
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 10(2): 187-191 (2017)
PCR based analysis of Haemobartonellosis (
Candidatus
mycoplasma haematoparvum
and
Mycoplacma
haemocanis
) and its prevalence in dogs in Isfahan, Iran
Sayed Reza Hosseini,
1
Arman Sekhavatmandi
2
and Faham Khamesipour
3,4
1
Department of Pathobiology, Faculty of Veterinary Medicine, Shahrekord Branch, Islamic Azad University,
Shahrekord, Iran
2
Graduated of Veterinary Medicine, Shahrekord Branch, Islamic Azad University, Shahrekord, Iran
3
Cellular and Molecular Research Center, Sabzevar University of Medical Sciences, Sabzevar, Iran
4
Department of Pathobiology, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
ABSTRACT
The present study was conducted to determine prevalence of Candidatus mycoplasma haematoparvum and Myco-
placma haemocanis in dogs in Isfahan (Central province of Iran). Total 294 dogs were the materials of the study.
To determine molecular and haematological parameters, 2 ml blood with and without anticoagulant were taken
according to technique from vena cephalica antebrachii. Candidatus mycoplasma haematoparvum and Mycoplacma
haemocanis was detected in blood smears preparations of 26 (8.82%) by Geimsa staining and (20.06%) by PCR. Based
on the 16S rDNA sequence, a speci c PCR assay was developed.
KEY WORDS: HAEMOBARTONELLOSIS, DOGS, IRAN, PCR
187
ARTICLE INFORMATION:
*Corresponding Author: borji_milad@yahoo.com
Received 21
st
March, 2017
Accepted after revision 21
st
June, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
The organisms formerly known as Haemobartonella and
Eperythrozoon spp. are small, pleomorphic bacteria that
parasitize red blood cells of a wide range of vertebrate
animals. Haemobartonellosis, infectious anaemia of
dogs is caused by Candidatus mycoplasma haematopar-
vum and Mycoplacma haemocanis, an anaplasma spe-
cies belonging to the rickettsia family. The infection is
characterized by extreme fatigue, depression, anorexia,
weight loss and anaemia and may cause death (Lumb,
2001; Neimark et al., 2001; Torkan et al., 2014).
The pathogen can be identi ed as small coccoids,
rings or strings on erythrocyte membrane or free in
188 PCR BASED ANALYSIS OF HAEMOBARTONELLOSIS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Hosseini, Sekhavatmandi and Khamesipour
plasma in Giemsa staining of blood smears (Murray
et al., 1995; Lumb, 2001).Mode of transmission has not
been clearly identi ed but bloodsucking arthropods like
ticks were the suspected vectors. Another possible mode
of transmission is close ghting among dogs. Intrauter-
ine and lactation-related transmission was also reported
(Neimark et al., 2002; Jane et al., 2005).Acute disease
presents with fever, anorexia, weight loss, jaundice,
apathy, adenopathy, motor incoordination and spleno-
megaly (North, 1978; Sykes et al., 2004). Chronic dis-
ease has atypical symptoms like anaemia, weight loss,
paraplegia, dehydration, hyperesthesia and depression
(Saykes et al., 2008). Latent form of the infection has
also been described (Pitulle et al., 1999).Diagnosis of
haemobartonellosis depends on clinical and hematologi-
cal ndings together with microscopic examination of
blood smears and speci c serological and PCR testing
for the pathogen (Lappin et al., 2006). Various antibiot-
ics were reported to be effective in the treatment of hae-
mobartonellosis. Haemobartonellosis was rst described
in 1953 in the United States (Splitter et al., 1956) but the
number of studies about incidence and prevalence of the
disease and the risk factors in transmission remains lim-
ited after 50 years (Kemming et al., 2004). In addition,
studies examining Candidatus mycoplasma haematopar-
vum and Mycoplacma haemocanis infection have not
been came across in this country except for one study
(Foley et al., 2001).Therefore, this study was planned to
investigate prevalence of Candidatus mycoplasma hae-
matoparvum and Mycoplacma haemocanis infection in
dogs.
MATERIAL AND METHODS
Study population consisted of 294 dogs (140 females,
154 males). To determine molecular and haematological
parameters, 2 ml blood with and without anticoagulant
were taken according to technique from V. cephalica. The
dogs were clinically examined and blood samples with
and without anticoagulant were drawn into tubes for
haematological and molecular analysis. Prepared blood
smears were stained with Geimsa method and examined
under light microscope according to the literature (Foley
et al., 2001).
Blood was processed for DNA extraction as described
by d’Oliveira et al. (1995). Brie y, 200: L of thawed
blood was washed 3-5 times by mixing with 0.5 mL PBS
(137mM NaCl, 2.6 mM KCl, 8.1mM Na2HPO4, 1.5 mM
KH2PO4, pH 7.4), each time followed by centrifugation
at maximum speed (13,000 rpm) for 5 min. After the
nal wash, the cell pellet was resuspended in 100: L of
lysis mixture (10mM Tris-HCl, pH 8.0, 50 mM KCl, 0.5%
Tween 20, and 100: g/mL of proteinase K). This mixture
was incubated overnight at 56ºC, followed by 10 min of
boiling to inactivate the Proteinase K and kept at -20ºC
until needed for PCR for ampli cation of the 16S rRNA
gene (Dean et al., 2008).
The DNA samples from cattle blood were used in PCR
reactions (Reverse line blot-PCR) to amplify any Ana-
plasma (or even any Ehrlichia) 16S rRNA gene present.
One primer set was used to amplify a 309-328 bp frag-
ment being part of the V1 region of the16S rRNA gene.
The forward primer was (EF416566) (5’-GAAACTAA-
GGCCATAAATGACGC -3’) for Mycoplacma haemocanis
and (HQ918288) (5’-ACGAAAGTCTGATGGAG-
CAATAC-3’) for Candidatus mycoplasma haematopar-
vum whereas the reverse primer was (5’- ACCTGTCAC-
CTCGATAACCTCTAC-3’) for Mycoplacma haemocanis
and (5’-TATCTACGCATTCCACCGCTAC-3’) for Candida-
tus mycoplasma haematoparvum. The 1xPCR reaction
constituents in a nal volume of 25: L were as follows:
1xPCR buffer (Invitrogen), 3.0 mM MgCl2 (Invitrogen),
200: M each dATP, dCTP, dGTP, 100: M dTTP (ABgene)
and 100: M dUTP (Amersham), 1.25 U of Taq polymerase
(Invitrogen), 0.1U of UDG (Amersham), 25 pmol of each
primer and 2.5: L of template DN A. This was over laid
with about 12.5: L of mineral oil. Positive control DNA
(E. canis) from Molecular Biology Laboratory, Makerere
University and negative control (reaction constituents
without DNA) tests were included. The reactions were
performed using a three phase touchdown program as
previously described (Barker et al., 2009; Brinson et al.,
2001). The possible presence of ectoparasites on the dogs
was also looked for carefully. Statistical analyses were
done using SPSS for Windows.
RESULTS AND DISCUSSION
Clinical signs including temperature, pulsation and
respiration rates were in normal ranges. Some of the
infected cats had anorexia and weight loss. Microscopic
examination revealed Candidatus mycoplasma haema-
toparvum and Mycoplacma haemocanis in 26 (8.82%)
cats. The animals had no ectoparasites on them. Baseline
haematological and biochemical ndings did not differ
after the treatment. Appearance of Candidatus myco-
plasma haematoparvum and Mycoplacma haemocanis in
Geimsa staining of blood smears is presented. According
to the results sex had no effect on the infection of the
dogs (P > 0.05) (Tables1, 2 and 3).
In this study, we used histological methods and novel
molecular techniques to determine the regional preva-
lence and identity of Candidatus mycoplasma haema-
toparvum and Mycoplacma haemocanis. Of 294 samples,
66 supported ampli cation of parasite rRNA by PCR. 25
samples were then examined in parallel by microscopic
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PCR BASED ANALYSIS OF HAEMOBARTONELLOSIS 189
Hosseini, Sekhavatmandi and Khamesipour
examination and PCR. One samples positive by initial
microscopic examination were not ampli ed by PCR.
Thirty-nine PCR-positive samples did not contain sarco-
cysts on initial microscopic examination, but additional
sections from these samples revealed sarcocysts in an
additional 12 samples. The partial sequence analysis of
the16S rRNA gene (both sequences had 150 bp) indi-
cated a homology of 100% between the PCR amplicons
of the positive samples and Candidatus Mycoplasma
haematoparvum.
The infection was most probably transmitted by
ectoparasites (Wood et al., 2006). In conclusion, a rate of
haemobartonellosis of 22.45% was determined in dogs
and it is believed that haemobartonellosis should always
be suspected in dogs presented to veterinary clinics with
non-speci c symptoms. In addition, serological investi-
gations should also be done in future studies to docu-
ment the prevalence of the disease in this country.
Polymerase chain reaction (PCR) has been increas-
ingly applied to detect these pathogens in both blood and
tick vectors instead of microscopy. Although a number
of publications report the use of PCR, most publications
are based on 6 original methods for all pathogens (Roura
et al.,2005; Sykes et al., 2005; Bauer et al., 2008; Sasaki
et al., 2008;). Many reports summarised compare PCR
detection with serology to demonstrate assay speci city.
However, the most suitable detection method depends
upon whether antigen or antibody detection is relevant
for the particular investigation, detection of parasites,
or current infection, prevalence studies or evidence of
exposure to parasites.
Although PCR is more sensitive than light microscopy
(Kemming et al., 2004), this method complicated post-
PCR detection methods to further enhance the sensitivity
and con rm the speci city of the PCR technique such as
PCR-ELISA and PCR-probe hybridisation. A quanti able
PCR technique referred to as real time PCR (also known
as 5’ Taq nuclease assays, uorogenic probe assays, or
TaqMan_ assays), are increasingly applied for the detec-
tion and identi cation of animal pathogens and do not
require post PCR electrophoresis or processing steps
(Gentilini et al.,2009).
Real time assays exploit the 5’ nuclease activity of
Taq DNA polymerase cleaving a dual labelled uores-
cent probe which has annealed to a speci c sequence
between two primers (Francino et al., 2006; Peters et
al., 2008; Wengi et al., 2008). To date, the applications
of real time PCR for the detection of tick borne disease
pathogens have been described for Theileria spp. and
Anaplasma spp. (Taber et al.,2009; Lako et al., 2010). It
may not be feasible for certain laboratories to use PCR-
ELISA, PCR-probe hybridisation or real time PCR assays
as the application of each of these methods requires spe-
ci c and expensive reagents and equipment. Microscopy
remains the most economic and sustainable method of
parasite detection for all laboratories.
This is the rst case of canine infection with Candi-
datus Mycoplasma haematoparvum in Iran. This organ-
ism, named Candidatus Mycoplasma haematoparvum is
smaller (0.3 _m in diameter) and does not form chains
on the erythrocyte surface of dogs (Sykes et al., 2005).
The infection has been con rmed by methods of molec-
ular biology (Jensen et al., 2001). In France, a 15.4%
prevalence of haemoplasma infection in dogs, as well as
the presence of coinfection of both haemoplasmas, has
been registered using the PCR test (Kenny et al., 2004).
Further researches on haemotropic mycoplasmas are
indispensable, regarding that: haemoplasmas might act
as cofactors in the progression of retroviral, neoplastic
and immunologically mediated diseases; the factors of
the virulence and pathogenic mechanisms in the devel-
opment of these infections, as well as the functions of
the immunologic system, which is in this case again
responsible for the occurrence of new opportunistic
infections, are not known (Messick, 2004). Until now,
there is no evidence that canine haemoplasmas cause
human diseases, but regarding the fact that feline and
swine haemotropic mycoplasmas have zoonotic poten-
tial, future researches should be conducted in this direc-
tion (Xavier Roura et al., 2010; Wu et al., 2006).
The hemoplasma sample prevalence was signi cantly
higher in Switzerland (8.7%) than in Spain (2.5%) (Willi
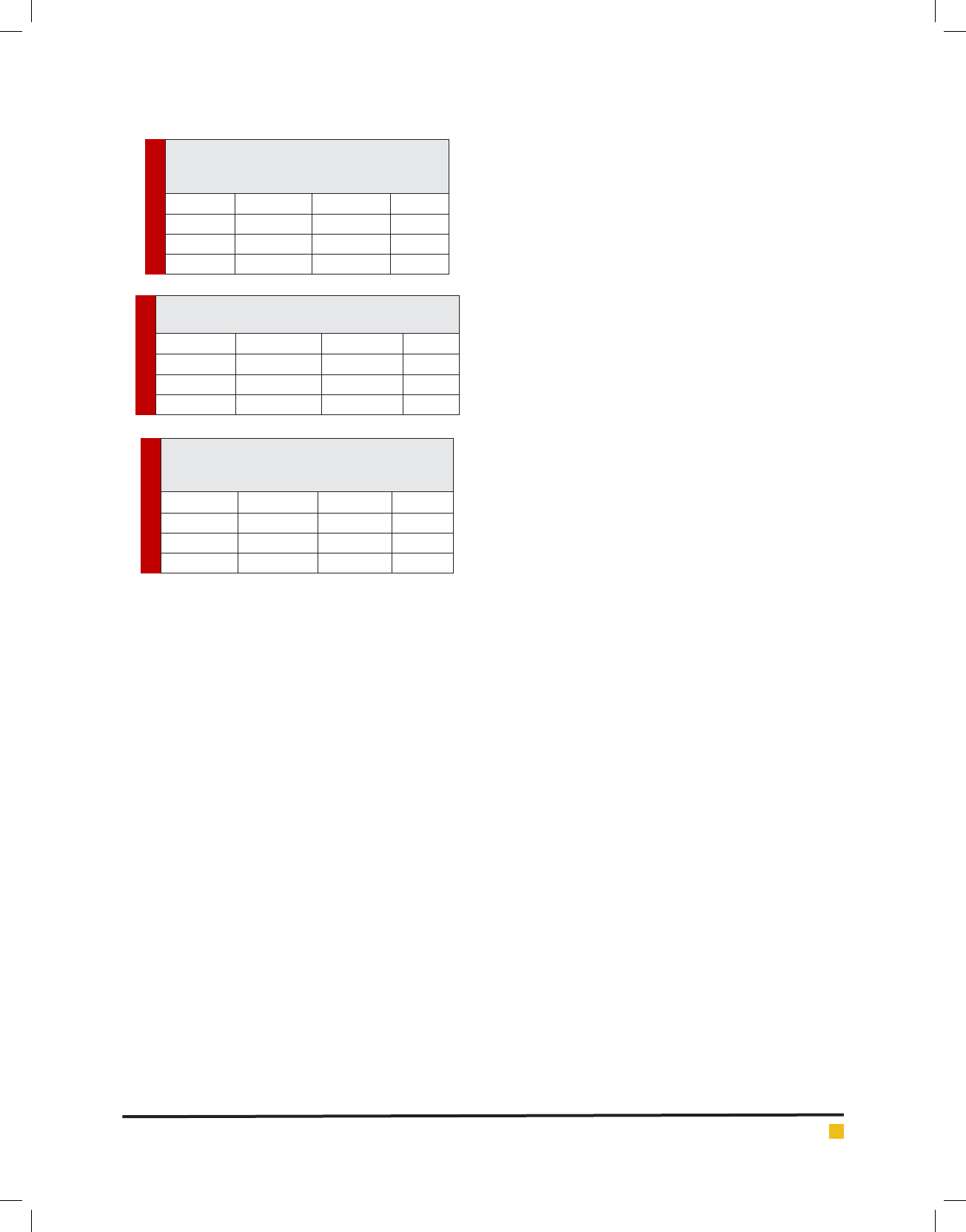
Table 1. Distributions of the ndings according
to the sex of the cats Candidatus mycoplasma
haematoparvum by pcr
Sex Number Positive %
Female 140 12 8.57
Male 154 17 11.03
Total 294 29 9.86
Table 2. Distributions of the ndings according to
the sex of the cats Mycoplacma haemocanis by pcr
Sex Number Positive %
Female 140 15 10.71
Male 154 18 11.68
Total 294 33 11.22
Table 3. Distributions of the ndings according to
the sex of the cats Mycoplacma haemocanis and
Candidatus mycoplasma haematoparvum by pcr
Sex Number Positive %
Female 140 26 18.57
Male 154 33 21.42
Total 294 59 20.06

190 PCR BASED ANALYSIS OF HAEMOBARTONELLOSIS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Hosseini, Sekhavatmandi and Khamesipour
et al.,2006; Lako et al., 2010; Xavier et al., 2010). Risk
factors for infection included living in kennels, young
age, crossbreeding, and mange infection. Among the
PCR-positive dogs, 40% were infested with blood-suck-
ing arthropods. No association was found with anemia.
ACKNOWLEDGEMENTS
We thank Hamid Reza Arshad Riahi and Manochehr
Momeni for their enthusiastic support of this work.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICT OF INTEREST
There are no con icts of interest.
RREFRENCES
Barker E.N., S. Tasker, M. Day, S.M. Warman, K. Woolley, R.
Birtles, K.C. Georges, C.D. Ezeokoli, A. Newaj-Fyzul, M.D.
Campbell, O.A.E. Sparagano, S. Cleaveland and C.R. Helps.
(2009) Development and use of real-time PCR to detect and
quantify Mycoplasma haemocanis and ‘Candidatus Myco-
plasma haematoparvum in dogs. Veterinary Microbiology Vol.
140: Pages 167–170.
Bauer N., H.J. Balzer, S. Thure and A. Moritz. (2008) Prevalence
of feline hemotropic mycoplasmas in convenience samples of
cats in Germany. Journal of Feline Medicine and Surgery Vol.
10: Pages 252–258.
Brinson J. J. and J. B. Messick., (2001) Use of a polymerase
chain reaction assay for detection of Haemobartonella canis in
a dog. Journal of the American Veterinary Medical Association
Vol. 218: Pages 1943–1945.
Dean R.S., C.R. Helps, T.J. Gruffydd Jones and S. Tasker. (2008)
Use of real-time PCR to detect Mycoplasma haemofelis and
Candidatus Mycoplasma haemominutum in the saliva and sali-
vary glands of haemoplasma-infected cats. Journal of Feline
Medicine and Surgery Vol. 10: Pages 413–417.
Foley J. E. and N. C Pedersen. (2001) Candidatus Mycoplasma
haemominutum, a low-virulence epierythrocytic parasite of
cats. International Journal of Systematic and Evolutionary
Microbiology Vol. 51: Pages 815–817.
Francino O., L. Altet, E. Sanchez-Robert, A. Rodriguez, L.
Solano-Gallego, J. Alberola, L. Ferrer, A. Sánchez and X.
Roura. (2006) Advantages of real-time PCR assay for diagnosis
and monitoring of canine leishmaniosis. Veterinary Parasitol-
ogy Vol. 137: Pages 214–221.
Gentilini F., M. Novacco, M.E. Turba, B. Willi, M.L. Bacci and
R. Hofmann-Lehmann. (2009) Use of combined conventional
and real-time PCR to determine the epidemiology of feline
haemoplasma infections in northern Italy. Journal of Feline
Medicine and Surgery Vol. 11: Pages 277–285.
Jensen W.A., M.R. Lappin, S. Kamkar and W.J. Reagan. (2001)
Use of a polymerase chain reaction assay to detect and dif-
ferentiate two strains of Haemobartonella felis in naturally
infected cats. American Journal of Veterinary Research Vol.
62: Pages 604-8.
Kemming G.I., J.B. Messick, G. Enders, M. Boros, B. Lorenz, S.
Muenzing, H. Kisch-Wedel, W. Mueller, A. Hahmann-Mueller,
K. Messmer and E. Thein. (2004) Mycoplasma haemocanis
infection—a kennel disease. Comparative Medicine Vol. 54:
Pages 404–409.
Kenny M.J., S.E. Shaw, F. Beugnet and S. Tasker. (2004) Dem-
onstration of two distinct haemotropic mycoplasmas in French
dogs. Journal of Clinical Microbiology Vol. 42: Pages 5397–
5399.
Lako B., A. Potkonjak, J. Balzer, A. Grozdana, D. Radsolava,
B. Topalski, M. Stevanevi and O. Stevanevi. (2010) First report
about canine infections with Candidatus mycoplasma haema-
toparvoum in Serbia. Acta Veterinaria (Beograd) Vol. 60, 5-6:
Pages 507-512.
Lumb W.V. (2001) More information on haemobartonellosis in
dogs. Journal of the American Veterinary Medical Association
Vol. 219: Pages 732–733.
Messick J.B. (2004) Hemotrophic mycoplasmas (hemoplasmas):
a review and new insights into pathogenic potential. Veteri-
nary Clinical Pathology Vol. 33: Pages 2-13.
Murray R.G.E. and E. Stackebrandt. (1995) Taxonomic note:
implementation of the provisional status Candidatus for
incompletelydescribed procaryotes. International Journal of
Systematic Bacteriology Vol. 45: Pages 186–187.
Neimark H., J.K.E Ohansson, Y. Rikihisa and J.G. Tully. (2001)
Proposal to transfer some members of the genera Haemobar-
tonella and Eperythrozoon to the genus Mycoplasma with
descriptions of ‘Candidatus Mycoplasma haemofelis’, ‘Can-
didatus Mycoplasma haemomuris’, ‘Candidatus Mycoplasma
haemosuis’ and ‘Candidatus Mycoplasma wenyonii’. Interna-
tional Journal of Systematic and Evolutionary Microbiology
Vol. 51: Pages 891–899.
Neimark H., K.E. Johansson, Y. Rikihisa and J.G. Tully. (2002)
Revision of haemotrophic Mycoplasma species names. Inter-
national Journal of Systematic and Evolutionary Microbiology
Vol. 52: Pages 683.
North D. C. (1978) Fatal haemobartonellosis in a non-splenec-
tomized dog – a case report. Journal of Small Animal Practice
Vol. 19: Pages 769–773.
Peters I.R., C.R. Helps, B. Willi, R. Hofmann-Lehmann and S.
Tasker. (2008) The prevalence of three species of feline haemo-
plasmas in samples submitted to a diagnostics service as deter-
mined by three novel real-time duplex PCR assays. Veterinary
Microbiology Vol. 126: Pages 142–150.
Pitulle C., D. M.Citron, B. Bochner, R. Barbers and M. D. Apple-
man. (1999) Novel bacterium isolated from a lung transplant
patient with cystic brosis. Journal of Clinical Microbiology
Vol. 37: Pages 3851–3855.
Roura X., E. Breitschwerdt, A. Lloret, L. Ferrer and B. Hegarty.
(2005) Serological evidence of exposure to Rickettsia,

Hosseini, Sekhavatmandi and Khamesipour
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PCR BASED ANALYSIS OF HAEMOBARTONELLOSIS 191
Bartonella and Ehrlichia spp. in healthy or Leishmania infan-
tum infected dogs from Barcelona. The International Journal
of Applied Research in Veterinary Medicine Vol. 3: Pages 129–
138.
Roura X., I.R. Peters, L. Altet, M.D. Tabar, E. N. Barker, M.
lanellas, C. R. Helps, O. Francino, S. E. Shaw and S. Tasker.
(2010) Prevalence of hemotropic mycoplasmas in healthy and
unhealthy cats and dogs in Spain. Journal of Veterinary Diag-
nostic Investigation Vol. 22: Pages 270–274.
Sasaki M., K. Ohta, A. Matsuu, H. Hirata, H. Ikadai and T. Oyam-
ada. (2008) A molecular survey of Mycoplasma haemocanis in
dogs and foxes in Aomori Prefecture, Japan. Journal of Proto-
zoology Research Vol. 18: Pages 57–60.
Seneviratna P., N. Weerasinghe and S. Ariyadasa. (1973)
Transmission of Haemobartonella canis by the dog tick, Rhipi-
cephalus sanguineus. Research in Veterinary Science Vol. 14:
Pages 112–114.
Sykes J.E., N.L. Bailiff, L.M. Ball, O. Foreman, J.W. George and
M.M. Fry. (2004) Identi cation of a novel haemotropic myco-
plasma in a splenectomized dog with hemic neoplasia. Jour-
nal of the American Veterinary Medical Association Vol. 224:
Pages 1946–1951.
Sykes J.E., L.M. Ball, N.L. Bailiff and M.M. Fry. (2005) Candida-
tus Mycoplasma haematoparvum, a novel small haemotropic
mycoplasma from a dog. International Journal of Systematic
and Evolutionary Microbiology Vol. 55: Pages 27-30.
Sykes J.E., J.C. Terry, L.L. Lindsay and S.D. Owens. (2008) Preva-
lences of various haemoplasma species among cats in the United
States with possible hemoplasmosis. Journal of the American
Veterinary Medical Association Vol. 232: Pages 372–379.
Splitter E. J., E. R. Castro and W. L. Kanawyer. (1956) Feline
infectious anemia. Veterinary medicine Vol. 51: Pages 17–22.
Tabar M.D., O. Francino, L. Altet, A. Sánchez, L. Ferrer and X.
Roura. (2009) PCR survey of vectorborne pathogens in dogs
living in and around Barcelona,an area endemic for leishmani-
osis, Veterinary Record Vol. 164: Pages 112–116.
Tasker S., C. R. Helps, M. J. Day, D. A. Harbour, S. E. Shaw,
S. Harrus, G. Baneth, R. G. Lobetti, R. Malik, J. P. Beau ls,
C. R. Belford and T. J. Gruffydd-Jones. (2003) Phyloge-
netic analysis of haemoplasma species – an international
study. Journal of Clinical Microbiology Vol. 41: Pages 3877–
3880.
Torkan, S., S. Nargesi and F. Khamesipour. (2014) The Com-
parative Study of the Treatment by Oxytetracycline and Home-
opathy on Induced Mycoplasma Haemofelis in Less than One-
Year-Old Cats. International Journal of Animal and Veterinary
Advances Vol. 6: Pages 97-102.
Wengi N., B. Willi, F.S. Boretti, V. Cattori, B. Riond, M.L.
Meli, C.E. Reusch, H. Lutz and R. Hofmann-Lehmann. (2008)
Real-time PCR-based prevalence study, infection follow-
up and molecular characterization of canine hemotro-
pic mycoplasmas. Veterinary Microbiology Vol. 126: Pages
132-41.
Willi B., S. Tasker, F.S. Boretti, M. G. Doherr, V. Cattori, M. L.
Meli, R. G. Lobetti, R. Malik, C. E. Reusch, H. Lutz and R. Hof-
mann-Lehmann. (2006) Phylogenetic analysis of ‘‘Candidatus
Mycoplasma turicensis’’ isolates from pet cats in the United
Kingdom, Australia, and South Africa, with analysis of risk
factors for infection. Journal of Clinical Microbiology Vol. 44:
Pages 4430–4435.
Woods J.E., M.M. Brewer, J.R. Hawley, N. Wisnewski and M.
R. Lappin. (2006) Evaluation of experimental transmission of
‘Candidatus Mycoplasma haemominutum’ and Mycoplasma
haemofelis by Ctenocephalides felis to cats. American Journal
of Veterinary Research Vol. 66: Pages 1008–1012.
Wu J., J. Yu, C. Song, S. Sun and Z. Wang. (2006) Porcine
eperythrozoonosis in China. Annals of the New York Academy
of Sciences Vol. 1081: Pages 280-5.