Environmental
Communication
Biosci. Biotech. Res. Comm. 10(2): 311-318 (2017)
Application of single-walled carbon nanotubes for
removal of aniline from industrial waste water
Davoud Balarak
1
, Ferdos Kord Mostafapour
1
and Ali Joghataei
2
1
Department of Environmental Health, Health Promotion Research Center, School of Public Health, Zahedan
University of Medical Sciences, Zahedan, Iran
2
Student Research Committee, Qom University of Medical Sciences, Qom, Iran
ABSTRACT
The adsorption behavior of aniline using single-walled carbon nanotubes (SWCNTS) as adsorbents is examined under
ambient conditions. The adsorption equilibrium of aniline on SWCNTS was evaluated by the Langmuir, Freundlich, Dubinin
Radushkevich and Tempkin isotherms. The results showed that the equilibrium data for aniline tted the Langmuir model
best within the concentration range studied. Experimental results showed that the time taken to attain adsorption equilib-
rium for aniline was 30 min. The adsorption energy obtained was 218.25 J/mg, which indicates that the adsorption process
is endothermic and a strong interaction between SWCNTS and Aniline molecules. The results showed that SWCNTS had
good potential for the removal of aniline from industrial wastewater.
KEY WORDS: ADSORPTION, ANILINE, SINGLE-WALLED CARBON NANOTUBES, ISOTHERMS
311
ARTICLE INFORMATION:
*Corresponding Author: alijoghatayi69@gmail.com
Received 10
th
March, 2017
Accepted after revision 18
th
June, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Rapid increasing of industries and subsequently increas-
ing the disposal of pollutants especially organic com-
pounds, to the water resources and environment caused
serious and adverse environmental impacts (Liu et al.,
2010, Delnavaz et al., 2009, Wu et al., 2012). With the
increasing concern for public health and environmental
quality, the stringent limits on the acceptable environ-
mental levels of organic pollutants have been estab-
lished (Liu et al., 2012, Jonidi Jafari et al., 2012). A com-
mon problem in most industries is the disposal of large
volume of wastewater containing organic compounds
(Jin et al., 2012, Shaobin et al., 2011).
Aniline is an important chemical compound which
is well own for its wide applications in the manufac-
ture of dyestuffs, rubbers, pesticides, plastics and paints
(Tang et al., 2012). However, the aniline-laden waste-
water discharged from these industries has become a
severe environmental problem as well (O’Neill et al.,

312 APPLICATION OF SINGLE-WALLED CARBON NANOTUBES FOR REMOVAL OF ANILINE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Balarak, Mostafapour and Joghataei
2000). It is highly toxic and has harmful influences on
human health and aquatic life (Leili M et al., 2013). Ani-
line is a blood toxin, causing hemoglobin to convert to
methemoglobin, resulting in cyanosis (Guo et al., 2012).
Lengthy or repeated exposures may result in decreased
appetite, anemia, weight loss, nervous system affects,
and kidney, liver and bone marrow damage. Any expo-
sure may cause an allergic skin reaction (Balarak et al.,
2016).
Due to the negative environmental impact caused by
the high toxicity of aniline, these wastewaters demand
thorough treatment. Traditionally, Aniline-containing
wastewater is usually treated by photocatalysis, cata-
lytic oxidation, biodegradation, advanced oxidation and
adsorption. Among these technologies, adsorption has
been proven to be effective in separating a wide variety
of organic contaminants from aqueous solutions (Shao-
bin et al., 2011). Compared to other treatment processes,
adsorption has the advantages of (1) removing a wide
variety of dissolved organic compounds and (2) not pro-
ducing any harmful byproducts (Ramavandi et al., 2015,
Ma et al., 2012). Various adsorbents have been devel-
oped for the removal of organic pollutants (e.g., dyes,
pesticides, pharmaceuticals/drugs, and phenols) from
water (Zazouli et al., 2014). Activated carbon (AC) is the
most commonly used commercial adsorbent because of
its excellent adsorption capacity for organic contami-
nants (Balarak et al., 2016).
However, it has certain shortcomings that include
limited availability, low adsorption capacity, and dif-
cult recovery (Zazouli et al., 2014). Recently, a great
deal of attention has been focused on the application of
nano-structured materials as adsorbents to remove toxic
and harmful organic substances from wastewater (Visa
et al., 2012, Jazayeri et al., 2010, Futalan et al., 2011).
Carbon nanotubes (CNTs) are one of the most widely
studied carbon nanomaterials and can serve as excellent
adsorbents. Because of their hollow and layered struc-
ture and large specific surface area, which is why CNTs
are the most commonly used nanomaterials for adsorb-
ing toxic material. CNT adsorbents can be classified into
three types: single-walled CNTs (SWCNTs), multi-walled
CNTs (MWCNTs), and functionalized CNTs (f-CNTs). Such
materials have already played an important role in the
effective removal of several organic contaminants from
water (Balarak et al., 2016, Nourmoradi et al., 2013).
In this study, SWCNTs were used to study the removal/
adsorption of aniline from an aqueous solution. The
effects of various operating parameters, such as adsor-
bent dose, temperature, initial aniline concentration and
contact time were studied and optimized. The kinetics
and thermodynamics of the adsorption process of ani-
line were studied. Thermodynamic calculations of the
adsorption process are required to understand the mech-
anism of adsorption, spontaneity, and heat of adsorption
using different thermodynamic parameters.
MATERIALS AND METHODS
The bulk solution of the aniline was prepared by dis-
solving a measured quantity of aniline (molecular for-
mula is C
6
H
7
N and formula weight is 93.13 g/mol) in
1 L of double distilled water.The single-walled carbon
nanotubes with average diameters of 10–20 nm [SMWC-
NTs (10–20)] was obtained from Research Institute of
Petroleum Industry (RIPI), Tehran, Iran and were used
as received. On the basis of the information provided
by the manufacturer, the SWCNTs were synthesized
by catalytic chemical vapor deposition (CVD) method.
All chemicals used in this study were obtained from
Sigma–Aldrich (analytical grade), and all solutions were
prepared using deionized water. Double distilled water
was used in all the experiments.The scanning electron
microscope (SEM) (JEOL JSM 6500F) and transmission
electron microscopy (TEM) (JEOL JEM-1230 operating
at 120 kV) were used to characterize the SWCNTs mor-
phological structure.
Adsorption kinetics was carried out by in batch tech-
nique. Batch experiments were carried out for determin-
ing the adsorption isotherms of aniline onto the various
adsorbent in a glass beaker. The aniline aqueous solutions
were magnetically stirred at a constant rate (180 rpm),
allowing suf cient time for reaching adsorption equilib-
rium. It was assumed that the applied stirring speed allows
all the adsorbent surface area to come in contact with ani-
line molecules over the course of the experiments. In each
experiment a xed volume (100 mL) of aniline aqueous
solution at constant aniline concentration (25-200 mg/L)
was used. The study was performed at room temperature
to be representative of environmentally relevant condi-
tion. All experiments were carried out in duplicate and
the average value was used for further calculation. The
removal ef ciency and sorption capacity of the SWCNTs
were determined by Eq. (1) and (2), respectively (Rawaj h
et al., 2006, Balarak et al., 2016):
(1)
(2)
Where; R (%) and q
e
(mg/g) are the removal ef ciency
and adsorption capacity, respectively. Co and Ce are the
concentrations of aniline in solution (mg/L) at time t=0
and t, respectively, M (g) is the mass of the sorbent and
V (L) is the volume of the aniline solution.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS APPLICATION OF SINGLE-WALLED CARBON NANOTUBES FOR REMOVAL OF ANILINE 313
Balarak, Mostafapour and Joghataei
RESULTS AND DISCUSSION
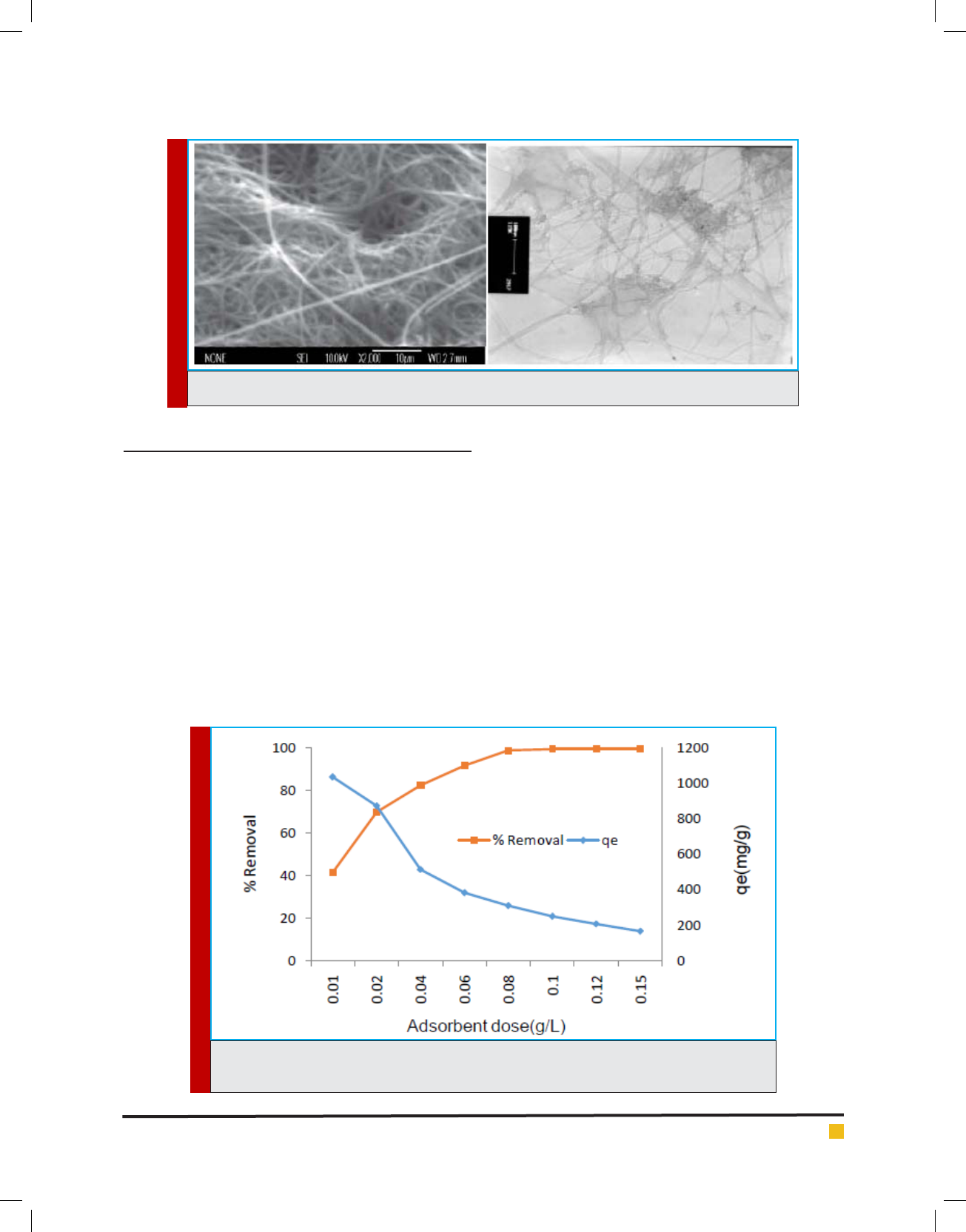
The scanning electron microscope and transmission
electron microscope imaging was used to study the mor-
phological structure of the pristine SWCNTs; representa-
tive images are presented in Fig. 1. The outer diameters
and inner cavities of SWCNTs (10–20) were 15–25 nm
and 6–10 nm, respectively. In addition, the TEM analysis
verified the hollow structure of the SWCNTs.
The removal of the Aniline was studied with different
dose of SWCNTS from (0.01 to 0.15 g/L) at the optimum
concentration of 25 mg/L with xed contact time (30
min), pH (7) and temperature of 30˚C ± 2˚ C. The effect
of the dose rate of SWCNTS on the removal of Aniline
is pictured in Figure 2. It was noted that the percentage
removal of the Aniline ion increases as the concentration
of SWCNTS increases owing to the enhanced total surface
area of the adsorbent. This means that the toxic ions can
be removed effectively from the contaminated water with
the proper amount of the adsorbent, which would pos-
sess more adsorption sites available for the Aniline ion
uptake from the solution (Mojovic
´
et al., 2011, Jadhav et
al., 2001). The optimum dose rate was found to be 0.8 g/L
for with the effective removal of 98.9%.
ADSORPTION ISOTHERMS
An isotherm describes the equilibrium relationship
between the adsorbate concentration in the liquid phase
and that on the adsorbent’s surface at a given condition
FIGURE 1. Micrographs of SWCNTs (a) SEM and (b) TEM
FIGURE 2. Effect of adsorbent dose on adsorption of aniline (C0= 25 mg/L, contact time=30 min,
pH=7, Temp= 30˚C)

314 APPLICATION OF SINGLE-WALLED CARBON NANOTUBES FOR REMOVAL OF ANILINE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Balarak, Mostafapour and Joghataei
(Suresh et al., 2012). It gives the most appropriate equi-
librium correlation. They are also important for compar-
ing biosorption performance, optimization, design and
prediction purposes. The biosorption of aniline on SWC-
NT
S
was optimized by analyzing equilibrium curve of
the following three isotherm models.
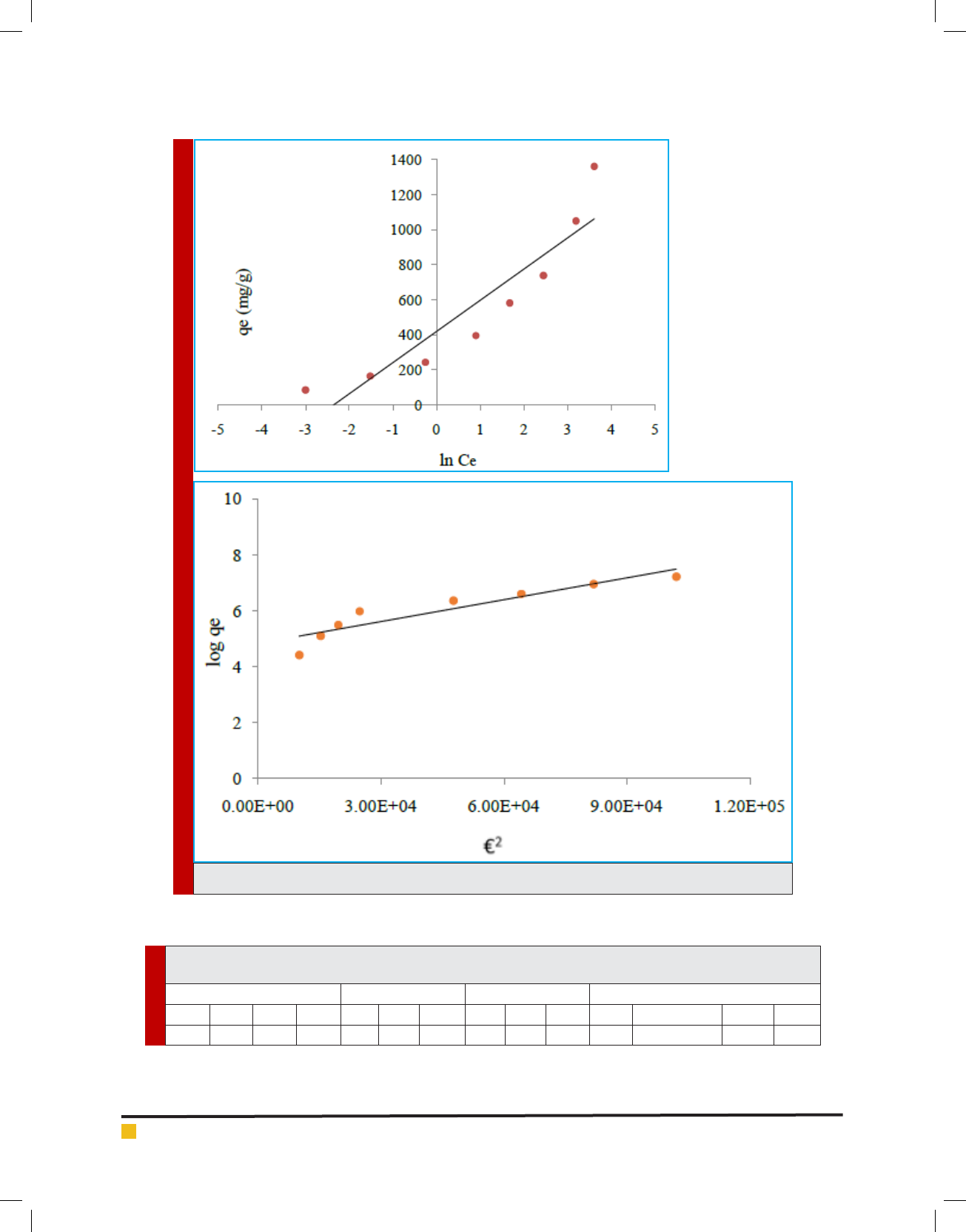
1. Langmuir Isotherm: The Langmuir Isotherm de-
veloped by Irving Langmuir was originally used to
describe the gas-solid phase and adsorption onto
activated carbon, but is now extended and gener-
ally applied to liquid-solid interaction. The equa-
tion is (Mojovic
´
et al., 2011, Jadhav et al., 2001):
(3)
Where q
e
(mg/g) and C
e
(mg/L) are the solid phase con-
centration and liquid phase concentration of aniline at
equilibrium, respectively. Q
m
(mg/g) is the maximum
sorption capacity and b (L/mg) is the Langmuir constant
related to the af nity of the adsorbate for the adsorbent.
The linearized form of Equation (3) is given as (Balarak
et al., 2015, Zazouli et al., 2015):
(4)
Values of q
m
and b are determined from the linear
regression plot of (C
e
/q
e
) versus C
e
, Fig 3 a. Linear plot in
negative direction indicates that Langmuir model fails to
explain the process of adsorption and absence of forma-
tion of monolayer.
FREUNDLICH ISOTHERM
The Freundlich isotherm relates the solute concentration
on the adsorbent surface to the solute concentration in
the liquid phase. The isotherm assumes that adsorption
occurs on a heterogeneous adsorbent surface (i.e. mul-
tilayer adsorption). Freundlich model is represented by
the equation (Balarak et al., 2016, Balarak et al., 2017):
(5)
Equation (5) can be linearized in logarithmic form, Equa-
tion (5) and the Freundlich constants can be determined
(Xin et al., 2011).
(6)
Where Kf and n are the Freundlich constants character-
istic of the system. Kf and n are indicators of adsorp-
tion capacity and adsorption intensity, respectively. A
linear regression plot of log qe versus log Ce, Figure 3b
gives the Kf and n values. The model is applicable to
the adsorption on heterogeneous surfaces by a uniform
energy distribution and reversible adsorption.
2. Tempkin Isotherm: The Tempkin isotherm takes
into account the interaction between adsorbate
and adsorbent and assumes a linear decrease in
the heat of adsorption instead of a logarithmic de-
crease. Tempkin isotherm is expressed as (Senturk
B et al., 2009, Ghaedi et al., 2012):
(7)
Equation can be expressed in a linear form as (Shen B
et al., 2009, Balarak et al., 2017):
(8)
Where , and B is a constant related to adsorption
heat, and Kt is the equilibrium binding constant (L/mol)
corresponding to maximum binding energy. K
t
and are
calculated from the slope and intercept of q
e
versus LnC
e
,
Fig 3C The Tempkin equation better holds for the predic-
tion of gas phase equilibrium rather than liquid phase.
The liquid phase is a more complex phenomenon since
the adsorbed molecules do not necessarily organized in
a tightly packed structure with identical orientation.
3. Dubinin Radushkevich Isotherm: This model is in-
volved to estimate the porosity, free energy and
the characteristics of adsorbents. The isotherm as-
sumes the surface heterogeneity and the variation
of adsorption potential during sorption process.
The model has commonly been applied in the fol-
lowing linear Equation (Balarak et al., 2016):
(9)
Polanyi potential, €, can be calculated according the fol-
lowing equation (Agarwal et al., 2016):
(10)
Where B is a constant related to the adsorption energy,
Q
m
the theoretical saturation capacity. The slope of the
plot of Ln qe versus €
2
gives B (mol
2
∙J
−2
) and the intercept
yields the adsorption capacity, Qm (mg∙g
−1
) as shown in
Figure 3d. The mean free energy of adsorption (E) which
is energy require to transfer one mole of the aniline from
in nity in solution to the surface of the solid can be

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS APPLICATION OF SINGLE-WALLED CARBON NANOTUBES FOR REMOVAL OF ANILINE 315
Balarak, Mostafapour and Joghataei
calculated from the B value using the following relation
(Balarak et al., 2016):
(11)
Linear plot with high regression factor indicating the
successful model in explaining the adsorption model.
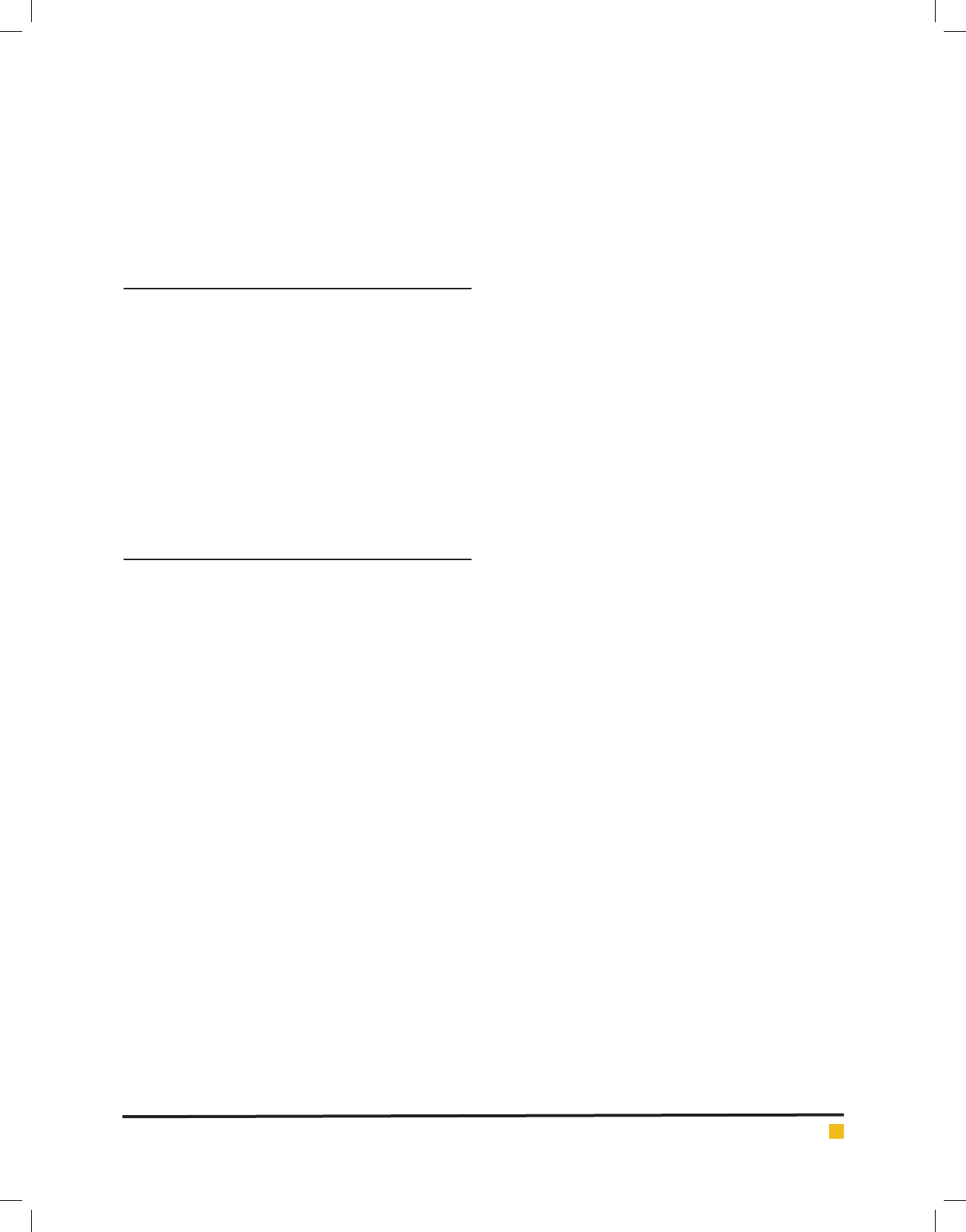
Table 1 summarizes different isotherms constants. The
graphs in Fig 3-6, the Langmuir Isotherm in Figure 3
gave the highest correlation coef cient (R
2
=0.8108) rate
to the Freundlich isotherm (R
2
=0.7999) and Dubinin
Radushkevich (R
2
=0.7999). The linear coef cient of
determination for the Tempkin Isotherm was not very
high (R
2
=0.014). The graphs show that both the Freun-
dlich and Langmuir Isotherms models can suf ciently
describe the adsorption data well for aniline. The fact
that the sorption process showed a good t to the Lang-
muir Isotherm suggests a nite adsorption capacity and
energetically equivalent sites.
FIGURE 3. Isotherm plots for adsorption of aniline on SWCNTS (a) Langmuir isotherm, (b) Freundlich
isotherm, (c) Tempkin isotherm, (d) Dubinin Radushkevich isotherm.
Balarak, Mostafapour and Joghataei
316 APPLICATION OF SINGLE-WALLED CARBON NANOTUBES FOR REMOVAL OF ANILINE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 3. (Continude)
Table 1. Langmuir, Freundlich, Tempkin and Dubinin–Radushkevich Isotherm constants for the adsorption of
Aniline unto SWCNTS
Langmuir Freundlich Tempkin Dubinin–Radushkevich
q
m
bR
L
R
2
nK
F
R
2
K
t
bR
2
q
m
B (×10
7
mol
2
∙J
−2
)E R
2
925.2 0.041 0.162 0.996 2.41 5.64 0.958 1.46 25.2 0.724 945.4 6.25 808.95 0.894
Balarak, Mostafapour and Joghataei
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS APPLICATION OF SINGLE-WALLED CARBON NANOTUBES FOR REMOVAL OF ANILINE 317
The adsorption energy obtained from Tempkin plot
218.25 J/mg which indicates that the adsorption process
is endothermic and a strong interaction between SWCNT
S
and Aniline molecules. Also value of energy obtained
from Dubinin Radushkevich isotherm was 808.95 J/mol,
revealing physisorption of Aniline on SWCNT
S
.
CONCLUSION
The results of the present study indicate that SWCNT
S
have good potential as adsorbents for the removal of
aniline from aqueous solution. The amount of aniline
uptake (mg/g) was found to increase with increase in
aniline concentration and adsorption time. The results
obtained from the plots show that the process of adsorp-
tion follows Langmuir and Freundlich Isotherm model
for adsorbent. Thus, it can be used to estimate the model
parameters. This con rms the fact that Langmuir Iso-
therm best explains the adsorption process of aniline
from aqueous solution. The maximum loading capacity
estimated was 37.59 (mg/g) for Langmuir model.
ACKNOWLEDGEMENT
The authors are grateful from deputy of research and
technology of Qom University of Medical Sciences due
to supporting of this research.
REFERENCES
Agarwal S, Tyagi I, Gupta VK, Dehghani MH, Jaafari J, Balarak
D. Rapid removal of noxious nickel (II) using novel gamma-
alumina nanoparticles and multiwalled carbon nanotubes:
Kinetic and isotherm studies. Journal of molecular liquids.
2016; 224; 618-623.
Balarak D, Mostafapour FK. Batch Equilibrium, Kinetics and
Thermodynamics Study of Sulfamethoxazole Antibiotics onto
Azolla liculoides as a Novel Biosorbent. British Journal of
Pharmaceutical Research. 2016; 13(2): 1-14.
Balarak D, Mahdavi Y, Bazrafshan E, Mahvi AH. Kinetic, iso-
therms and thermodynamic modeling for adsorption of acid
blue 92 from aqueous solution by modi ed azolla licoloides.
Fresenius Environmental Bulletin.2016;25(5); 1321-30.
Balarak D, Mahdavi Y, Bazrafshan E, Mahvi AH, Esfandyari
Y. Adsorption of uoride from aqueous solutions by carbon
nanotubes: Determination of equilibrium, kinetic and thermo-
dynamic parameters. Fluoride. 2016;49(1):35-42.
Balarak D, Mostafapour FK, Joghataei A. Kinetics and mecha-
nism of red mud in adsorption of cipro oxacin in aqueous
solution. Biosci. Biotech. Res. Comm. 2017; 10(1): 241-248.
Balarak D, Mahdavi Y, Maleki A, Daraei H and Sadeghi S.
Studies on the Removal of Amoxicillin by Single Walled Car-
bon Nanotubes. British Journal of Pharmaceutical Research.
2016;10(4): 1-9.
Balarak D, MahdaviY and Mostafapour FK. Application of
Alumina-coated Carbon Nanotubes in Removal of Tetracycline
from Aqueous Solution. British Journal of Pharmaceutical
Research.2016; 12(1): 1-11.
Balarak D, Mostafapour F, Bazrafshan E, Saleh TA. Studies on
the adsorption of amoxicillin on multi-wall carbon nanotubes.
Water science and technology. 2016; 75(7);1599-1606.
Balarak D, Jaafari J, Hassani G, Mahdavi Y, Tyagi I, Agarwal
S, Gupta VK. The use of low-cost adsorbent (Canola residues)
for the adsorption of methylene blue from aqueous solution:
Isotherm, kinetic and thermodynamic studies. Colloids and
Interface Science Communications.2015; 7;16–19.
Balarak D, Joghataei A, Azarpira H, Mostafapour FK. Biosorp-
tion of amoxicillin from contaminated water onto palm bark
biomass. International journal of life science and pharma
research. 2017; 7(1); 9-16.
Delnavaz M, Ayati B, Ganjidoust H. Reaction kinetics of ani-
line synthetic wastewater treatment by moving bed bio lm
reactor. Iranian J Health Environ 2009; 2 (1):76-87.
Futalan CM, Kan CC, Dalida ML, Pascua C, Wan MW. Fixed-bed
column studies on the removal of copper using chitosan immo-
bilized on bentonite. Carbohydrate Polymers. 2011;83(2):697-
704.
Ghaedi M, Sadeghian B, Pebdani AA, Sahraei R, Daneshfar A,
Duran C. Kinetics, thermodynamics and equilibrium evalua-
tion of direct yellow 12 removals by adsorption onto silver
nanoparticles loaded activated carbon. Chem Eng J 2012;
187(0):133-41.
Guo J, Chen S, Liu L, Li B, Yang P, Zhang L, et al. Adsorption
of dye from wastewater using chitosan–CTAB modi ed ben-
tonites. J Colloid Interface Sci 2012; 382(1):61-6.
Jadhav S, Verma N, Sharma A, Bhattacharya P. Flux and
retention analysis during micellar enhanced ultra ltration
for the removal of phenol and aniline. Sep Purif Technol
2001;24(3):541-57.
Jazayeri SH, Hayati Ashtiani M, Ashra zadeh SN, Ghannadi
Maragheh M, Nozad Golikand A. Heavy metal removal from
synthetics wastes by natural and acid-activated bentonites. J
Nuclear Sci Technol 2010; 51: 18-27
Jin Q, Hu Z, Jin Z, Qiu L, Zhong W. Biodegradation of aniline
in an alkaline environment by a novel strain of the halophilic
bacterium, dietzia natronolimnaea JQ-AN. Bioresour Technol
2012;117:148-54.
Jonidi Jafari A. Photocatalytic removal of aniline from
synthetic wastewater using Zno nanoparticle under ultra-
violet irradiation. Iranian J Health Environ 2012; 5(2):167-
78.
Leili M, Moussavi G, Nadda K. Degradation and minerali-
zation of furfural in aqueous solutions using heterogeneous
catalytic ozonation. Desalin Water Treat. 2013; 51(34-36):
6789-97.
Liu J, Guan J, Lu M, Kan Q, Li Z. Hemoglobin immobilized with
modi ed “ sh-in-net” approach for the catalytic removal of
aniline. J Hazard Mater 2012; 217:156-63.

Balarak, Mostafapour and Joghataei
318 APPLICATION OF SINGLE-WALLED CARBON NANOTUBES FOR REMOVAL OF ANILINE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Liu QY, Liu YX, Lu XJ. Combined Photo-Fenton and biologi-
cal oxidation for the treatment of aniline wastewater. Procedia
Environ Sci. 2010; 12:341-8.
Ma J, Qi J, Yao C, Cui B, Zhang T, Li D. A novel bentonite-
based adsorbent for anionic pollutant removal from water.
Chem Eng. J. 2012; 200:97-103.
Mojovi
c
´
Z, Jovic
´
-Jovicˇic
´
N, Milutinovic
´
- Nikolic
´
A, Bankovi
c
´
P, Rabi-Stankovic
´
AA, Jovanovic
´
D. Phenol determination
on HDTMA bentonite-based electrodes. J Hazard Mater 2011;
19:178-84.
Nourmoradi H, Nikaeen M, Pourzamani H, Nejad MH. Com-
parison of the ef ciencies of modi ed clay with polyethylene
glycol and tetradecyl trimethyl ammonium bromide for BTEX
removal. Int J Environ Health Eng 2013; 2(1):7-14.
O’Neill FJ, Bromley-Challenor KCA, Greenwood RJ, Knapp JS.
Bacterial growth on aniline: implications for the biotreatment
of industrial wastewater. Water Res 2000; 34: 4397–409.
Rawaj h Z, Nsour N. Characteristics of phenol and chlorinated
phenols sorption onto surfactant modi ed bentonite. J Colloid
Interface Sci 2006;298(1):39-49.
Ramavandi B, Leili M. Ef ciency of shrimp shell to remove
methylene blue from aqueous solutions. Iranian J Health Envi-
ron 2015;5(4):310-25.
Senturk HB, Ozdes D, Gundogdu A, Duran C, Soylak M.
Removal of phenol from aqueous solutions by adsorption onto
organo modi ed tirebolu bentonite: equilibrium, kinetic and
thermodynamic study. J Hazard Mater 2009; 172:353– 62.
Shaobin H, Hussain I, Yongqing Z. Kinetics of the degrada-
tion of aniline using zero-valent iron in aqueous solution. Int
Poster J Sci Technol 2011; 1(2-3): 64.
Shen D, Fan J, Zhou W, Gao B, Yue Q, Kang Q. Adsorption
kinetics and isotherm of anionic dyes onto organo-bentonite
from single and multisolute systems. J Hazard Mater. 2009;
172(1):99-107
Suresh S, Srivastava V, Mishra I. Adsorptive removal of ani-
line by granular activated carbon from aqueous solutions
with catechol and resorcinol. Environ Technol 2012;33(7):
773-81.
Tang B, Lin Y, Yu P, Luo Y. Study of aniline/ caprolactam mix-
ture adsorption from aqueous solution onto granular activated
carbon: Kinetics and equilibrium. Chem Eng J. 2012; 187:69-
78.
Visa M. Tailoring y ash activated with bentonite as adsor-
bent for complex wastewater treatment. Appl Surf Sci 2012;
263:753-62.
Wu G-Q, Zhang X, Hui H, Yan J, Zhang Q-S, Wan J-L. Adsorp-
tive removal of aniline from aqueous solution by oxygen
plasma irradiated bamboo based activated carbon. Chem Eng
J. 2012; 185:201-10.
Xin X, Si W, Yao Z, Feng R, Du B, Yan L, et al. Adsorption of
benzoic acid from aqueous solution by three kinds of modi ed
bentonites. J Colloid Interface Sci 2011; 359:499–504.
Zazouli MA, Mahdavi Y, Bazrafshan E, Balarak D. Phytodeg-
radation potential of bisphenolA from aqueous solution by
Azolla Filiculoides. Journal of Environmental Health Science
& Engineering 2014;12(66):1-5.
Zazouli MA, Mahvi AH, Mahdavi Y, Balarak D. Isothermic and
kinetic modeling of uoride removal from water by means
of the natural biosorbents sorghum and canola. Fluoride.
2015;48(1):15-
Zazouli MA, Mahvi AH, Dobaradaran S, Barafrashtehpour
M, Mahdavi Y, Balarak D. Adsorption of uoride from aque-
ous solution by modi ed Azolla Filiculoides. Fluoride.
2014;47(4):349-58.