Environmental
Communication
Biosci. Biotech. Res. Comm. 10(2): 287-296 (2017)
On the analysis of phenol removal from drinking water
by batch reactor using powdered eggshell
Giti Kashi
Department of Environmental Health, Tehran Medical Branch, Islamic Azad University, Tehran, Iran
ABSTRACT
Phenol is a known prejudicial exotoxin and nds place in the environment due to intense activity of petrol industry.
The aim of this applied-analytical study is to investigate phenol removal from urban drinking water using batch
reactor with using powdered eggshell. Various operating variables are tested for their effects on phenol removal;
these include pH, contact time, adsorbent doses, initial phenol concentration, reaction kinetics, and powdered egg-
shell characteristics. Sample of urban drinking water is prepared containing 5-15 mg/L phenol. Powdered eggshell
is prepared in a laboratory oven at 105˚C for 12 h. The phenol-containing water enters batch reactor and phenol
removal ef ciency is studied in different cases of the variables pH (3-11), contact time (0-120 min), and adsorbent
doses (3-5 gr/dl). Characteristics of the eggshell powder show that the average diameter size of eggshell powder is 2
μm. The main component of eggshell powder is calcium carbonate (CaCO
3
). The best conditions for phenol removal
are obtained to be pH 3, contact time 80 min, and adsorbent dose 4 gr, and phenol concentration 5 mg/L. The adsorp-
tion of phenol on powdered eggshell is obtained from Langmuir isotherm.
KEY WORDS: ADSORPTION, ISOTHERM, PHENOL, POWDERED EGGSHELL, URBANE DRINKING WATER
287
ARTICLE INFORMATION:
*Corresponding Author: gitikashani11@gmail.com
Received 12
th
April, 2017
Accepted after revision 28
th
June, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Chemically, phenol is acidic because of the in uence of
the aromatic ring. Phenol is an organic pollutant that
nds in the industrial wastewater. Phenol has been
measured in ef uents (up to 53 ppm), ambient water (1.5
- >100 ppb), groundwater (1.9 - >10 ppb), rain (0.075-
1.2 ppb), sediment (>10 ppb), and ambient air (0.03-44
ppb) (Al-Khalid, 2012 and American Public Health Asso-
ciation, 2015). The increase in phenol levels in ground-
water has been mainly attributed to pro igate utilization
of phenolic pesticides, municipal and industrial sew-
age treatment systems such as oil re nery, and decay-
ing organic matter or producing toxic poly chlorinated

288 ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Giti Kashi
phenols during water chlorination process (Bazrafshan,
2016).
Phenol and its derivatives like Dinitrophenol and pen-
tachlorophenol are very toxic substances with a toxicol-
ogy rating of 4 (Cabaj, 2016). Phenol is famous due to
its high toxicity for human life and aquatic life (Chand
Meena, 2015). Phenol-laced water may lead to liver, kid-
ney, and respiratory disorders in humans (Dakhil, 2013).
Phenol-contaminated water above the permissible drink-
ing water limit leads to phenolic tastes and doors prob-
lem, may be a cause of lethal to humans, and the drink-
ing water standard have been promulgated as >1 μg/l at
point of chlorination (Daraei, 2013). Processes such as
activated carbon adsorption, ion exchange, liquid-liquid
extraction, and chemical oxidation methods are effec-
tive for removing phenol and phenolic compounds from
water (Dehghani, 2016).
The main disadvantage of ion exchange technique
is low selectivity against anions. The main disadvan-
tage of these techniques is high capital and operational
cost (Fan, 2011). The advantages of adsorption technol-
ogy are applicable in batch and continue arrangements,
easier accessibility, designing exibility, economical,
and retain very effectiveness on high concentration and
low of phenolic industrial wastewater. Many natural
adsorbents are experimented as removal phenol agents
including sawdust Yeast (Liao, 2010), and almond shell
(Hsieh, 2008, Loganathan, 2013, Kashi, 2015 and Giti,
2015). The potential of annual egg, as biosorption of
organic materials, production in 2011 is estimated to be
5.2 million tons in Iran country. The aim of this applied-
analytical study is to investigate feasibility of phenol
removal from drinking water using the batch reactor
with using eggshell powder. The variables under study
involve pH, contact time, adsorbent doses, initial phenol
concentration, reaction kinetics, and eggshell powder
characteristics.
MATERIAL AND METHODS
Phenol-contaminated water samples used for adsorp-
tion experiments are obtained from urbane distribution
system situated the site of a laboratory in Islamic Azad
University Tehran Medical Sciences Branch, in Tehran
city. The samples are tested for the main physicochemi-
cal characteristics. The mean values of these water char-
acteristics are presented in Table 1. All the reagents used
are of analytical grade. A solution of 5, 10, and 15 mg/L
phenol is prepared by dissolving appropriate amount of
phenol (Merck, Germany) in deionized water.
After collecting chicken eggshells from local markets
of Tehran city, removing waste matters such as colour
and fat, boiling in deionized water for 30 minutes, and
washing with deionized water, eggshell powder is pre-
pared by heating the collected eggshells in a hot air
oven (Dena, Iran) at 105˚C for 12 h, maybe, temperature
higher than 105˚C leads to decreasing phenol removal
due to damaging calcium carbonate structure, while
temperature below 105˚C leads to developing bad taste
and odour in nished water. Heat pre-treatment removes
the organic matter, cause of taste and colour problems
in water. After heating, the eggshells are crushed by a
laboratory electrical crusher (AIKA, Germany) for 20-30
second and are sieved several times to get a uniform
fraction of eggshell in a speci c size (60-100 mesh/
0.25-0.104 mm), according to ASTM standard (Mijan,
2014). The powdered eggshell is stored in the desicca-
tor after pre-treating with a solution of sodium hypo
chloride (NaOCl) (Merck, Germany), to eliminate the dust
particles (Mourão, 2011).
Scanning Electron Microscopy (SEM) image (Philips,
XL 30, Holand) is prepared from powdered eggshell.
X-ray diffraction patterns are measured using RINT
2000 (Rigaku Instrument Corp.) with Cu K radiation
for con rming the structure and mineral composition
of powdered eggshells (Philips, Xpert, Holand). Pow-
dered eggshell composition is obtained by energy dis-
persive X-ray (EDX) analysis. The surface area of pow-
dered eggshell is analysed through nitrogen adsorption
measurements at 77 K using Micromeritics Gemini 2370
equipment. The zeta potential is analysed with a Nano
Zetasizer (Philips, Holand).
The batch reactor is a 250 ml glass rectangular con-
tainer (10×6×6 cm). To evaluate the effect of adsorption,
on the phenol removal process, samples undergo with
different pH (ca. 3-11), different times (0-120 min), dif-
ferent concentrations of F (5-15 mg/L), and adsorbent
dosage (3-5 gr/dl). The sample number is obtained 192.
Magnetic stirrer (AIKA, Germany) is used for homogene-
ous mixing of water samples (120 rpm). For each test,
200 ml of sample water is poured into the reactor. All
tests are performed at laboratory temperature (20˚C).
Chloride acid and sodium hydroxide solutions (0.1 N)
(Merck, Germany) are used for pH adjustment.
All tests were performed in triplicate, and the mean
data values are reported. The water samples are tested
for phenol after adsorption process by using spectropho-
tometer (Hach DR5000, America) at a wavelength of 500
nm. Phenol is determined by standard method 5530D
(Ngah, 2011). The percentage phenol removal is calcu-
lated according to the following equation (1):
(1)
Where the percentage of phenol removal (R, percent-
age) and the phenol value before and after treatment (C
t0
and C
t
, mg/L) expressed.

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR 289
Giti Kashi
The removal phenol capacity of the regenerated pow-
dered eggshell is calculated according to the following
equation (2):
(2)
Where the removal phenol capacity of the regener-
ated powdered eggshell (DC
FC
, mg/g) and the phenol con-
centration before and after treatment (S
0
and S
t
, mg/L)
expressed. To optimize runs and data analysis, based on
Taguchi model, is used to study vestiges of the selected
variables and minimize the number of experimental runs
(Tzvetkova, 2016).
RESULTS AND DISCUSSION
The reduction of phenol from urbane drinking water is
investigated in an adsorption reactor with ller particles
made of charring chicken eggshells in batch mode. Sev-
eral operational variables are examined for the effects
on process reduction ef ciency. The following results
are obtained from the experiments.
Figure 1 illustrates SEM images of the eggshell pow-
der. As is observed that, the average diameter size of
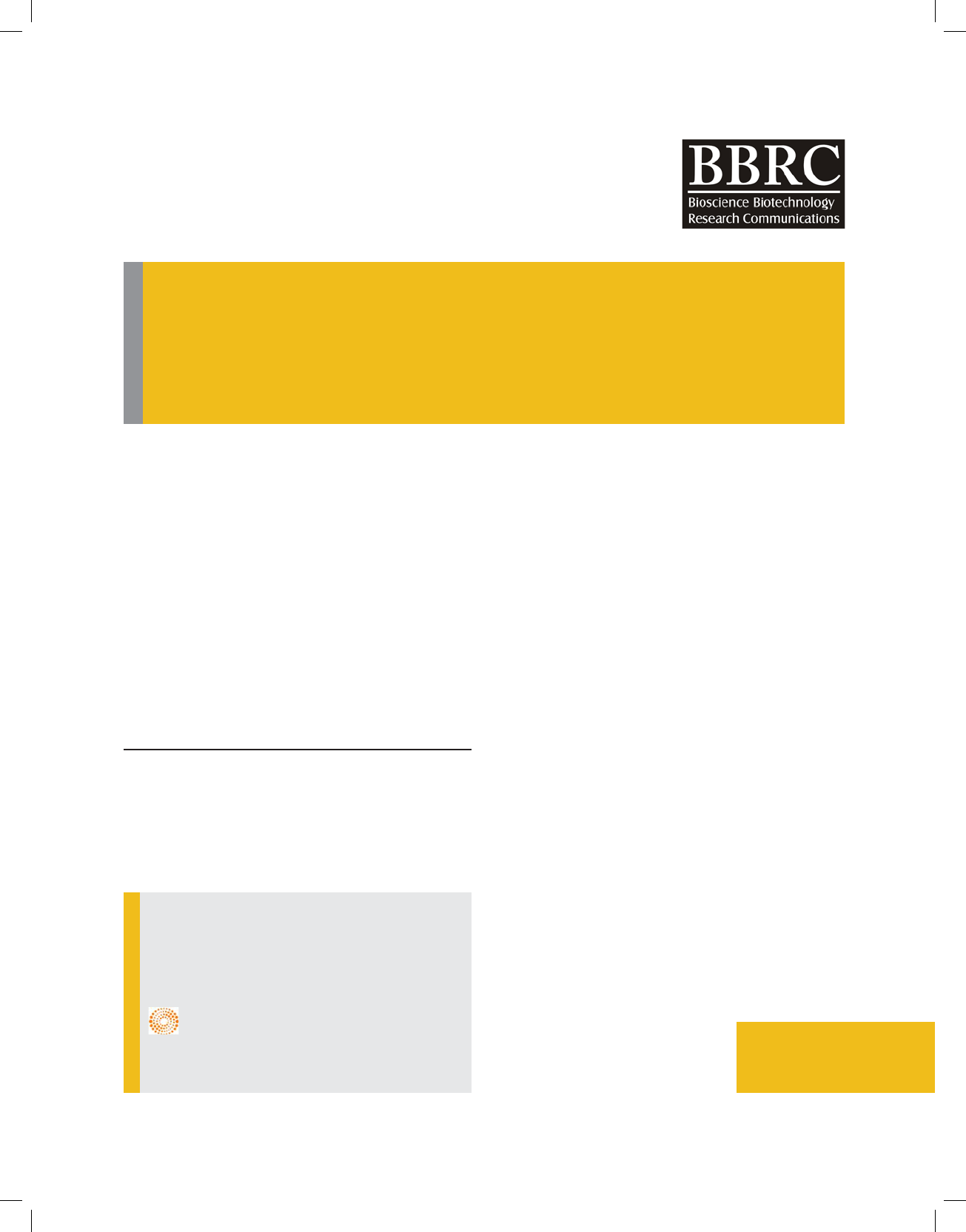
eggshell powder is 2 μm. Figure 2 illustrates X-ray dis-
persive (XRD) analysis of the eggshell powder. As is
found in gure 2, the analysed eggshell powder is com-
posed of the elements including calcium (Ca) and phos-
phor (P) as the main and partial elements respectively.
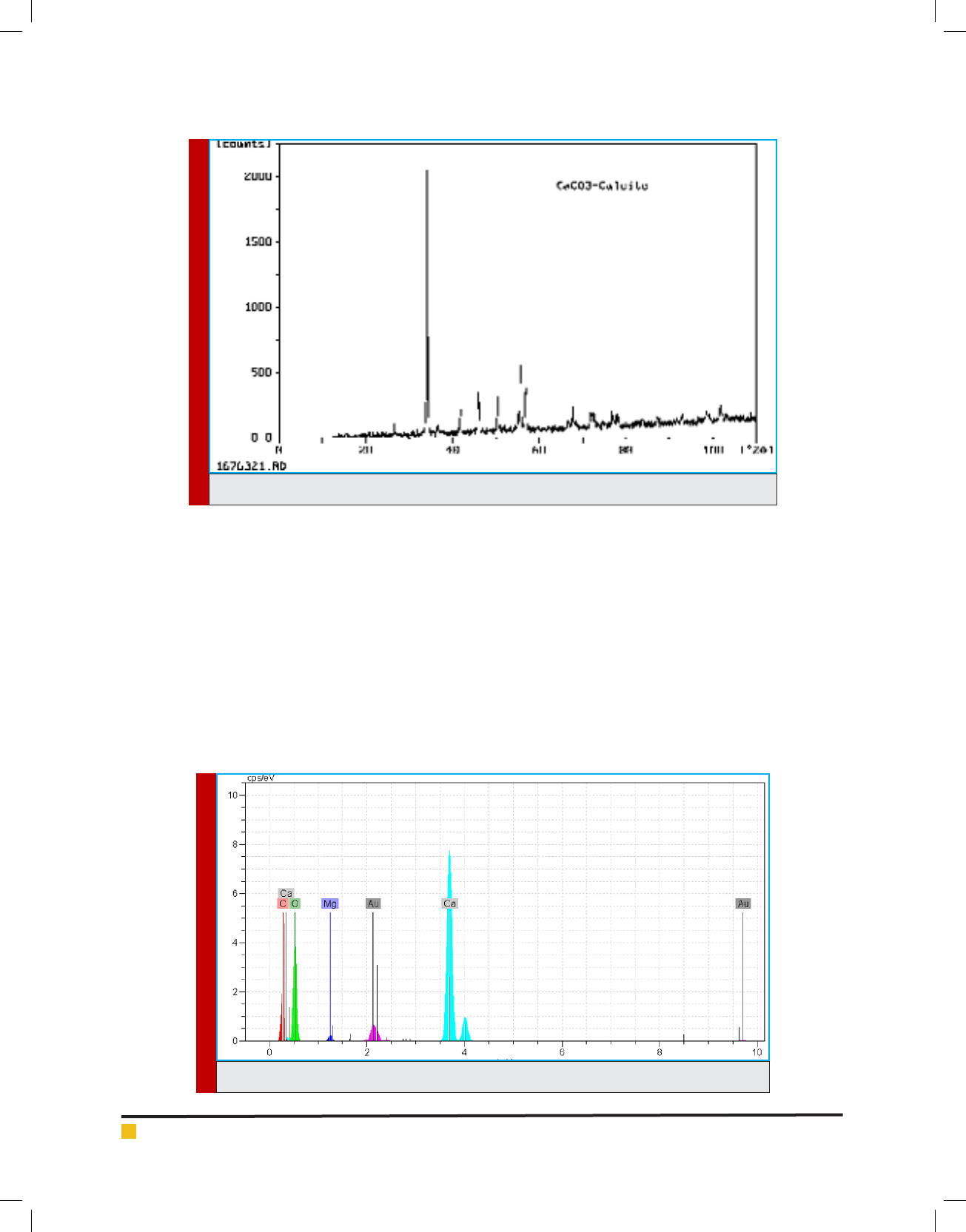
Figure 3 illustrates EDX analysis of the eggshell powder.
As is found in gure 3, the analysed eggshell powder is
composed of elements including calcium (Ca), oxygen
(O), magnesium (Mg), carbon (C), and so on.
The characteristics of eggshell powder are showed in
Table 2. Speci c surface area, BET measurements has
also been performed, and the highest BET is obtained for
eggshell powder 7.43 m
2
/g. The spectrum of the analysed
eggshell powder is adapted to 2370 standards. Therefore
these peaks con rmed that the main component of egg-
shell powder is calcium carbonate (CaCO
3
). The calcium
to carbon (Ca/C) ratio of eggshell powder is 2.9. SEM
analysis is another helpful instrument for the analysis of
the surface morphology of an adsorbent. The agglomer-
ate, non-adhesive, porous and irregular surface structure
of the adsorbent can be distinctly shown in SEM image
indicates in gure 1a. Furthermore, the pores on the sur-
face of the adsorbent are regular, adhesive, and very het-
erogeneous as indicates in gure 1b. The heterogeneous
pores leads to producing a lager exposed surface area
for the adsorption of phenol. In other hand the hetero-
Table 1. The Main Physicochemical Characteristics
of Phenol-Contaminated Urbane Water
Parameter Unit Value
Calcium mg/L as CaCO
3
162
Dissolved oxygen mg/L 8.05
Nitrate mg/L 9.5
ORP mV 272
pH - 7.19
Sulphate mg/L 93.8
Temperature ˚C 20
Total Alkalinity mg/L as CaCO
3
122
Table 2. Characterization of Eggshell Particles
Particle pHZPC
Density
(g/cm
3
)
Speci c
Surface
Area, BET
(m
2
/g)
Diameter
(μm)
Eggshell 8.2 1.148 7.43 2
(a) (b)
FIGURE 1. SEM image of eggshell powder (a) before treatment, (b) after treatment
290 ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Giti Kashi
FIGURE 2. XRD analysis of eggshell powder
FIGURE 3. EDX analysis of eggshell powder
geneous pores leads to producing a large af nity for the
adsorption of phenol.
Mijan et al. (2014) report that the eggshell pow-
der particles are irregular in shape and their surface is
rough. The diameter of pore is symptom of the antici-
pated adsorption of phenol molecule onto the surface of
the adsorbent. It distinctly indicates the porous surface
formation which supports the adsorbent with enhanced
surface area and very adsorption capacity. The SEM
analysis shows that the removal of phenol affects the
orientation of the eggshells powder. The treated sample
illustrates regular, adhesive appearance causing higher
adsorption of phenol. Bhaumik et al report that the par-
ticle size of eggshell powder is 150-350 μm. Zul kar
et al. (2013) report that BET of eggshell powder is 3.23
m
2
/g. Gaonkar and Chakraborty report that composition
of eggshell consists of calcium carbonate (91%). Agarwal
and Gupta report that the most compound in the egg-
shell powder is calcium carbonate. Functional groups of
eggshells are diagnosed by infrared analysis. The peaks
at about 710, 875, 1420, 1807, and 2520 cm
-1
adopt
with those of pure CaCO
3
. The XRD pattern displays six
characteristic peaks at 2=34.5°, 42.2°, 46°, 50.9°, 56°,
and 57.8° in crystalline structure and corroborates the
Giti Kashi
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR 291
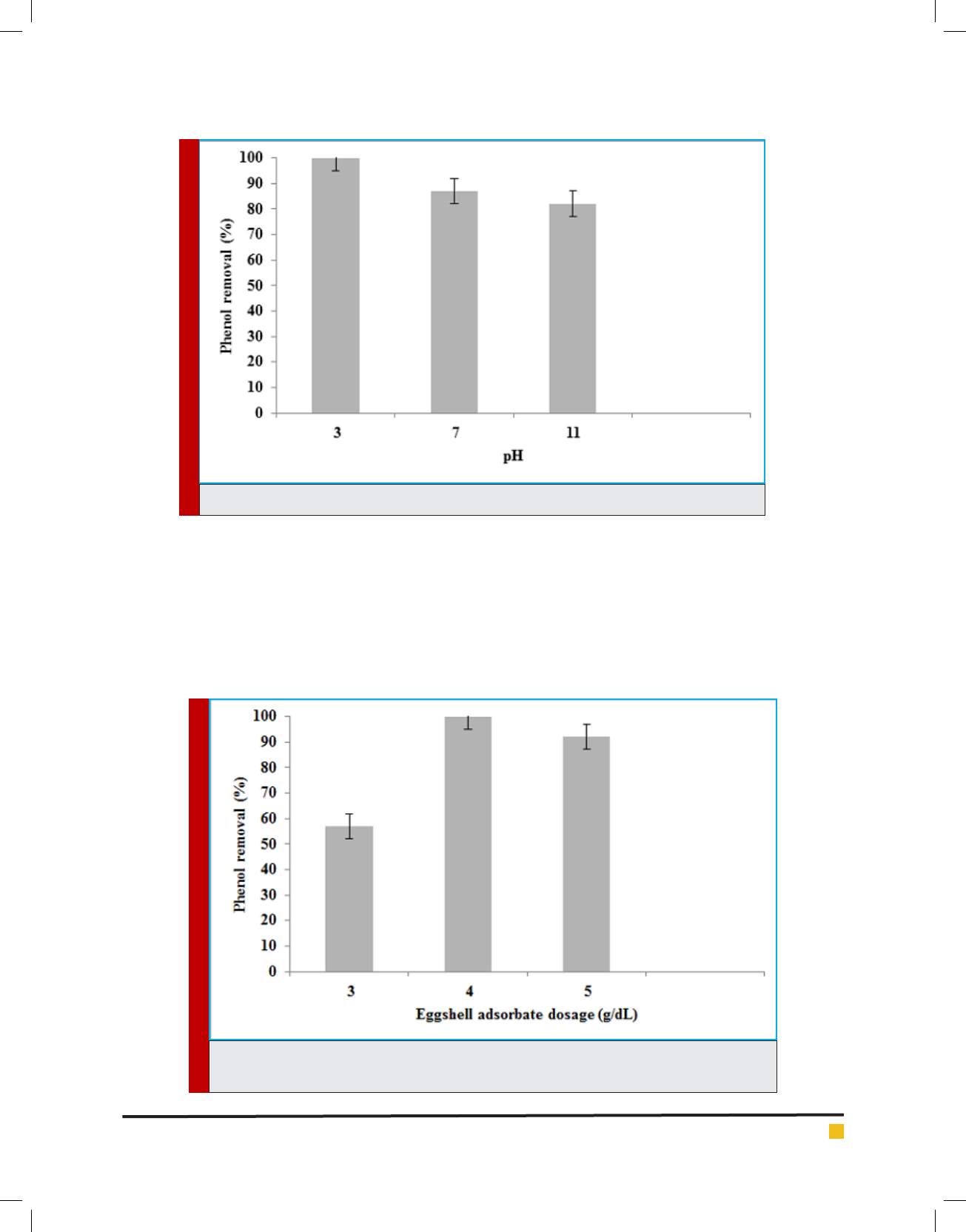
FIGURE 4. The effect of water pH on the phenol removal in the batch adsorption reactor
FIGURE 5. The effect of eggshell powder adsorbent dosage on the phenol removal in the batch
adsorption reactor
presence of calcite (CaCO3) in the eggshell (Zul kar,
2013).
Adsorption experiments are carried out an initial
pH values in the range of 3 to 11 at the experimen-
tal conditions such as contact time (80 min), phenol
concentration (5 mg/L) and amount of eggshell powder
adsorbent (4 g). The mean phenol removal decreases
from 100% to 87% when the pH increased from 3 to
7 (Figure 4). Phenol removal in the adsorption reactor
is mainly in uenced by the water pH. The pH has a
signi cant effect on phenol reduction due to surface
charge of the absorbent, with the highest reduction
obtained at pH 3. It is related to phenol ionization, and
adsorbent surface. Phenol is a weak acid and produces
phenoxide ions. Due to more exchangeable ion rate,
and better adsorbent, the optimum pH for reaching to
Giti Kashi
292 ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
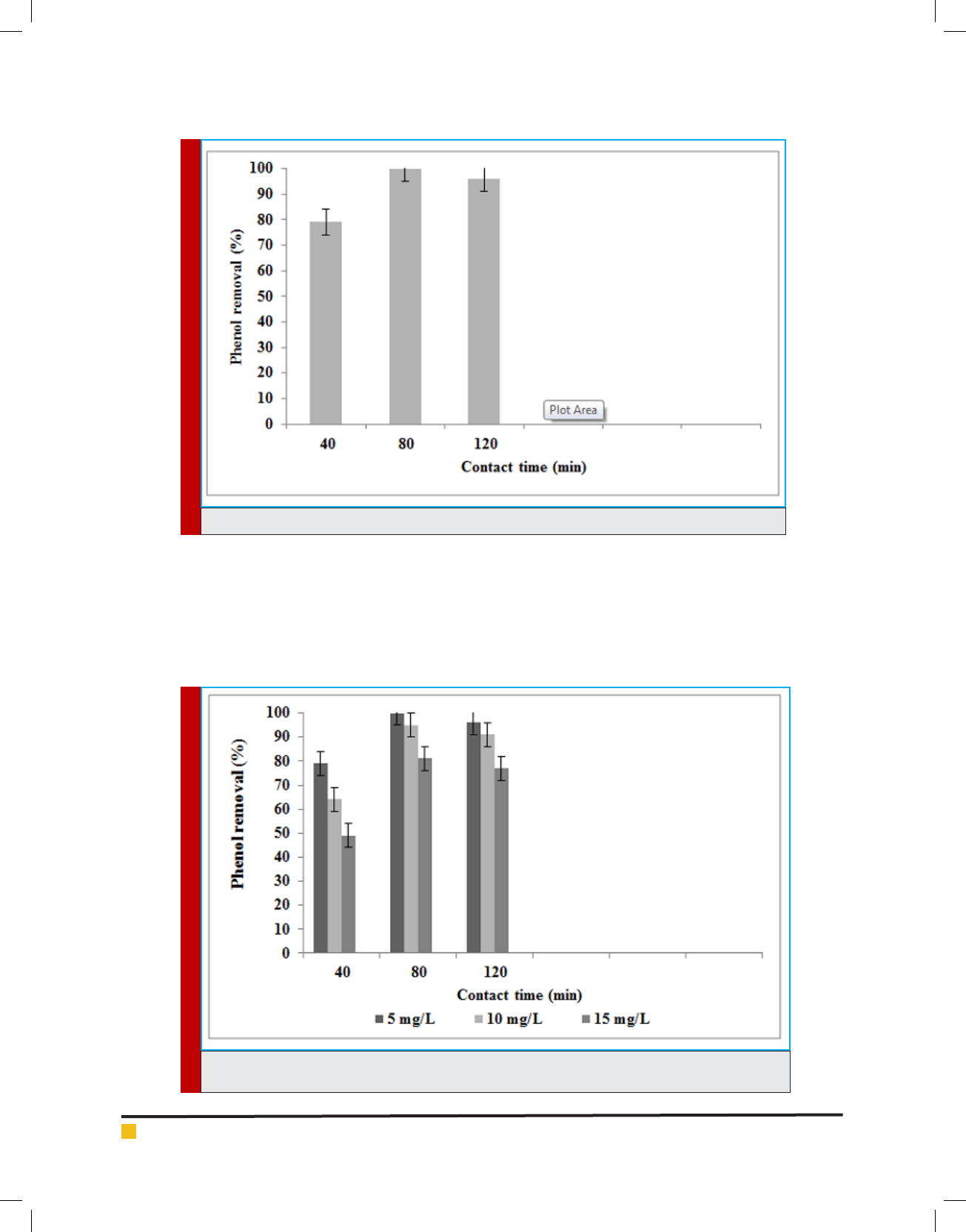
FIGURE 6. The effect of contact time on the phenol removal in the batch adsorption reactor
FIGURE 7. The effect of initial phenol concentration on the uoride removal in the batch adsorption
reactor
phenol standard (>1 μg/L) is pH 3. The phenol removal
of in alkaline condition diminishes which can be due to
increasing turbidity.
This is in agreement with Daraei et al. (Daraei, 2013),
who report that the fastest removal rate occurs at pH
equal to 3.5. The pH of point of zero charge (pHZPC) is
an important parameter in phenol sorption, for it is the
pH in which the sorbent has a neutral charge. When pH
is increased above the pHZPC, phenol sorption decreases
due to electrostatic repulsion between the surface and
phenoxide ions as well as a result of competition with
hydroxides in solution.
Giti Kashi
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR 293
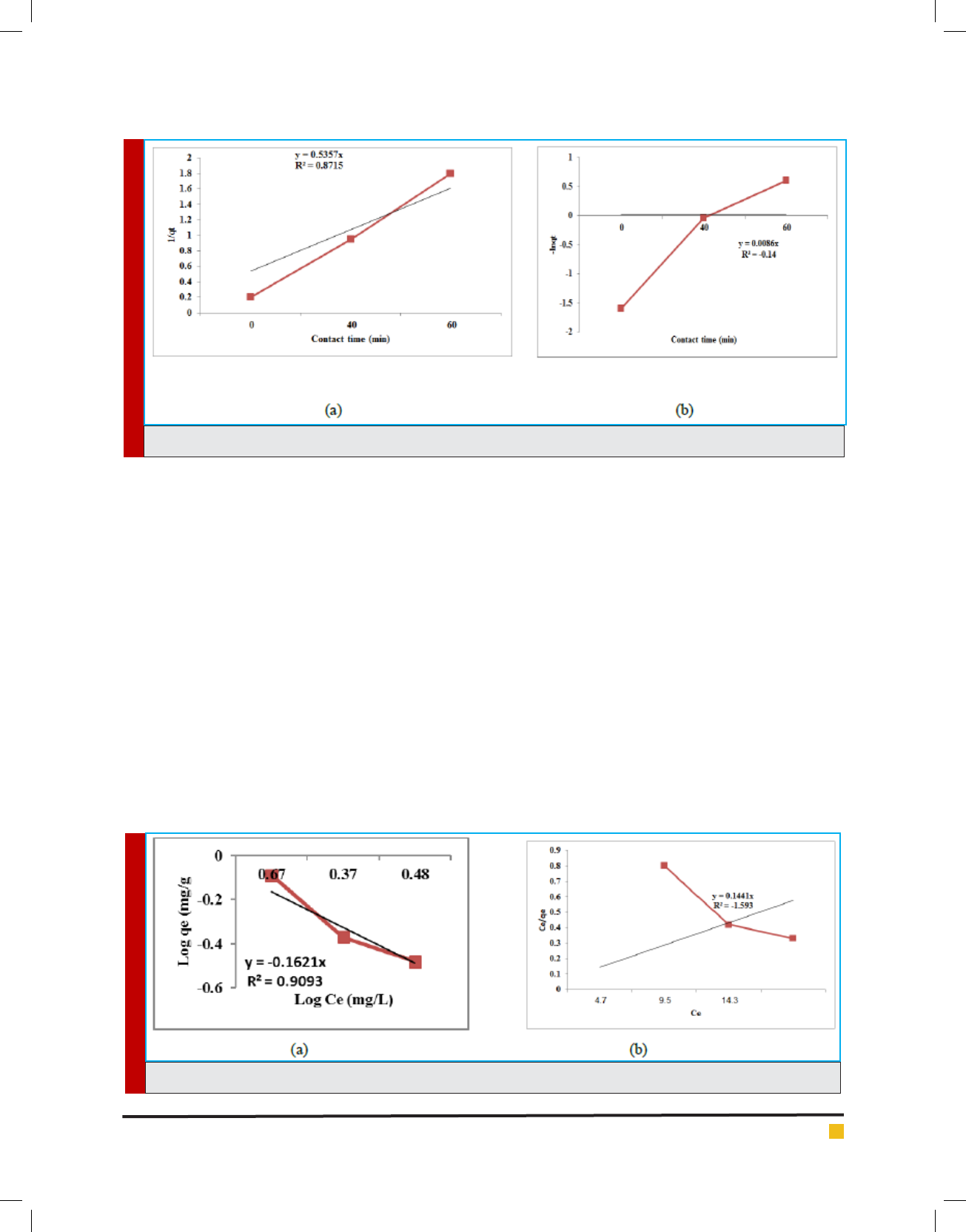
FIGURE 8. Kinetic models: (a) rst order (b) second order
FIGURE 9. Conceptual model of DPSIR framework
Adsorption experiments are carried out an ini-
tial adsorbent dosage in the range of 3 to 5 g at the
experimental conditions such as contact time (80 min),
phenol concentrations (5 mg/L) and pH value (3). The
mean phenol removal increases from 57% to 100%
when the adsorbent dosage increases from 3 to 4 (Fig-
ure 5). Enhancing the adsorbent dosage increases the
percentage of phenol that is attributed to enhancing of
sportive surface area, and sorption capacity since more
active adsorption sites, more exchangeable sites, and
more proper porosity are available. The optimum dos-
age of eggshell powder adsorbent is 4 g. It is seen that
4 g is a better adsorbent dosage than 3 g. This phe-
nomenon can be due to exposure of the active sites of
adsorbent which allow phenoxide ions of water to have
a direct contact with eggshell powder, therefor increas-
ing adsorption capacity. Increasing the adsorbent dosage
also leads to decreasing surface area between eggshell
powder adsorbent and phenol adsorbate due to the for-
mation of aggregates. Any change in the adsorbent dos-
age will increase pH. Zul kar et al (2013) reported that
the optimum dosage of eggshell is 5.0 g. At constant
pH, activity of phenoxide is directly proportional with
the concentration of Ca
+2
and thereby increasing the
adsorption of phenol over eggshell surface signi cantly
(Zul kar, 2013).
Adsorption experiments are carried out as a func-
tion of the time levels in the range of 0 to 120 min
at the experimental conditions such as amount of egg-
shell powder adsorbent (4 g/dL), phenol concentration (5
mg/L) and pH value (3). The ef ciency of phenol removal
increases as the contact time increases. The mean phe-
nol removal increases from 79% to 100% when the con-
tact time increases from 40 to 80 min (Figure 6). The
ef ciency of phenol removal initially increases as the
contact time increases, but then gradually approaches
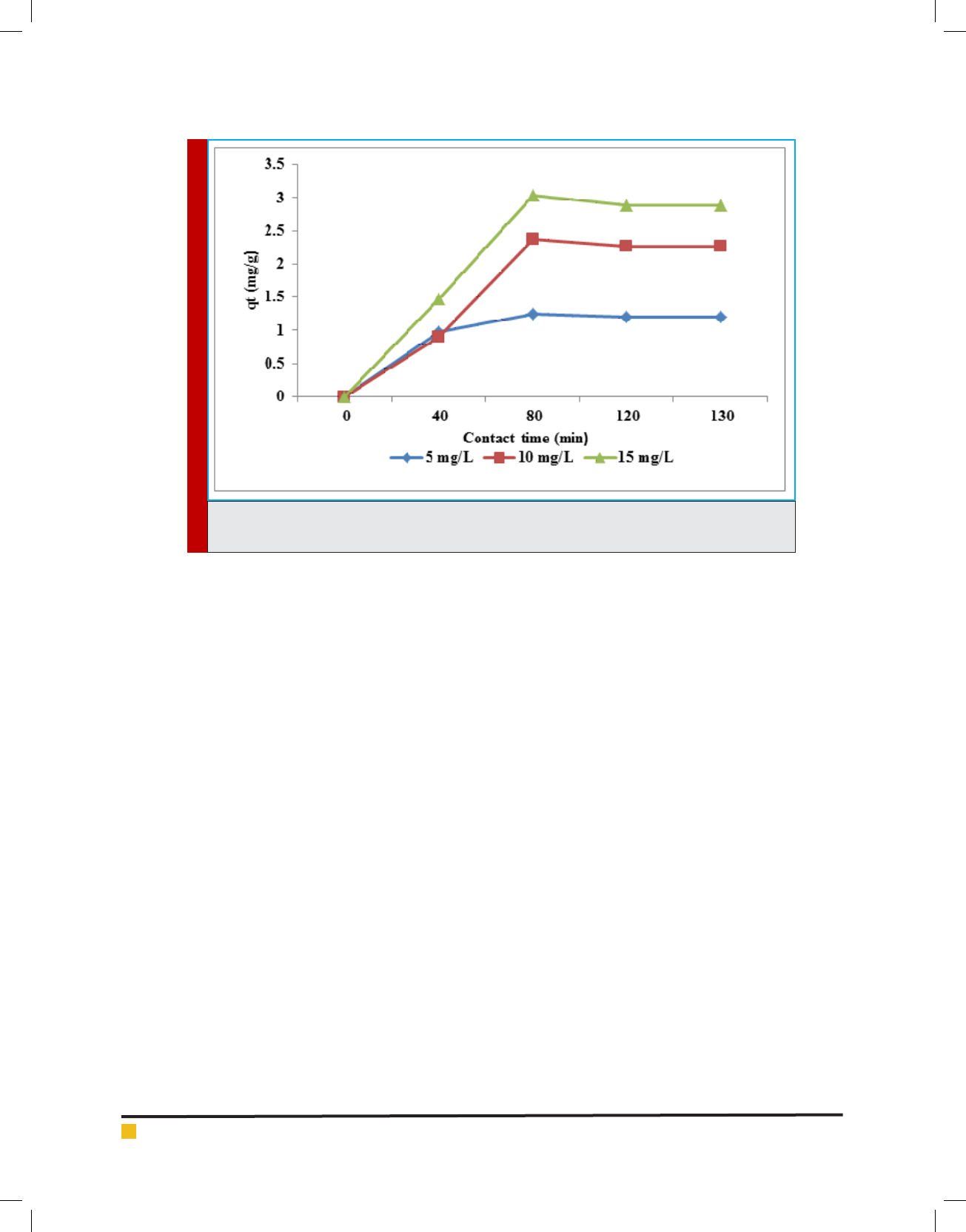
Giti Kashi
294 ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 10. Effect of contact time and initial concentration on the phenol removal (Sorbent dosage 4
g; pH 3)
a more or less constant value, indicating obtain-
ment of equilibrium. As there is no enhance in phenol
removal ef ciency between 80 and 120 minute, equilib-
rium time of 80 minute is selected for eggshell powder
adsorbents.
These variations can be due to the fact that initially,
all absorbent sites are empty and active, the solute con-
centration gradient is high and attraction of active func-
tional groups towards phenol which results in stronger
surface binding is high. Then, the phenol adsorption rate
on to eggshell powder adsorbent noticeably diminishes
due to decreasing in absorbent sites. This phenomenon
indicates a monolayer of phenoxide ions on the external
surface and pores of eggshell powder and pore diffu-
sion on to the internal surface eggshell powder through
the lm due to continues turbulent maintains during the
experiments. During this period residual phenol uctu-
ates from a maximum value of 1.05 mg/L at 40 min
contact time to a minimum value of 0.0 mg/L for a con-
tact time of 80 min. The highest removal capacity of
eggshell powder (4 mg/g) is obtained for duration of 80
min, respectively. An equilibrium time of 60 minute is
selected for chitosan-H
2
SO
4
beads by Ngah et al., (2011).
An equilibrium time of 120 minute is selected for car-
bonate hydroxyapatite are extracted from eggshell waste
by Liao (Liao, 2010). If the phenoxide ion is more than
absorbent sites, adsorption decreases due to saturating
absorbent sites at a constant concentration.
Adsorption experiments are carried out an initial
phenol concentration in the range of 5 to 15 mg/L at
the experimental conditions such as contact time (40-
120), amount of eggshell powder adsorbent (4 g/dL)
and pH value (3). The mean phenol removal decreases
from 100% to 81% when the initial phenol concentra-
tion increases from 5 to 15 mg/L
for duration 80 minute
(Figure 7). The phenol removal as a function of con-
tact time is proportional to the phenol ions in water. At
higher concentration, due to saturating active adsorp-
tion sites by phenol the mass transfer rate decreases.
Due to enhancing concentration gradient, performed as
enhancing driving force to predominant all mass trans-
fer resistances of the phenol between the solution and
solid phase, resulting in an enhancing equilibrium sorp-
tion until sorbent saturation is obtained. This is in agree-
ment with Balasubramani and Sivarajasekar who report
that the removal rhodamine B ef ciency decreases when
the initial rhodamine B concentration increases. In other
hands, increasing concentration of phenol leads to shift-
ing the pH
ZPC
to lower value and decreasing the electro-
static attraction between sorbent surfaces and phenox-
ide ions.
The proposed mechanism of phenol uptake rate onto
eggshell powder surface involves the replacement (ion
exchange adsorption) of calcium of the eggshell pow-
der, by phenoxide ion to form an insoluble matter. The
regression coef cient for the tted line is calculated to

Giti Kashi
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR 295
be R
2
= 0.8715 for phenol. The apparent rate constant,
K
1
and the half-life time, t
1/2
are calculated to be 0.5357
min
-1
and 1.29 min. Phenol reduction follows a rst
order kinetic model. Phenol reduction follows a Freun-
dlich isotherm model (R
2
>0.9). This is agreement with
Kumaraswamy et al who report that chromium by egg-
shell powder follows a Langmuir isotherm model. There-
fore, the eggshell powder adsorption reactor, in batch
mode, is showed to be an ef cient and viable process for
meeting a high degree of phenol reduction from drink-
ing water and be considered as a promising technology
for treating phenol-polluted drinking water in develop-
ing countries (Figure 8, Figure 9).
CONCLUSION
The experimental results suggest that batch chicken
eggshell powdered reactor is a practical and promis-
ing method for the phenol-contaminated water. Phenol
removal is affected by pH, the concentration of phenol,
the concentration of adsorbent, and reaction time. This
reactor are capable of phenol removal at the pH value
(3) investigated, with a reaction time 80 min. It is pur-
posed that performance of process is studied the other
material.
ACKNOWLEDGMENTS
The authors thank the Department of Environmental
Health of Islamic Azad University, Tehran Medical Sci-
ences Branch for nancial and instrumental supports.
REFERENCES
Al-Khalid, T. and El-Naas, M.H., 2012. Aerobic biodegrada-
tion of phenols: a comprehensive review. Critical reviews
in environmental science and technology, 42(16), pp.1631-
1690.
American Public Health Association, American Water Works
Association, Water Pollution Control Federation and Water
Environment Federation, 2015. Standard methods for the
examination of water and wastewater(Vol. 2). American Pub-
lic Health Association..
Bazrafshan, E., Amirian, P., Mahvi, A.H. and Ansari-
Moghaddam, A., 2016. Application of adsorption process for
phenolic compounds removal from aqueous environments: a
systematic review.Global NEST Journal,18(1), pp.146-63.
Cabaj, J., J
e
˛drychowska, A., Zajłc, D., Krawiec, S. and
Sołoducho, J., 2016. Phenolic Compounds Determination
Using Laccase-based Electrode Modi ed with Conducting Pol-
ymer Support. International Journal of Electrochemical Sci-
ence,11(1), pp.609-620.
Chand Meena, M., Band, R. and Sharma, G., 2015. Phenol
and Its Toxicity: A Case Report. Iranian Journal of Toxicol-
ogy,8(27), pp.1222-1224.
Dakhil, I.H., 2013. Removal of phenol from industrial wastewa-
ter using sawdust.
International Journal of Engineering And
Science,
3(1), pp.25-31.
Daraei, H., Mittal, A., Noorisepehr, M. and Daraei, F., 2013.
Kinetic and equilibrium studies of adsorptive removal of phe-
nol onto eggshell waste.
Environmental Science and Pollution
Research,
20(7), pp.4603-4611.
Dehghani, M.H., Mosto , M., Alimohammadi, M., McKay,
G., Yetilmezsoy, K., Albadarin, A.B., Heibati, B., AlGhouti,
M., Mubarak, N.M. and Sahu, J.N., 2016. High-performance
removal of toxic phenol by single-walled and multi-walled
carbon nanotubes: Kinetics, adsorption, mechanism and opti-
mization studies.Journal of Industrial and Engineering Chem-
istry,35, pp.63-74.
Fan, J., Zhang, J., Zhang, C., Ren, L. and Shi, Q., 2011. Adsorp-
tion of 2, 4, 6-trichlorophenol from aqueous solution onto
activated carbon derived from loosestrife.Desalination,267(2),
pp.139-146.
Giti, K. and Narges, J., 2015. Optimization electrophotocata-
lytic removal of acid red 18 from drinking water by the Tagu-
chi model.
Hsieh, F.M., Huang, C., Lin, T.F., Chen, Y.M. and Lin, J.C., 2008.
Study of sodium tripolyphosphate-crosslinked chitosan beads
entrapped with Pseudomonas putida for phenol degrada-
tion.
Process Biochemistry,43(1), pp.83-92.
Kashi, G., Mehree, A., Zaeimdar, M., Khoshab, F. and Madaree,
A.M., 2015. Removal of uoride from urban drinking water by
eggshell powder.
Liao, D., Zheng, W., Li, X., Yang, Q., Yue, X., Guo, L. and Zeng,
G., 2010. Removal of lead (II) from aqueous solutions using
carbonate hydroxyapatite extracted from eggshell waste.Jour-
nal of Hazardous Materials,177(1), pp.126-130.
Loganathan, P., Vigneswaran, S., Kandasamy, J. and Naidu, R.,
2013. De uoridation of drinking water using adsorption pro-
cesses.Journal of Hazardous materials,248, pp.1-19.
Mijan, M.A., Kim, D.H. and Kwak, H.S., 2014. Physicochemical
properties of nanopowdered eggshell.International Journal of
Food Science & Technology,49(7), pp.1751-1757.
Mourão, P.A.M., Laginhas, C., Custódio, F., Nabais, J.V., Car-
rott, P.J.M. and Carrott, M.R., 2011. In uence of oxidation
process on the adsorption capacity of activated carbons from
lignocellulosic precursors.Fuel Processing Technology,92(2),
pp.241-246.
Ngah, W.W., Fatinathan, S. and Yosop, N.A., 2011. Isotherm
and kinetic studies on the adsorption of humic acid onto chi-
tosan-H 2 SO 4 beads.Desalination,272(1), pp.293-300.
Tzvetkova, P.G., Nickolov, R.N., Tzvetkova, C.T., Bozhkov,
O.D. and Voykova, D.K., 2016. Diatomite/carbon adsorbent for
phenol removal.Journal of Chemical Technology and Metal-
lurgy,51(2), pp.202-209.

Giti Kashi
296 ON THE ANALYSIS OF PHENOL REMOVAL FROM DRINKING WATER BY BATCH REACTOR BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Zazouli, M.A., Taghavi, M. and Bazrafshan, E., 2012. In u-
ences of Solution Chemistry on Phenol Removal From Aque-
ous Environments by Electrocoagulation Process Using Alu-
minum Electrodes.Health Scope,1(2), pp.66-70.
Zul kar, M.A., Mariske, E.D. and Djajanti, S.D., 2012.
Adsorption of lignosulfonate compound using powdered
eggshells. Songklanakarin Journal of Science and Technol-
ogy,34(3), pp.309-316.
Zul kar, M.A., Novita, E., Hertadi, R. and Djajanti, S.D., 2013.
Removal of humic acid from peat water using untreated pow-
dered eggshell as a low cost adsorbent.International Journal of
Environmental Science and Technology,10(6), pp.1357-1366.