
Medical
Communication
Biosci. Biotech. Res. Comm. 10(3): 354-358 (2017)
Effect of heptane food simulating liquid on surface
microhardness of 4 composites (Filtek Z250, Aelite,
Filtek Z350 and Clear l ST)
Niusha Golbari
1
, Morad Sadaghiani
2
*, Anahit Afrasiabi
3
, Mahdi Allahdadi
4
, Elmira Najafrad
5
and Ehsan Sadeghi Ziaratgahi
6
1
DDS. Post Graduate Student of Restorative Dentistry Department, Dental School, Shahid Beheshti University
of Medical Sciences, Tehran, Iran
2
Department of Restorative Dentistry, Islamic Azad University, Dental Branch, Tehran, Iran
3
DDS. Post Graduate Student of Restorative Dentistry Department, Dental School, Shahid Beheshti University
of Medical Sciences, Tehran, Iran
4
DDS. Post Graduate Student of Restorative Dentistry Department, Dental School, Shahid Beheshti University
of Medical Sciences, Tehran, Iran
5
DDS. Post Graduate Student of Restorative Dentistry Department, Dental School, Hamadan University of
Medical Sciences, Tehran, Iran
6
DDS. Post Graduate Student of Restorative Dentistry Department, Dental School, Shahid Beheshti University
of Medical Sciences, Tehran, Iran
ABSTRACT
Resin based composites are became more and more popular in restorative dentistry, particularly because of their
esthetic aspects. Decreasing the microhardness of dental restorative composites after curing in oral environment can
in uence their clinical durability. The aim of the current study was to determine effect of food simulating liquids 50%
heptane on surface microhardness of Z250 microhybrid, Aelite nano lled Z350 and Clear l nanohybrid composites.
20 specimens of each composite were prepared in a prefabricated mold with 5 diameter and 2 mm depth. All the
specimens composite were stored in distilled water, immediately after curing for 24 hours as the control group. Then
the specimens were taken out of the solution and washed, dried and then surface microhardness of specimens was
evaluated by the microhardness device based on Vickers. These specimens were divided into two groups randomly;
354
ARTICLE INFORMATION:
*Corresponding Author: Morad_Sadaghiani@yahoo.com
Received 23
rd
July, 2017
Accepted after revision 28
th
Sep, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF: 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
DOI: 10.21786/bbrc/10.3/3
Niusha Golbari et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EFFECT OF HEPTANE FOOD SIMULATING LIQUID ON SURFACE MICROHARDNESS OF 4 COMPOSITES 355
each of them was immersed in one of the following solutions distilled water, 50% heptane for 7 days at 37 ºC. After
one week conditioning period microhardness testing was carried out. The data were analyzed by 2way ANOVA and
Tucky HSD test. According to the results, there were signi cant differences on the initial microhardness of all com-
posites in water (p<0.05). Microhardness of the Z250 was higher than the other groups in water and heptane (p<0.05).
A signi cant decrease observed on the secondary microhardness of the Aelite and Clear l composites in heptane
compared to the rst time (p<0.05). The Clear l had higher decrease on microhardness in water and heptane com-
pared to the other composites (p<0.05). The microhardness of composite resin materials used in this study in uenced
after immersion in Heptane food simulation solution and distilled water. The effect of heptane on change in surface
microhardness is material dependent.
KEY WORDS: COMPOSITE,NANOHYBRID, MICROHYBRID,NANOFILL, HEPTANE, FOOD SIMULATING
INTRODUCTION
The administration of resin-based restorative materials
in dentistry has increased recently because of their good
aesthetic appearance, improvements in formulations,
ease of handling, and ability to establish a bond to dental
hard tissues. The mechanical property of the dental com-
posites depends on the ller particles and particle size.
Recent advancements on the organic matrix and inor-
ganic llers have led to the development of new materi-
als with reduced particle size and increased ller loading
which improved mechanical properties and aesthetics on
the current composite resin materials.Restorative materi-
als are required to have long-term continuousness while
the oral cavity is a complex aqueous environment and
restorative material contacts with saliva, (Catelan et al.
2010, Hengtrakool et al. 2011, Erdemir et al. 2013 George
and Kavyashree 2017).
Also, low pH due to acidic foods and drinks may
in uence the mechanical and physical characteristics of
the materials (Miranda et al. 2011). Physical character-
istics of restorative materials are an important concern
when determining suitable restorative materials because
they strongly in uence the clinical longevity of restora-
tions (Seifert et al. 2011). In clinical environment, micro-
hardness of materials decrease might contribute to its
deterioration. Under in vivo conditions, composite resin
materials may be exposed either discontinuously or con-
tinually to chemical agents found in saliva, food and
beverages (Topcu et al. 2010). In the short- or long-term,
these conditions have adverse effect on its physical and
chemical structure (Valinoti et al. 2008). The material’s
microhardness is one of the most important properties,
which correlates with resistance to intra-oral softening,
compressive strength and degree of conversion (Volta-
relli et al. 2010). A low surface microhardness value is
largely related to inadequate wear resistance and pro-
clivity to scratching, which can compromise fatigue
strength and lead to failure of the restoration (Erdemir
et al. 2013). So, the aim of the current study was to
determine effect of food simulating liquids 50% heptane
on surface microhardness of Z250 microhybrid, Aelite
nano lled Z350 and Clear l nanohybrid composites
MATERIAL AND METHODS
In this experimental in vitro study 4 composite types
were used (n=10). The composites allocated in stainless
steel (5mm diamater×2mm thickness). A smooth plate
put on the composite and the produced collected at 40 s
by SDS Kerr (1000mW/cm
2
) and polymerized (2×2) and
polished using aluminum oxide (3M ESPE) by spraying
the water. Then samples stored in distilled water 37ºC
for 24 h. Then microhardness of the samples determined
using Intender (6100 Vickers, USA).
COMPOSITES
The information of the composites used in the study
was Filtek z250 Micro hybrid ( ller weight 82%, ller
volume 60%) Zirconia silica (0.6μm) Bis-EMA, UDMA
Bis-GMA. The Filtek Z350 was Nano lled ( ller weight
78.5%, ller volume 59.5%) Zro2/sio2 nanocluster, Sio2
nano ller (5-20nm) Bis-GMA Bis-EMA UDMA TEG-
DMA. The Aelite was Nano lled ( ller weight 73%, ller
volume 54%) Glass frit Amouphous silica (0.04-5μm)
Exhoxylated Bisphenol A Dimethacrylate TEGDMA.
The Clear lMajesty ES-2 was Nanohybrid ( ller weight
93%, ller volume 81%) Silanatedbarium glass ller
Pre-polymerized organic ller (0.04-1μm) hydrophobic
aromatic dimethacyilate TEG-DMA Bis-GMA. The 50 gr
force for 15 s is done using Intender on 3 points in each
sample. Then the microhardness of the samples deter-
mined. The 10 samples allocated into the heptane and 10
in distilled water for 7 days. After one week condition-
ing period microhardness testing was carried out.
STATISTICAL ANALYSIS
The data were analyzed by 2way ANOVA and Tucky
HSD test using SPSS 16.0 for Windows (SPSS, Inc., Chi-
cago, IL, USA). P< 0.05 was considered as signi cant
differences between treatments.
Niusha Golbari et al.
356 EFFECT OF HEPTANE FOOD SIMULATING LIQUID ON SURFACE MICROHARDNESS OF 4 COMPOSITES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
RESULTS AND DISCUSSION
According to the results, there were signi cant differ-
ences on the initial microhardness of all composites in
water (p < 0.05). Microhardness of the Z250 was higher
than the other groups in water and heptane (p < 0.05).
No signi cant difference observed on primary micro-
hardness of Aelite and Clear l (p > 0.05). A signi cant
decrease observed on the secondary microhardness of
the Aelite and Clear l composites in heptane compared
to the rst time (p < 0.05). The microhardness of Clear l
signi cantly decreased compard to the other composites
in water and heptane conditions.
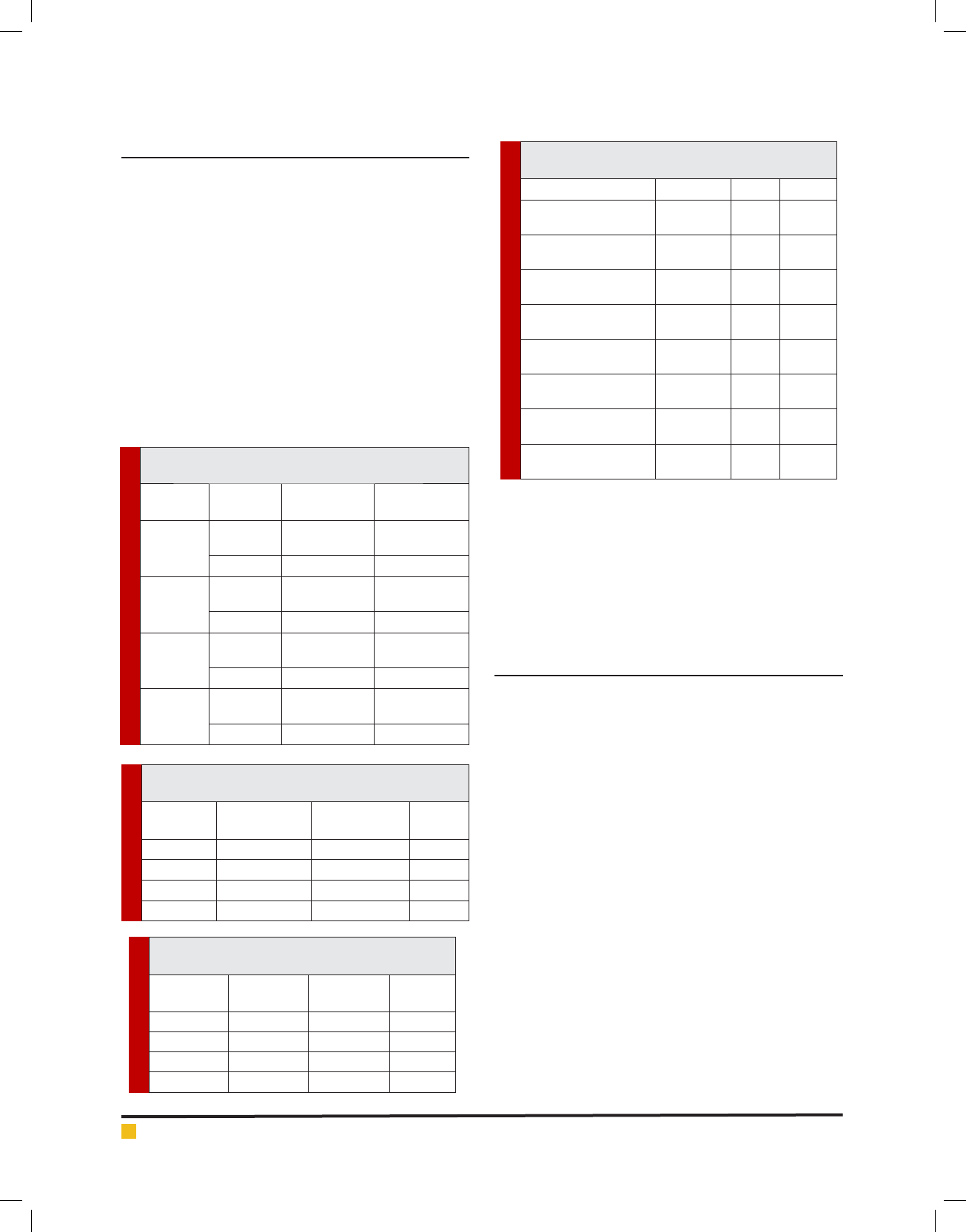
As seen in table 2, a signi cant differences observed
between primary and secondary microhardness of
the Z350 (65.30±6.19 and 75.84±4.25), 75.84±4.25
(50.88±7.47 and 39.87±5.07), Z250 (73.69±3.69 and
85.22±9.33) and Clear l (43.66±4.99 and 35.47±4.61).
As seen in table 3, signi cant difference was observed
on microhardness of Aelite (0.006) and Clear l (0.0001)
stored in heptane.
The primary and secondary microhardness of materi-
als is presented in table 4.
DISCUSSION
During consumption of food or drink contacts teeth or
restoration surfaces for only a short time before it is
washed away by saliva. Usually contact of teeth with
acidic food or drink for a prolonged period of time
and the situation did not account for the role of saliva
(Erdemir et al. 2013). As observed in the current study,
surface microhardness of Z250 was higher than the other
groups. After 24 hours distilled water had signi cant
effect on all the specimens. After 7days distilled water
had signi cant effect on all groups however, Heptane
had signi cant effect on Aelite and Clear l specimens.
According to analyses after both 24 hours and 7 days
Z250 and Z30 specimens showed increase in micro-
hardness while Aelite and Clear l showed signi cant
decrease in microhardness. Clear l presented the low-
est microhardness values. Distilled water was selected
instead of arti cial saliva to simulate the aching effect
of saliva because the arti cial saliva storage medium is
not considered to be a more clinically relevant environ-
ment (Erdemir et al. 2013).
The surface microhardness index of all restorative
materials after a week of storage in distilled water was
higher than the baseline surface microhardness val-
Table 1. The microhardness of the different composited
composite stored in distilled water or heptane
Composite Food
suspension
Primary
microhardness
Secondary
microhardness
Z350
distilled
water
65.3000 75.8450
Heptane 63.8390 67.3380
Aelite
distilled
water
73.6970 85.2210
Heptane 77.3370 82.7360
Z250
distilled
water
50.8810 39.8760
Heptane 50.8720 39.8550
Clear l
distilled
water
43.6690 35.4780
Heptane 43.4300 33.5460
Table 2. the primary and secondary microhardness of
composite stored in distilled water
Composite Primary
distilled water
Secondary
distilled water
P value
Z350 65.30±6.19 75.84±4.25 0.0001
75.84±4.25 50.88±7.47 39.87±5.07 0.015
Z250 73.69±3.69 85.22±9.33 0.0001
Clear l 43.66±4.99 35.47±4.61 0.013
Table 3. the primary and secondary microhardness
of composite stored in heptane
Composite
Primary
heptane
Secondary
heptane P value
Z350 63.83±3.55 67.33±5.95 0.226
Aelite 50.87±6.41 39.85±6.90 0.006
Z250 77.33±6.27 82.73±3.68 0.064
Clear l 43.43±4.46 33.54±2.62 0.0001
Table 4. the primary and secondary microhardness of
materials
Compared materials t-Test P value
Z350 & Distilled water
(primary & secondary)
-10.54±1.33 -7.906 0.001
Z350 & Heptane
(primary & secondary)
-11.52±6.86 -5.312 0.226
Aelite & Distilled water
(primary & secondary)
11.00±3.67 2.991 0.015
Aelite & Heptane
(primary & secondary)
11.01±3.083 3.573 0.006
Z250 & Distilled water
(primary & secondary)
-9.09±8.90 -3.231 0.001
Z250 & Heptane
(primary & secondary)
-9.55±10.71 -2.820 0.064
Clear l & Distilled water
(primary & secondary)
8.19±2.66 3.072 0.013
Clear l &Heptane
(primary & secondary)
9.88±1.35 7.280 0.001

Niusha Golbari et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EFFECT OF HEPTANE FOOD SIMULATING LIQUID ON SURFACE MICROHARDNESS OF 4 COMPOSITES 357
ues. This could possibly be explained by the ampli-
ed monomer conversion and additional post-curing
cross-linking reactions in the resin phase over the time.
Compoglass F, Filtek Z250, Filtek Supreme and Premise
specimens stored in distilled water had lower surface
microhardness reductions compared to the specimens
immersed in sports and energy drinks (Erdemir et al.
2013). In a study using Meliodent, FuturaGen and hard
GC reline.
Rajaee et al. (2014) reported heptane conditioning
decreased the exural strength of Meliodent and Futur-
aGen and microhardness of FuturaGen. Ethanol solution
had the most adverse effect on the microhardness and
exural strength of the tested resin materials (Rajaee
et al. 2014). Takahashi et al. (1998) reported that water
immersion had different effects on the exural strength
and microhardness of different denture base and reline
resin materials. They concluded that the results could
be due to the fact that the intrinsic strength of the
resin and the amount of water sorption in the system
in uences the mechanical strength of water absorbed
acrylic resins. It is reported two days of immersion in
the water lead to a reduction in the microhardness of
the resin samples. As mentioned, water absorption and
continuation of the acrylic polymerization process is
time-dependent and diffusion-controlled Azevedo et
al. (2005). Organic solutions may damage the resin
matrix (heptane and aqueous ethanol solution). On the
other hand, water and citric acids can damage organic
llers. Therefore organic solutions could decrease ex-
ural strength and microhardness of dental resins (Yesi-
lyurt et al. 2009).
In a study, Yaniko
g
˘lu et al. (2009) determined the
surface microhardness of lled (Estelite), nano l (Ælite),
un lled (Valux Plus), hybrid (Tetric ceram) and Ormocer-
based (Admira) composite resins in tea, coffee, Turk-
ish coffee, mouthwash, cola, and distilled water. Based
on their report the microhardness values of composite
materials were statistically different in different immer-
sion solutions. The acidity may change the polymeric
matrixes of composite resin affecting dimethacrylate
monomer present in their compositions (Al-Samadani,
2013). A previous study suggested that, by lowering the
solutions’ pH, there is production of methacrylic acid
that results in the sorption and hygroscopic expansion
as a consequence of enzymatic hydrolysis and biodeg-
radation (Sripetchdanond and Leevailoj, 2014). It was
observed that sodium uoride containing mouth rinses
also reduce the surface microhardness (Sripetchdanond
and Leevailoj, 2014).
In a recent study, George et al (2017) on effect of four
mouth rinses on microhardness of resin composite (Filtek™
P60) material (3M ESPE St. Paul, MN, USA) reported all the
mouth rinses showed reduction in surface microhardness
of the esthetic restorative material. Yesilyurt et al. (2009)
reported microhardness of silorane-based composite was
not in uenced by ethanol signi cantly, which could be
due to the hydrophobicity of the resin matrix. Except for
Bis-EMA, all other molecules (Bis-GMA, UDMA, and TEG-
DMA) have hydroxyl groups, which promote water sorp-
tion. As for silorane-based composite, it has 3,4-epox-
ycyclohexyl-cyclopolymethylsiloxane. In conclusion, the
microhardness of composite resin materials used in this
study in uenced by food simulation solutions. The effect
of heptane on change in surface microhardness is material
dependent.
REFERENCES
Al-Samadani KH. 2013 Color stability of restorative materials
in response to Arabic coffee, Turkish coffee and Nescafe. J
Contemp Dent Pract. 14:681-90.
Azevedo A, Machado AL, Vergani CE, Giampaolo ET,Pavarina
AC. 2005 Hardness of denture base and hard chair-side reline
acrylic resins. J Applied Oral Sci. 13: 291-295.
Catelan KA, Briso AL, Sundfeld RH, Santos PH. 2010 Effect of
artifcial aging on the roughness and microhardness of sealed
composites. J Esthet Restor Dent. 22:324-30.
Erdemir U, Yildiz E, Eren MM, Ozel S.2013 Surface hardness
evaluation of different composite resin materials: in uence of
sports and energy drinks immersion after a short-term period.
J Appl Oral Sci. 21(2):124-31.
George R. and Kavyashree G. 2017 Effect of four mouth rinses
on microhardness of esthetic restorative material: An in vitro
study. J Int Oral Health 2017;9:55-9.
Hengtrakool C, Kukiattrakoon B, Kedjarune-Leggat U. 2011
Effect of naturally acidic agents on microhardness and surface
micromorphology of restorative materials. Eur J Dent. 5:89-
100.
Miranda DA, Bertoldo CE, Aguiar FH, Lima DA, Lovadino JR.
2011 Effects of mouthwashes on Knoop hardness and surface
roughness of dental composites after different immersion
times. Braz Oral Res. 25:168-73.
Rajaee N, Vojdani M, Adibi S. 2014 Effect of food simulating
agents on the exural strength and surface hardness of den-
ture base acrylic resins. OHDM. 13(4): 1041-1047.
Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE. 2011
Health effects of energy drinks on children, adolescents, and
young adults. Pediatrics. 127:511-28.
Sripetchdanond J, Leevailoj C. 2014 Wear of human enamel
opposing monolithic zirconia, glass ceramic, and composite
resin: An in vitro study. J Prosthet Dent 112:1141-50.
Takahashi Y, Chai J, Kawaguchi M. 1998 Effect of water sorp-
tionon the resistance to plastic deformation of a denture base
material relined with four different denture reline materials.
Intern J Prosthods. 11: 49-54.
Topcu FT, Erdemir U, Sahinkesen G, Yildiz E, Uslan I, Acikel
C. 2010 Evaluation of microhardness, surface roughness, and

Niusha Golbari et al.
358 EFFECT OF HEPTANE FOOD SIMULATING LIQUID ON SURFACE MICROHARDNESS OF 4 COMPOSITES BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
wear behavior of different types of composite resins polymer-
ized with two different light sources. Biomed Mater Res B Appl
Biomater. 92:470-8.
Valinoti AC, Neves BG, Silva EM, Maia LC. 2008 Surface deg-
radation of composite resins by acidic medicines and pH-
cycling. J Appl Oral Sci. 16:257-65.
Voltarelli FR, Santos-Daroz CB, Alves MC, Cavalcanti AN,
Marchi GM. 2010 Effect of chemical degradation followed by
tooth brushing on the surface roughness of restorative com-
posites. J Appl Oral Sci. 18:585-90.
Yaniko
g
˘
lu N, Duymu
s
¸ ZY, Yilmaz B. 2009 Effects of different
solutions on the surface hardness of composite resin material.
Dent Mater J 28(3): 344–351
Yesilyurt C, Yoldas O, Altintas SH, Kusgoz A. 2009 Effects of
food simulating liquids on the mechanical properties of a sil-
orane-based dental composite. Dent Mater J 28:362-7.