophosphamide, Cisplatin, Bleomycine, Etoposide,
Cytarabin, Erinotekan and Methoterexate with con-
sidering excration pattern and discharge in to hospital
sewage, have been increased toxicity of aquatic envi-
ronment (EPA, 2013).
Ghafuri and Nabizadeh
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COMPOSITION AND QUANTITY OF CYTOTOXIC WASTE FROM ONCOLOGY WARDS 443
Implementation of cytotoxic waste management plan
and selection of treatment methods
Risk control measures for cytotoxic waste management
including: identi cation, containment and segregation.
Other requirements contain handling or storage of cyto-
toxic waste, disposal and treatment, in the process of
waste management plan must be considered.
The choice of treatment system involves considera-
tion of waste characteristics, technology capabilities
and requirements, environmental and safety factors,
and costs, many of which depend on local conditions.
The results of study about solubility and degradability
of conventional cytotoxic waste is depicted in Table 4.
For decrease of environmental risks and degradation
of these compounds by chemical degradation methods
as an option that appropriate for developing countries
were investigated. results determined that over 66% of
residuals cytotoxic drug compounds can be converted
in to nontoxic and no genotoxic by chemical degrada-
tion including oxidation by potassium permanganate
(KMNO
4
) or sodium hypochlorite (NaCLO,5.25%) readily
available in Iran hospitals. It must be noted that this
process is not suitable for other cytotoxic waste contain-
ing vial, syringe, and gloves for which the appropriate
process is handling (Drug, 2015).
CONCLUSION
The implementation of medical waste management is
one of the most significant healthcare issues currently
requiring attention in Iran. Hospital waste materials pose
FIGURE 3. Daily average dosage versus rate of other cytotoxic waste from contact
a wide variety of health and safety hazards for patients
and healthcare workers. Many of hospitals in Iran have
neither a satisfactory cytotoxic waste disposal system
nor a waste management and disposal policy. Provision
of a cytotoxic waste management planning and mon-
itoring systems in hospitals is a prerequisite issue for
effective reduction of health care waste associated risks.
ACKNOWLEDGEMENTS
This research has been carried by cooperation of Qom
University of Medical Science and the authors express
gratitude to all hospital staff of Qom University of Medi-
cal Science for their support through this study.
REFERENCES
Abduli M.A., ET. Al, 2010. Municipal Waste Reduction Poten-
tial and Related Strategies in Tehran, Int. J. Environ. Res., 4(4):
901-912, 1735-6865.
Almuneef, M., Memish, Z.A., 2003. Effective medical waste
management: it can be Done, American Journal of Infection
Control, 31, 188–192.
Andrew C. Johnson, Monika D. Ju rgens a, 2008. Richard J.
Williams a, Kummerer. K, Kortenkamp. A, John P. S. ,Do cyto-
toxic chemotherapy drugs discharged into rivers pose a risk
to the environment and human health ? An overview and UK
case study, Journal of Hydrology, 348, 167– 175
Anju Priya Toolaram, Klaus Ku¨mmerer.2014. Environmental
risk assessment of anti-cancer drugs and their transformation
products: A focus on their genotoxicity characterization-state

Ghafuri and Nabizadeh
444 COMPOSITION AND QUANTITY OF CYTOTOXIC WASTE FROM ONCOLOGY WARDS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
of knowledge and short comings. 2014- Mutation Research
760, 18–35
Ansell Healthcare Products LLC, 2015. Handling Cytotoxic
Drugs; A HEALTH AND SAFETY REVIEW. www.ansellhealth-
care.com
Bdour, A., Altrabsheh, B., Hadadin, N., Al-Shareif, M., 2007.
Assessment of medical wastes management practice: a case study
of the northern part of Jordan. Waste Management, 27, 46–759
Blenkharn, J.I, 2007. Standards of clinical waste management
in hospitals – a second look. Public Health, 121, 540–545.
Blenkharn, J.I., 2007. Lowering standards of clinical waste
management: do the hazardous waste regulations conflict with
the CDC’s universal/standard precautions?, Journal of Hospital
Infection 62, 467–472.
Brewer. B, Antell .A, 2013. A case study of the management
of hazardous waste drugs in a large university hospital,http://
dx.doi.org/10.1016/j.jchas.10.003
Bureau of National Health Insurance, National Health Insur-
ance Annual Statistical Report ,Bureau of National Health
Insurance,2003, Taiwan, ROC.
Cheng, Y.W., Sung, F.C., Yang, Y., Lo, Y.H., Chung, Y.T., Li,
K.-C, 2009, Medical waste production at hospitals and associ-
ated factors, Waste Management, 29: 440–444.
Cheng, Y.W., Sung, F.C., Yang, Y., Lo, Y.H., Chung, Y.T., Li,
K.-C., 2009. Medical waste Production at hospitals and associ-
ated factors. Waste Management, 29, 440– 444.
Clair N. Sawer, McCarty .Perryl, 2003. Chemistry for Environ-
mental Engineering and Science, McGraw -Hill
Dehghani. M.H, Azam, K., Changani, F., DehghaniFard.E.,
2008, Assessment of medical waste management in educa-
tional hospitals of Tehran University Medical Sciences. Iranian
Journal of Environmental Health Science and Engineering,
5(2): 131–136.
Drug and Related waste, 2015. Government of south Auster-
alia Cytotoxic epa.gov/opptintr/exposure/pubs/episuitedl.htm
(accessed 1.11.13.).
Farzadkia .M, Moradi.A, Shah Mohammadi.M., Jor .S, Hospi-
tal waste management status in Iran: a case study in the teach-
ing hospitals of Iran, 2009, University of Medical Sciences.
Waste Management and Research, 27: 384–389.
Ferrando-Climent. L, S. Mozaz. R, Barcel. D, 2014. Incidence
of anticancer drugs in an aquatic urban system: From hospital
ef uents through urban wastewater to natural environment,
Environmental Pollution 193, 216e223
Ferreira. V, Ribau Teixeira. S, 2010. Healthcare waste manage-
ment practices and risk perceptions: Findings from hospitals
in the Algarve region, Portugal, Waste Management 30. 2657–
2663
Jang, Y.C., Lee, C., Yoon, O.S., Kim, H., 2006, Medical waste
management In Korea. Journal of Environmental Manage-
ment, 80: 107–115.
Jean-Philippe Besse, Jean-François Latour, Jeanne Garric.
2012. Anticancer drugs in surface waters, what can we say
about the occurrence and environmental signi cance of cyto-
toxic, cytostatic and endocrine therapy drugs? 2012- Environ-
ment International 39. 7386
Johannessen.L.M, Dijkman.M, Bartone.C, Hanrahan.D,
Boyer.M.G, Chandra, C., 2000. Health Care Waste Management
Guidance Note. The World Bank, Washington, DC.
Mehrdad Askarian, Peigham Heidarpoor, Ojan Assadian, 2010.
A total quality management approach to healthcare waste
management in Namazi Hospital, Iran. waste Management,
2010, 30, 2321-2326
Nussbaumer. S, Bonnabry. P, Jean-Luc Veuthey, Fleury-Sou-
verain. S, 2011. Analysis of anticancer drugs: A review, Jour-
nal home page: www.elsevier.com/locate/talanta, Talanta 85,
2265– 2289
Ra ee. A, Nabizadeh. R. 2016. Infectious Waste Management
in Imam Khomeini Hospital Complex in Tehran and Recom-
mending Appropriate Managerial Solutions” Journal of Health,
Vol. 7, No. 4, autumn, Pages 446-457
Tsakona. M, Anagnostopoulou. E, Gidarakos.E . 2010. Hospi-
tal waste management and toxicity evaluation: A case study.
Waste Management 27. 912–920
U.S. EPA, 2013. Exposure Assessment Tools and Models. EPI
Suite v4.1. http://www.
Vijay R, Puneet G. and M C Joshi, Practical guidelines for dis-
posing cytotoxic waste. India’s No.1 Weekly for the Pharma-
ceutical Industry
Voudrias. E, Goudakou.L, Kermenidou. K, Softa. A, 2012. Com-
position and production rate of pharmaceutical and chemical
waste from Xanthi General Hospital in Greece, Waste Manage-
ment 32. 1442–1452
WHO, 2014. Safe management of wastes from Health-care
activities. 2nd edition.
Zhang.J, Victor .W.C, Chang, 2013. Removal of cytostatic
drugs from aquatic environment: A review, 2013- Science of
the Total Environment 445–446. 281–298