
Medical
Communication
Biosci. Biotech. Res. Comm. 10(2): 324-329 (2017)
Comparative studies on the enamel demineralization
of cemented orthodontic bands using four different
cements
Mehrnaz Moradinejad
1
, S. Hojjat Shakib
2
* and Zeinab Eftekhari
1
1
Assistant Professor, Department of Orthodontics, School of Dentistry, Ahvaz Jundishapur University of
Medical Science, Iran
2
Postgraduate Orthodontic Resident, School of Dentistry, Ahvaz Jundishapur University of Medical Science, Iran
ABSTRACT
This study assessed the demineralization depth of the buccal and lingual surfaces of the premolars cemented by zinc
phosphate, glass-ionomer, resin-modi ed glass-ionomer (RMGI) and resin cement to receive orthodontic bands in
vitro. In this in vitro experimental trial, 80 intact premolars were collected and after cleaning, they were randomly
assigned into 4 groups. Orthodontic bands were cemented to the teeth using zinc phosphate, glass-ionomer, resin-
modi ed glass-ionomer and resin cements. The teeth were stored in the arti cial saliva at 37 °C for 7 days to simulate
cement solubility in the oral cavity while they were kept in the acidic gelatin solution (gelatin 17%, 1g/L hydroxyapa-
tite, 0.1% thymol, pH=4.3) for in vitro caries stimulations. These procedures were repeated for 4 times (total 8 weeks).
After bands removal, the teeth were coated by a nail varnish and only 2 small windows (2×2 mm) in the buccal (which
was not under the band and with no contact with cement) and lingual surfaces (under the band with a contact with
cement) were exposed. The teeth were kept in 10% methylene blue for 24 hours and after being washed by deionized
water, they were sectioned buccolingually. The sections were examined by a microscope in 50x magni cation and
depth of methylene blue penetrations were calculated. The depths of the demineralization were analyzed by one-sided
analysis of variance (ANOVA) and post hot Tukey tests. The demineralization depth in the lingual surfaces using the
cements of zinc phosphate, glass-ionomer, resin modi ed glass- ionomer and resin cement were 17.85 (±11.59), 15.55
(±9.44), 8.55 (±8.04) and 11.8 (±8.88) microns respectively while the values were 26.95 (±6.72), 25.75 (±5.66), 24.35
(±6.77) and 22.65 (±8.19) for the buccal surfaces. Signi cant differences were found regarding the demineralization
depth in the lingual surfaces in different cements (p=0.02), however, the differences were not signi cant in the buccal
324
ARTICLE INFORMATION:
*Corresponding Author: s.ho.shakib@gmail.com
Received 1
st
April, 2017
Accepted after revision 29
th
June, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/

Moradinejad, Shakib and Eftekhari
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COMPARATIVE STUDIES ON THE ENAMEL DEMINERALIZATION OF CEMENTED ORTHODONTIC BANDS 325
surfaces as a control group. In lingual surfaces, signi cant differences were observed between zinc phosphate and
resin-modi ed glass-ionomer which was higher for zinc phosphate cement (p=0.02). Resin-modi ed glass-ionomer
showed the best results to prevent enamel demineralization adjacent to the orthodontic bands.
KEY WORDS: DEMINERALIZATION, BANDS, RESIN MODIFIED GLASS-IONOMER, DYE PENETRATION
INTRODUCTION
Enamel demineralization and adhesive bond strength are
quite controversial in orthodontic treatments. Although
brackets are a certain part of x orthodontic treatment,
more than 85% of the orthodontists use orthodontic
bands for molars. If the bond linking the band and tooth
fails, decalci cation of the tooth surface, unplanned and
long visits as well as unsuccessful treatment mechan-
ics will be inevitable (Millett 2003 and Craig 2006). To
increase the resistance against occlusal forces, a more
stable treatment is applied to use the bands. Hence, it
is essential to place an appropriate retention in bands
using a mechanical or chemical method. The cement
used not only does provide chemical retention, but also
it provides a mechanical retention by lling the pores
(Kvam 1983, Mosby 2002 and Millett 2009 Kashani
etal., 2012 Prabhavathi 2015).
One of the foremost causes of band loosening or why
a band has a bond failure is the dissolution of cement in
oral cavity. Clinically speaking, the degree of band reten-
tion and the extent to which cements dissolve in oral
cavity are important since the bands with low retention
and cements of high dissolution can accumulate plaque
under the bands in a way that enamel decays within
three weeks. Therefore, using cements with low disso-
lution in oral cavity can increase the ef ciency of the
orthodontic mechanics and reduce caries and microleak-
age (Norris 1986, Johnson 2000, Millett 2003, Buchalla
2000, Hajmiragha 2008, Sabouhi Prabhavathi 2015).
Since 1878, zinc phosphate cement has been used
to cement orthodontic bands. It has a high compres-
sive strength, low tensile strength and it dissolves more
quickly adjacent to organic acids (Norris 1986, Johnson
2000, Millett 2003). When cemented orthodontic bands
are removed by zinc phosphate, in some cases decal-
ci cations are observed a great deal which can be due
to the cement loss between the bands and tooth and a
more favorable environment for bacterial activity, (Craig
2006, Buchalla 2000).In addition to the biocompatibil-
ity with enamel and dentin, glass-ionomer cement has
various cariostatic effects. Fluoride ion activity in these
cements can cause remineralization although their bond
strength is clinically limited (Pithon 2006 Prabhavathi
etal., 2015).
To try to release uoride and to improve bond
strength, resin modi ed glass-ionomer cements were
introduced by Rix (2001). This cement requires moisture
because of its speci c chemical composition and it can
be used in moisture atmospheres as a suitable element to
be applied in areas where dry isolation cannot be used.
It is chemically bonded to enamel and dentin. Also, it
has a similar thermal expansion coef cient compat-
ible with tooth structure (Valente 2002). Resin cements
are composites with small ller particles and low ller
ensuring there is a thin lm thickness. They are much
stronger than light-cure glass-ionomers but they cannot
expand as light-cure glass-ionomers do. Resin cements
can be micromechanically bonded to a prepared enamel,
dentin, alloy and ceramics. They are offered in different
dyes (O’Brien 2002).The current study aims at comparing
the incidences of enamel decalci cation of zinc phos-
phate, glass-ionomer, resin-modi ed glass-ionomer and
resin cement to receive orthodontic bands.
MATERIALS AND METHODS
In this experimental research 80 intact premolars which
had no cracks, fractures or restorations were collected
and were extracted during a 6-month period for ortho-
dontic treatments. To remove debris, a non- uoride
lotion (13% hypochlorite for 24 hours) was used. After
dis-infecting them either in a normal saline or deionized
water, they were kept in room temperature (Foley 2002).
The teeth were then divided up into 4 groups with 20 in
each. The groups were zinc phosphate cements (Hoff-
mann, Germany), RMGI (GC Fuji, Japan), glass-ionomers
cements (GC Fuji, Japan) and Resin Cements (GC Fuji,
Japan).
Around the collected teeth, stainless steel orthodontic
bands (DENTAURUM,Germany) were installed and were
tightly xed to reduce enamel dissolution. In each band
and each group, cement was added in a way that its
maker asserted. Care was taken in each group to remove
additional cement from the edges of cervical and occlusal
bands before they were polymerized so as to they do not
affect the results. Afterwards, cements were remained
untouched for 2 minutes in 25° C to solidify. (bench set)
After sub-dividing, the teeth were kept in plastic con-
tainers and then using an acrylic resin, they were lled
high up to normal bones. Only the crowns were exposed
and the bands were located a few millimeters higher
than acrylic resin. We put the teeth in plastic bags and
the following procedures were followed for 4 times (in
an 8-week period):
Moradinejad, Shakib and Eftekhari
326 COMPARATIVE STUDIES ON THE ENAMEL DEMINERALIZATION OF CEMENTED ORTHODONTIC BANDS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
To simulate in vitro caries in oral cavity, the samples
were put in an incubator at 37° C in arti cial saliva for
7 days. Then, all the teeth were kept in an acidic gela-
tin solution (17% gelatin,1 g/L hydroxyapatite, 0.1 %
thymol, 4/3 pH) in an incubator at 37°C for 7 days (fol-
lowing the recommendations made by Silverstone etal,
1985) (17). They were kept in deionized water subject to
demineralization gel.
In the next stage, the teeth were taken out of the
acrylic and bands were removed from them. Hav-
ing removed the leftover cements, the teeth were then
cleaned. Following that, they were protected applying
nail-varnish. Only two small windows of the enamel
(2×2 mm) were exposed. One was beneath the covering
band in the lingual surface which was subject to cement
and the other outside the covering band in the buccal
surface with no touch with the cement. The second one
is considered as the control surface for all the molars. In
this case, the enamel which was directly connected to
the cement and also the enamel which was 2 millimeters
far from cement was tested.
The teeth were then put in a 10% methylene blue
at 37°C for 24 hours and were washed using deionized
water. In this stage, all of them were kept in plastic con-
tainers to be prepared for cutting and using epoxy res-
ins, we lled them one millimeter down to the cusps.
The mounted teeth of the epoxy resins were buccolin-
gually cut by a diamond disc with cold water along the
line between the buccal and lingual windows. The cuts
which were 50 times magni ed by a stereomicroscope
were then analyzed. Finally, the depth of caries lesions
was examined in microns via measuring penetration
rate of methylene blue.
We then compared the demineralization depth meas-
ures of the caries lesions in different sub-groups through
the one-way analysis of variance. In the lingual surfaces
of molars, the ANOVA test results were signi cant so
having used Tukey comparisons; we compared them two
by two.
RESULTS AND DISCUSSION
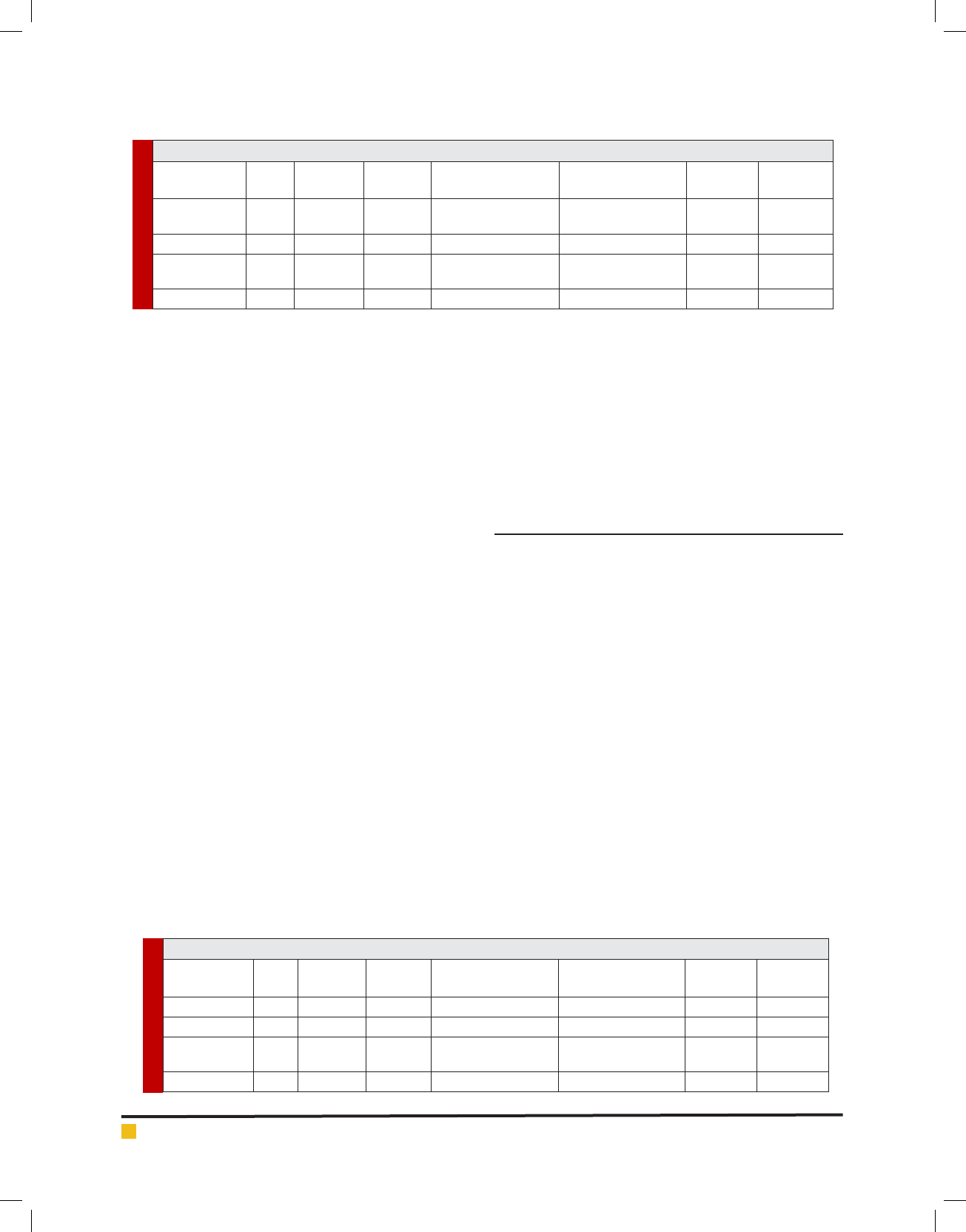
The demineralization depth of the caries lesions in lin-
gual surfaces cemented by zinc phosphate, glass-iono-
mer, resin-modi ed glass-ionomers and resin cements
were 17.85 (± 11.59), 15.55 (±9.44), 8.55 (± 8.04) and
11.8 (± 8.88) microns. (Table 1)
The demineralization depth of the caries lesions in
buccal surfaces cemented by zinc phosphate, glass-iono-
mer, resin-modi ed glass-ionomers and resin cements
were 26.95 (± 6.72), 25.75 (±5.66), 24.35 (± 6.77) and
22.65 (± 8.19) microns. (Table 2)
The results of the one-way ANOVA revealed signi -
cant differences in demineralization depth of the caries
lesions in lingual surfaces. (p=0.02). On the other hand,
the post hot Tukey test aiming at comparing two by
two groups showed signi cant differences between zinc
phosphate cements and resin modi ed glass-ionomer
(p=0.02), but in other two-by-two comparisons no sig-
ni cant difference was seen. (Table 3).
In buccal surfaces (control group), there was no sig-
ni cant difference in terms of demineralization depth
Table 1. Distribution indices of demineralization depth (in microns) in lingual surface of teeth for various cements
Group Mean Standard
Deviation
Standard
Error
95% con dence
interval/Low range
95% con dence
interval/High range
Minimum Maximum
Zinc
Phosphate
17.85 11.59 2.59 12.43 23.27 0 46.0
Glass-ionomer 15.55 9.44 2.11 11.13 19.97 0 48.0
Resin-modi ed
glass ionomer
8.55 8.04 1.79 4.79 12.31 0 24.0
Resin cement 11.8 8.88 1.99 7.64 15.96 0 26.0
Table 2. Distribution indices of demineralization depth (in microns) in buccal surface of teeth for various cements
Group Mean Standard
Deviation
Standard
Error
95% con dence
interval/Low Range
95% con dence
interval/high range
Minimum Maximum
Zinc Phosphate 26.95 6.72 1.5 23.8 30.09 9.0 38.0
Glass-ionomer 25.75 5.66 1.26 23.1 28.39 16.0 37.0
Resin-modi ed
glass ionomer
24.35 6.77 1.51 21.18 27.52 15.0 44.0
Resin cement 22.65 8.19 1.83 18.82 26.48 0 40.0

Moradinejad, Shakib and Eftekhari
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COMPARATIVE STUDIES ON THE ENAMEL DEMINERALIZATION OF CEMENTED ORTHODONTIC BANDS 327
values of caries lesions in using various cements (one-
way ANOVA: p=0.24). As there was no signi cant dif-
ference in any of the comparisons, we did not compare
the results of the demineralization depth two by two.
Obviously, under these circumstances no signi cant dif-
ferences will be available among cement groups in terms
of demineralization depth values.
On the other hand, the demineralization depth aver-
age of caries lesions (Standard deviation ±) in all cements
was analyzed and it was 13.44 microns ±) 10.05) for
lingual surface of the teeth; whereas in buccal surface
it was 24.93 microns (±6.95) According to the results
driven from Student t test, signi cant differences were
observed in buccal and lingual surfaces of the molars
and in lingual surface it was signi cantly lower than
buccal surface. (p=0.0001)
What makes teeth capable for decalci cation and
caries when orthodontic bands are applied is that these
bands and their connections provide a suitable place
for plaque accumulation. It was discovered that 85% of
cervical and occlusal margins of orthodontic bands are
exposed to caries lesions (Radlanski 2003). Therefore,
using cements which can release uoride can be effective
in preventing enamel decalci cation. As the ndings of
this study indicate, signi cant differences were observed
in lingual surfaces in terms of demineralization depth
of the caries lesions when zinc phosphate cements and
resin modi ed glass ionomer were used (17.85 and 8.55
microns respectively). In addition, resin modi ed glass
ionomer had the most preventive effects of deminerali-
zation. The least capability of preventing demineraliza-
tion of caries lesions was for zinc phosphate and glass
ionomer cements and resin cements respectively. (15.55,
11.8 microns for glass ionomer and resin cements).
In buccal surfaces, which were the control group for
all the teeth, there was not any signi cant difference
in demineralization depth of the arti cial caries lesions
in zinc phosphate, glass ionomer, resin modi ed glass
ionomer and resin cements. Nevertheless, the lowest
demineralization depth with an average 22.65 microns
was observed for resin cements whereas resin modi ed
glass ionomers, glass ionomer and zinc phosphate fol-
lowed in ranking respectively, (demineralization depth
values: 24.35, 25.75 and 26.95 microns). The variations
in demineralization depth in different groups together
with high amount of released uoride from resin modi-
ed glass ionomer cements can be indicative of suitable
bond strength, high tensile strength and low solubility
(Foley 2002, Silverstone 1985, Radlanski 2003).
In this study, the highest values of demineralization
depth were observed around the cemented orthodontic
bands which used zinc phosphate. This can be due to
the fact that zinc phosphate cement did not contain any
uoride and as a result it provided no excessive protec-
tion of enamel against acidic attacks of in vitro bacteria.
On the other hand, the dissolution of zinc phosphate
cement in in vitro cavity can make the teeth vulnerable
to caries (Foley 2002).
Also, resin cements have had comparatively more
suitable preventive effects of caries around orthodon-
tic bands than glass ionomer cements. Pressure strength
and higher tensile strength as well as low solubility and
micromechanical bond to the teeth are among the ben-
e ts of resin cements compared to glass ionomers. Resin
cements are recommended to be used under certain
conditions in which RMGIs cannot cause any retention
(Weiner 2008).
In 2015 in India, Prabhavathi etal. carried out an in
vitro experiment and analyzed demineralization values
of orthodontic cements (zinc phosphate, glass ionomer
and resin modi ed glass ionomer cement); and having
used acidic gelatin solution, they then stimulated acidic
cariogenic conditions and cut the teeth to measure car-
ies lesions by an electron microscope (Prabhavathi etal.,
2015). In this research, zinc phosphate cement had the
highest demineralization value while the value for glass
ionomer was the lowest. These results correspond with
the ndings obtained from our study about zinc phos-
phate; nevertheless, RMGI cement of the current study
had the minimum demineralization depth and that was
different from the recent study.
In an in vitro study conducted in 2014 in India,
Hedge etal. (2014) used a similar research protocol and
they found out RMGI had the minimum demineraliza-
Table 3. The results of various comparisons of cement groups in terms of demineralization
depth in lingual surfaces of teeth. (Tukey test)
First Group Second Group Average differences P value
Zinc Phosphate Glass ionomer Resin modi ed
glass ionomer Resin cement
2.3
9.3
6.05
0.87
0.02
Signi cant
0.19
Glass-ionomer Resin modi ed glass ionomer
Resin cement
7.0
3.75
0.11
0.61
Resin modi ed glass ionomer Resin cement 3.25 0. 71
Moradinejad, Shakib and Eftekhari
328 COMPARATIVE STUDIES ON THE ENAMEL DEMINERALIZATION OF CEMENTED ORTHODONTIC BANDS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
tion whereas zinc phosphate had the highest enamel
demineralization in banded teeth (Hegde 2014). It can be
concluded that the ndings taken from this study cor-
respond with the current research. In 2012, in another
study in India, Goje et al. investigated the strength
against enamel demineralization after banding 4 ortho-
dontic cements in in vitro conditions and they suggested
that banded teeth which were cemented by glass ionomer
and RMGI had the lowest values followed by zinc poly
carboxylate and zinc phosphate (Goje etal. 2012). Same
ndings could be observed from the current research.
Kashani etal., in 2012, investigated the enamel deminer-
alization depth of adjacent cemented orthodontic bands
using zinc polycarboxylate, glass ionomer and RMGI in
Iran. In their ndings, the highest depth was for zinc
polycarboxylate; and the best result of preventing caries
in orthodontic bands was for RMGI (Kashani 2012).
The results taken from the recent study have been
reported in the current research in which RMGI was a
more suitable cement to prevent caries lesions. In another
research carried out in Canada in 2002, Foley etal. also
found out that zinc phosphate had more dye penetration
compared to zinc poly carboxylate cements and RMGI.
They also reported that RMGI is the best for long-term
orthodontic treatment (Foley 2002).As resin modi ed
glass ionomer possesses speci c features, researchers
consider it as a more suitable cement to prevent caries
lesions around and beneath orthodontic bands. There-
fore, considering the results of the observations (Foley
2002, Weiner 2008). We can conclude that resin modi-
ed glass ionomer could be used as an intermediary for
orthodontic banding purposes. However, it is important
to consider all the consequences of this replacement
including clinical inspections.
It is proven that in the rst three days of cement-
ing much more uoride is released from orthodontic
cements. However, after three weeks uoride release
decline considerably (Ogaard 1989). Having said that, it
is essential to conduct long term evaluations of demin-
eralization depth of caries lesions after applying ortho-
dontic cements.The lesions of enamel caries which are
arti cially created have all the histologic characteristics
of natural caries and they are successfully applied in in
vitro enamel demineralization researches (Casals 2007).
Moreover, stimulated enamel caries lesions are prepared
in a more homogenous way. As a result, a much more
reliable laboratory model is provided to survey deminer-
alization and remineralization depth values. Under these
condition the area in enamel in which carries form and
has a xed depth in subsurface, can be used to evalu-
ate remineralization (Queiroz 2008). Generalizing lab
research results to oral cavity has its own limitations.
First of all, in oral cavity variables such as uoride
weakening by saliva play an important role and hence
gaining access to various uoride products and clean-
ing them cannot be stimulated in experimental lab stud-
ies (Damato 1990). Moreover, in oral cavity conditions,
there are variables related to host such as the mineral
concentrations of tooth and pellicle or the conditions
in which plaque can be formed that can affect demin-
eralization value. The factors related to saliva includ-
ing salivary ow rate, its composition and buffering
capacity can have protective effects in tooth surfaces
(Marsh 1999). Increasing the remineralization capability
of saliva is also clinically important. As saliva is widely
found in oral cavity, the demineralization rate is de -
nitely lower than lab conditions; so more evaluations
are recommended in clinical environments and in situ.
CONCLUSION
It can be concluded that using resin modi ed glass iono-
mer has had the best results in preventing enamel dem-
ineralization under orthodontic bands.
REFERENCES
Buchalla W, Attin T, Hellwig E. (2000). Brushing abrasion of
luting cements under neutral and acidic conditions. Oper Dent;
25: 482-487.
Casals E, Boukpessi T, McQueen CM, Eversole SL, Faller RV.
(2007). Anticaries potential of commercial dentifrices as deter-
mined by uoridation and remineralization ef ciency. J Con-
temp Dent Pract Nov; (7): 1-10.
Craig R. (2006). Restorative dental materials. 12th Ed. St. Louis:
The CV Mosby Co. Chap20: 486-491.
Damato FA, Strang R, Stephen KW. (1990). Effect of uoride
concentration on remineralization of carious enamel: an in
vitro pH-cycling study. Caries Res; 24: 174-180.
Foley T, Aggarwal M, Hatibovic-Kofman S. (2002). A compari-
son of in vitro enamel demineralization potential of 3 ortho-
dontic cements. Am J Orthod Dentofacial Orthop; 121:526–
530.
Goje SK, Sangolgi VK, Neela P, Lalita CH. (2012). A comparison
of resistance to enamel demineralization after banding with
four orthodontic cements: an in vitro study. J Ind Orthod Soc;
46(3): 141-147.
Hajmiragha H, Nokar S, Alikhasi M, Nikzad S, Dorriz H. (2008).
Solubility of three luting cements in dynamic arti cial saliva.
Journal of Dentistry, Tehran University of Medical Sciences;
5:95-98.
Hegde AB, Patil T, Khandekar S, Gupta G, Krishna Nayak US.
(2014). Comparative assessment of resistance to enamel dem-
ineralization after orthodontic banding with three different
cements: an in vitro study. Cumhuriyet Dent J; 17(2): 159-165.
Johnson N. (2000). Currents products and practice orthodontic
banding cements. J Orthod; 3: 283-284.

Moradinejad, Shakib and Eftekhari
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COMPARATIVE STUDIES ON THE ENAMEL DEMINERALIZATION OF CEMENTED ORTHODONTIC BANDS 329
Kashani M, Farhadi S, Rastegarfard N. (2012). Comparison of
the effect of three cements on prevention of enamel demin-
eralization adjacent to orthodontic bands. J Dent Res, Dent
Clinics Dent Prospects (JODDD); 6(3): 89-93.
Kvam E, Broch J, Harvold I. (1983). Comparison between a zinc
phosphate cement and a glass ionomer cement for cementation
of orthodontic bands. Eur Orthod; 5:307-313.
Marsh PD. (1999). Microbiologic aspects of dental plaque and
dental caries. Dent Clini North Am; 43: 599-614.
Millett D, Mandall N, Hickman J, Mattick R, Glenny AM.
(2009). Adhesives for xed orthodontic bands. A systematic
review. Angle Orthod; 79: 193-199.
Millett DT, Cummings A, Letters S, Roger E, Love J. (2003).
Resin-modi ed glass ionomer, modi ed composite or conven-
tional glass ionomer for band cementation? An in vitro evalu-
ation. Eur J Orthod 25: 609–614.
Millett DT, Duff S, Morrison L, Cummings A, Gilmour WH.
(2003). In vitro comparison of orthodontic band cements. Am
J Orthod Dentofacial Orthop Jan; 123:15-20.
Mosby F. (2002). Inc: Resin-modi ed glass-ionomer cement
can be used to bond orthodontic brackets. J Base Dent Pract;
2:209-210.
Norris DS, McInnes-Ledoux P, Schwaninger B, Weinberg R.
(1986). Retention of orthodontic bands with new uoride-
releasing cements. Am J Orthod; 89: 206-211.
O’Brien WJ. (2002). Dental materials and their selection. 3rd
Ed. Chicago, US: Quintessence Pub Co.
Ogaard B. (1989). Prevalence of white spot lesions in 19-year-
olds: a study on untreated and orthodontically treated persons
5 years after treatment. Am J Orthod Dentofacial Orthop; 96:
423-427.
Pithon MM, Dos Santos RL, Oliveira MV, Ruellas AC, Romano
FL. (2006). Metallic brackets bonded with resin-reinforced
glass ionomer cements under different enamel conditions.
Angle Orthod Jul; 76(4):700-704.
Prabhavathi V, Jacob J, Kiran MS, Ramakrishnan M, Sethi E,
Krishnan CS. (2015). Orthodontic cements and demineraliza-
tion: An in vitro comparative scanning electron microscope
study. J Int Oral Health; 7(2): 28-32.
Queiroz CS, Takeo Hara A, Paes Leme AF, Cury JA. (2008). pH
cycling models to evaluate the effect of low uoride dentifrice
on enamel de- and remineralization. Braz Dent J; 19 (1).
Radlanski RJ, Renz H, Reulen A. (2003). Distribution of the
cement lm beneath the orthodontic band: a morphometric in
vitro study. J Orofac Orthop; 64:284-292.
Rix D, Foley TF, Mamandras A. (2001). Comparison of bond
strength of three adhesives: composite resin, hybrid GIC,
and glass- lled GIC. Am J Orthod and Dentofac Orthop
Jan;119(1):36-42.
Sabouhi M, Taher M, Yarahmadi A. (2009). The comparison of
solubility of zinc phosphate and polycarboxilate based on Ira-
nian standard no 2725 and 2726. Shiraz Univ Dent J; 10:153-
159.
Silverstone LM. (1985). Fluorides and remineralization in clini-
cal uses of uorides. In: Weis HY, editor. A state of the art
conference on the uses of uorides in clinical dentistry. Phila-
delphia: Lea & Febiger:153-175.
Valente RM, De Rijk WG, Drummond JL, Evans CA. (2002).
Etching conditions for resin-modi ed glass ionomer cement
for orthodontic brackets. Am J Orthod Dentofacial Orthop
May;121(5):516-520.
VanMiller EJ, Donly KJ. (2003). Enamel demineralization inhi-
bition by cements at orthodontic band margins. Am J Dent
Oct;16(5): 356-358
.
Weiner R. (2008). Liners, bases, and cements: an in-depth
review, Part 3. Dent Today; 27(11): 65-70.