
Toxicological
Communication
Biosci. Biotech. Res. Comm. 10(2): 165-172 (2017)
The protective effect of chlorogenic acid on arsenic
trioxide induced hepatotoxicity in mice
Javad Ghahhari
1
, Gholamhassan Vaezi
2
, Gholamhossein Riazi
3
, Ali Abbasi
4
, Mona
Modanloo
5
and Mohammad Shokrzadeh
6
*
1
Department of Biology, Damghan Branch, Islamic Azad University, Semnan, Iran
2
Department of Biology, Damghan Branch, Islamic Azad University, Semnan, Iran
3
Institute of Biochemistry and Biophysics, University of Tehran, Tehran, Iran
4
Department of Pathology, Sari Branch, Islamic Azad University, Sari, Iran
5
Pharmaceutical Sciences Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
6
Department of Toxicology and Pharmacology, Faculty of Pharmacy, Mazandaran University of Medical
Sciences, Sari, Iran
ABSTRACT
Several studies have shown that chronic exposure to arsenic trioxide risk factor for many cancers such as lung, liver,
kidney and bladder. Free radicals in various ways such as lipid peroxidation, protein oxidation and DNA damage that
leads to many diseases. Chlorogenic acid, an antioxidant plant and prevent many diseases caused by oxidative stress
such as cancer. In this study, the protective effect of chlorogenic acid on arsenic trioxide-induced liver toxicity were
studied. Biochemical parameters including Alkaline phosphatase (ALP), Alanine aminotransferase (ALT), Aspartate
aminotransferase (AST), and Glutathione (GSH) were assessed for liver damage.In compare to positive control group
(arsenic trioxide, 10 mg/kg), the serum levels of ALP, ALT, and AST have signi cantly decreased (p<0.05) and the
level of GSH has signi cantly increased (p<0.05) in the groups administered with chlorogenic acid (10, 50, and 100
mg/kg).The results showed that chlorogenic acid has signi cant protective effects against the hepatotoxicity caused
by arsenic trioxide.
KEY WORDS: ALANINE AMINOTRANSFERASE, ALKALINE PHOSPHATASE, ARSENIC TRIOXIDE, ASPARTATE AMINOTRANSFERASE,
GLUTATHIONE
165
ARTICLE INFORMATION:
*Corresponding Author:
Received 1
st
March, 2017
Accepted after revision 19
th
June, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/

166 THE PROTECTIVE EFFECT OF CHLOROGENIC ACID ON ARSENIC TRIOXIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Javad Ghahhari et al.
INTRODUCTION
Exposure to arsenic trioxide take place on variety ways,
for example, fuel, drinking water, pesticides, air, and
food that is a threat to human health (Holt et al., 2009;
Mittal et al., 2012). Researches have shown that expo-
sure to arsenic can cause acute and chronic effects on
different organs of the body, like cardiovascular sys-
tem, digestive system, respiratory system, and nervous
system(Centeno et al., 2002; Abernathy et al., 2003).
Kidney and liver are the most important organs for met-
als and have the highest level of metallothionein bind-
ing to metals (Sarvankumar et al., 2011). Liver is an
important site for the metabolism of arsenic trioxide and
chronic exposure to arsenic trioxide cause liver disease
(Flora et al., 2007). Arsenic toxicity is due to its ability
to react with sulfhydryl groups in proteins and enzymes
and can be replaced phosphorus in many biochemical
reactions (Stevens et al., 2010, Šeruga and Tomac, 2014
and Aoyama and Nakaki, 2015).
Damages caused by arsenic in the liver are deter-
mined by changes in liver enzymes, including Alkaline
phosphatase (ALP), Alanine aminotransferase (ALT), and
Aspartate aminotransferase (AST) (De Azevedo et al.,
2008). Free radicals can lead to various injuries at cells,
such as DNA damage, inhibition of mitochondrial respi-
ratory chain enzymes, and membrane lipid peroxidation
(Umamaheswari and Chatterjee, 2008). The free radicals
by creating oxidative stress and toxic oxidative leads to
various diseases (Halliwell and Whiteman, 2004). Anti-
oxidants by neutralizing free radicals prevent damages
caused by free radicals in the body organs (Gradecka
et al., 2001). Glutathione synthesis of glutamate, cysteine,
and glycine has done with the help of two enzymes,
including glutamylcysteine synthetase and glutathione
synthetase. The liver is an important location for manu-
facturing and exporting of glutathione (Wu et al., 2004).
Glutathione is caused failure of reactive oxygen species
such as lipid peroxyl radical, peroxynitrite, hydroxyl
radical, and other free radicals (Fang et al., 2002). Glu-
tathione de ciency leads to oxidative stress. Oxidative
stress plays an important role in various diseases such
as cancer, diabetes, Alzheimer’s disease, kwashiorkor,
seizures, liver disease, and Parkinson’s disease (Wu et
al., 2004) .
Researches have shown that phenolic compounds as
natural antioxidants play an important role in protect-
ing the body’s cells against the toxic oxidative (Bralley et
al., 2008). Chlorogenic acid as a polyphenol compound
is an ester between caffeic acid and quinic acid, and
there is in many foods like coffee and apple. Chlorogenic
acid is a strong antioxidant that has pharmacological
properties like anti cancer and antibacterial (Um et al.,
2006; Das et al., 2012). The present study evaluates the
protective effects of chlorogenic acid on the liver toxic-
ity caused by arsenic trioxide in vitro by assessing the
biochemical parameters including Alkaline phosphatase
(ALP), Alanine aminotransferase (ALT), Aspartate ami-
notransferase (AST), and Glutathione (GSH).
MATERIAL AND METHODS
42 male mice (27 ± 2 g) procured from the animal house
of the Mazandaran University of Medical Sciences, Sari,
Iran. They were maintained in a controlled environment
(12 h light/dark cycles) and temperature (28 ± 1°C).
The mice were fed with drinking water and standard
diet.
Chlorogenic acid was obtained from Sigma-Aldrich
Company (USA). Arsenic trioxide was purchased from
Merck Company (Germany).Mice were divided in 7
groups and 6 mice in each group. In these experiments,
the effects of intraperitoneal administration of different
doses of chlorogenic acid on the biochemical param-
eters of the kidney were investigated. The rst group
was administered with normal saline (0.9%) (10 mg/
kg) as control, the second group was administered with
arsenic trioxide (10 mg/kg) as positive control, the third
group was administered with chlorogenic acid (100 mg/
kg) as negative control, and the fourth to the seventh
groups were administered with different doses of chlo-
rogenic acid (5, 10, 50, 100 mg/kg), then after 2 hours
the fourth to the seventh groups were administered with
arsenic trioxide (10 mg/kg) (Sabath and Robles-Osorio,
2012).
Blood samples were collected of heart with the
syringe, then transferred to centrifuge tubes, and cen-
trifuged at 4000 rpm for 15 minutes. After centrifuging,
the serum was separated and stored in the refrigerator
(Tapio and Grosche, 2006). The level of serum Alkaline
phosphatase (ALP), Alanine aminotransferase (ALT),
and Aspartate aminotransferase (AST) were measured
on a chemistry auto analyzer (Tchounwou et al., 2004).
Homogenized 0.1 g of liver tissue sample with 1ml assay
buffer (EDTA) was taken then, its contents were trans-
ferred to centrifuge tubes and 0.5 ml EDTA was added
to those. In the next step, 1.5 ml TCA 10% was added to
centrifuge tubes. Centrifuge samples for 15 min at 3000
rpm. Remove 1 ml of supernatants and place in new
tubes. Then, was added 2.5 ml Tris buffer (0.4 M) and 0.5
DTNB. Absorbance was measured of solutions at 412 nm
with spectrophotometry (Saeedi Saravi and Shokrzadeh,
2008; Shokrzadeh et al., 2015). The data were analyzed
with SPSS 16 software. Statistical analysis of data was
carried out with one way analysis of variance and Tukey
test. The differences were considered signi cant at p <
0.05.

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE PROTECTIVE EFFECT OF CHLOROGENIC ACID ON ARSENIC TRIOXIDE 167
Javad Ghahhari et al.
RESULTS AND DISCUSSION
The serum level of ALP has signi cantly increased
(p<0.05) in the groups administered with of chlorogenic
acid (5, 10, 50, and 100 mg/kg) when compared to the
control group (normal saline, 10 mg/kg) (Figure 1). The
serum level of ALP has signi cantly decreased (p<0.05)
in the groups administered with of chlorogenic acid (10,
50, and 100 mg/kg) when compared to the positive con-
trol group (arsenic trioxide, 10 mg/kg) but didn’t show
signi cant difference in dose of 5 mg/kg (Figure 2). The
serum level of ALP has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid (5,
10, 50, and 100 mg/kg) when compared to the negative
control group (chlorogenic acid, 100 mg/kg) (Figure 3).
The serum level of ALT has signi cantly increased
(p<0.05) in the groups administered with of chlorogenic
acid (5, 10, 50, and 100 mg/kg) when compared to the
control group (normal saline, 10 mg/kg) (Figure 4). The
serum level of ALT has signi cantly decreased (p<0.05)
in the groups administered with of chlorogenic acid (10,
50, and 100 mg/kg) when compared to the positive con-
trol group (arsenic trioxide, 10 mg/kg) but didn’t show
signi cant difference in dose of 5 mg/kg (Figure 5). The
serum level of ALT has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid (5,
10, 50, and 100 mg/kg) when compared to the negative
control group (chlorogenic acid, 100 mg/kg) (Figure 6).
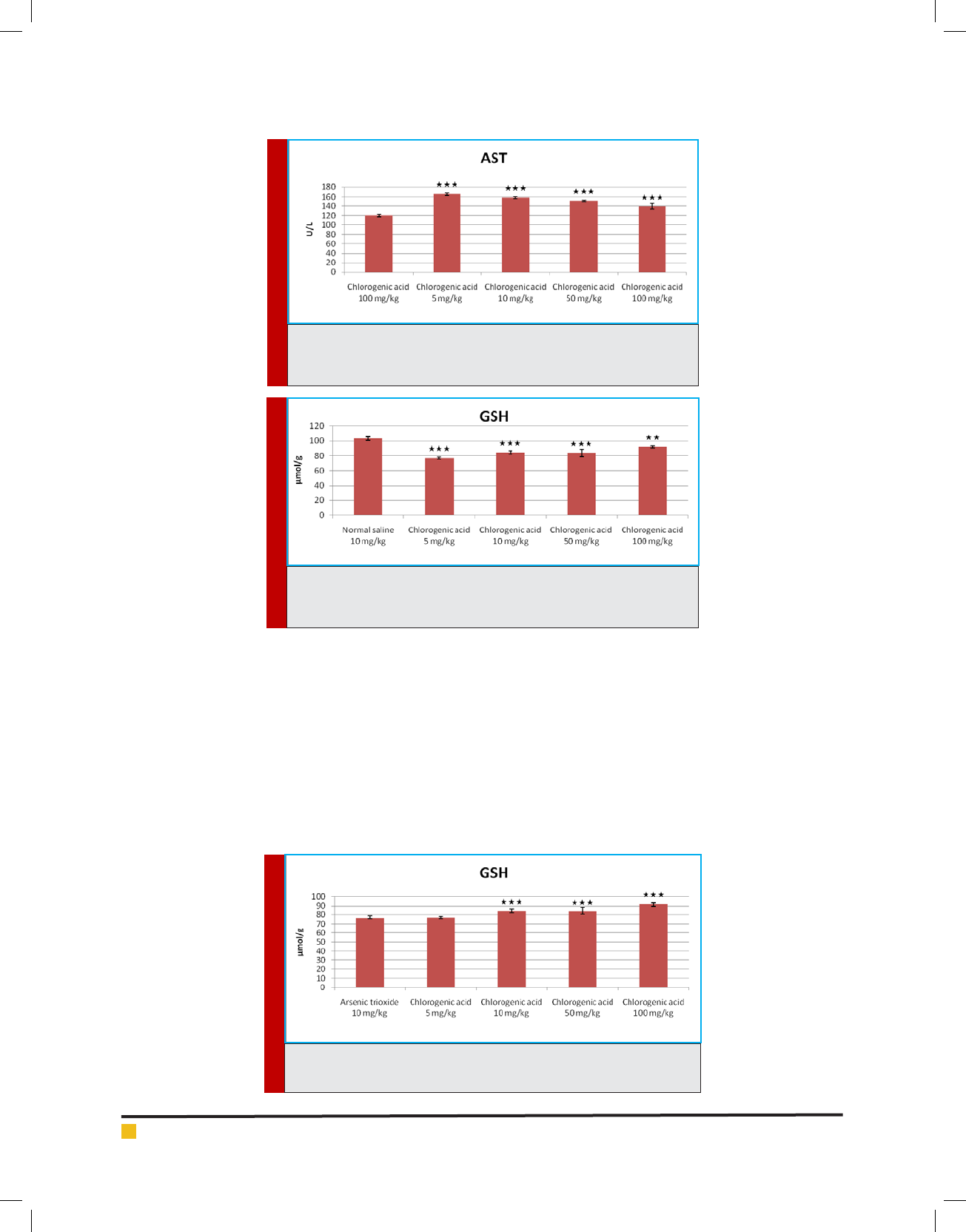
The serum level of AST has signi cantly increased
(p<0.05) in the groups administered with of chlorogenic
acid (5, 10, 50, and 100 mg/kg) when compared to the
control group (normal saline, 10 mg/kg) (Figure 7). The
serum level of AST has signi cantly decreased (p<0.05)
in the groups administered with of chlorogenic acid (10,
50, and 100 mg/kg) when compared to the positive con-
trol group (arsenic trioxide, 10 mg/kg) but didn’t show
signi cant difference in dose of 5 mg/kg (Figure 8). The
serum level of AST has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid (5,
10, 50, and 100 mg/kg) when compared to the negative
control group (chlorogenic acid, 100 mg/kg) (Figure 9).
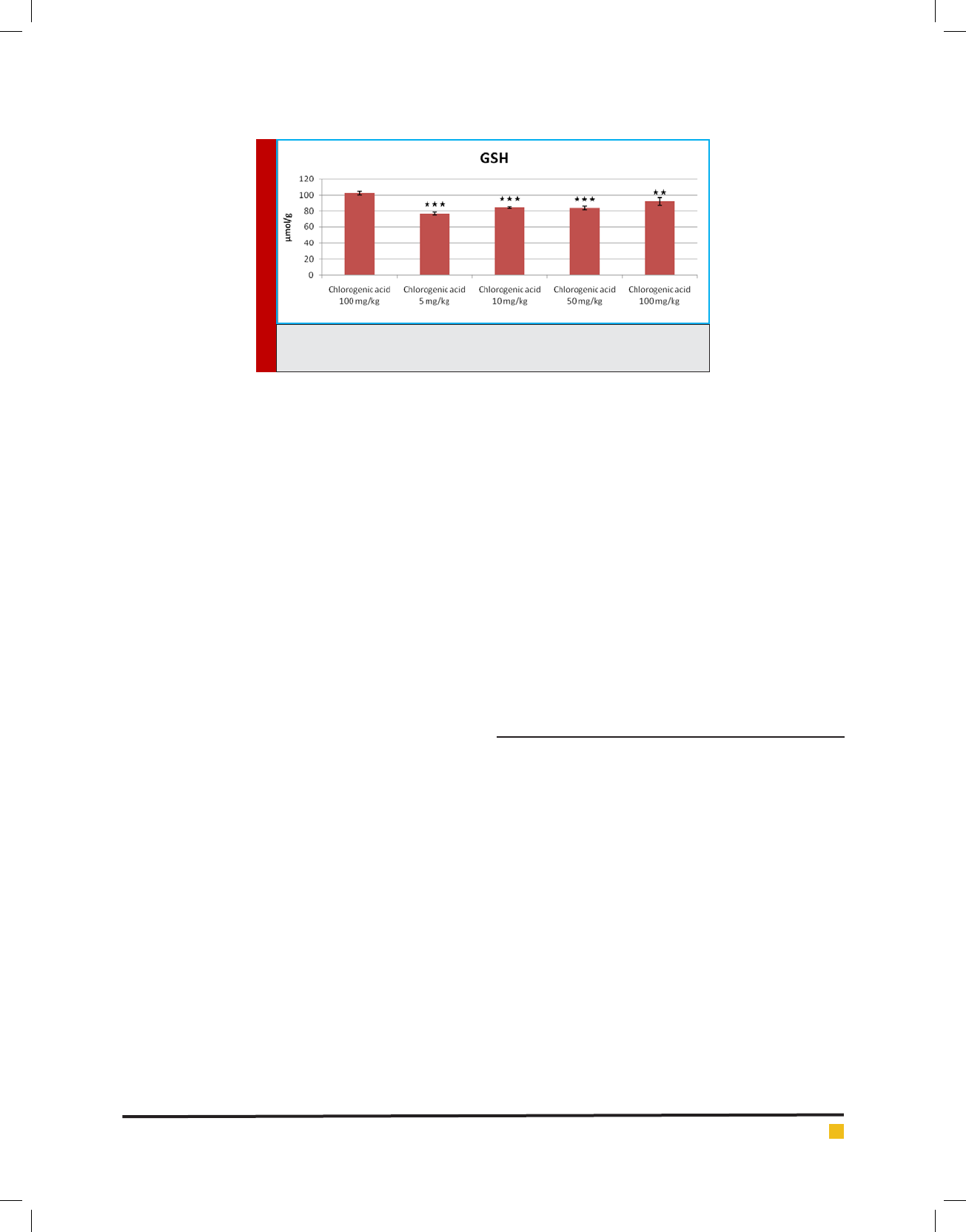
The level of GSH has signi cantly decreased (p<0.05)
in the groups administered with of chlorogenic acid (5,
10, 50, and 100 mg/kg) when compared to the control
group (normal saline, 10 mg/kg) (Figure 10). The level of
GSH has signi cantly increased (p<0.05) in the groups
administered with of chlorogenic acid (10, 50, and 100
mg/kg) when compared to the positive control group
FIGURE 1. The serum level of ALP has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid (5, 10, 50, and
100 mg/kg).
FIGURE 2. The serum level of ALP has signi cantly decreased (p<0.05)
in the groups administered with of chlorogenic acid (10, 50, and 100
mg/kg).

168 THE PROTECTIVE EFFECT OF CHLOROGENIC ACID ON ARSENIC TRIOXIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Javad Ghahhari et al.
FIGURE 3. The serum level of ALP has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid (5, 10, 50, and 100
mg/kg).
FIGURE 4. The serum level of ALT has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid (5, 10, 50, and 100
mg/kg).
FIGURE 5. The serum level of ALT has signi cantly decreased (p<0.05)
in the groups administered with of chlorogenic acid (10, 50, and 100
mg/kg).
(arsenic trioxide, 10 mg/kg) but didn’t show signi cant
difference in dose of 5 mg/kg (Figure 11). The level of
GSH has signi cantly decreased (p<0.05) in the groups
administered with of chlorogenic acid (5, 10, 50, and 100
mg/kg) when compared to the negative control group
(chlorogenic acid, 100 mg/kg) (Figure 12).
Arsenic compounds are one of the pollutants of envi-
ronment which are a serious threat to human health.
Millions of people around the world through drinking
water are exposed to arsenic compounds (Sabath and
Robles-Osorio, 2012). Exposure to arsenic and its com-
pounds can have dangerous effects on health (Tapio and

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE PROTECTIVE EFFECT OF CHLOROGENIC ACID ON ARSENIC TRIOXIDE 169
Javad Ghahhari et al.
FIGURE 6. The serum level of ALT has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid (5, 10, 50, and 100
mg/kg).
FIGURE 7. The serum level of AST has signi cantly increased
(p<0.05) in the groups administered with of chlorogenic acid (5, 10,
50, and 100 mg/kg).
FIGURE 8. The serum level of AST has signi cantly decreased (p<0.05)
in the groups administered with of chlorogenic acid (10, 50, and 100
mg/kg).
Grosche, 2006) and can lead to many types of cancers,
such as skin, liver, kidney, lung, intestine, and bladder
(Tchounwou et al., 2004). Inorganic arsenic compounds
as carcinogenic compounds are known. Epidemiological
studies have shown that these compounds are related
to the types of cancers like liver, kidney, and bladder
(Gradecka et al., 2001). Researches have shown that con-
sumption of drinking water containing inorganic arsenic
compounds is related to liver diseases such as liver can-
cer (Islam et al., 2011). In clinical studies of the liver,
liver enzymes, including Alkaline phosphatase (ALP),
Alanine aminotransferase (ALT), and Aspartate ami-
Javad Ghahhari et al.
170 THE PROTECTIVE EFFECT OF CHLOROGENIC ACID ON ARSENIC TRIOXIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 9. The serum level of AST has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid (5, 10, 50, and 100
mg/kg).
FIGURE 10. The level of GSH has signi cantly decreased (p<0.05) in
the groups administered with of chlorogenic acid (5, 10, 50, and 100
mg/kg).
FIGURE 11. The level of GSH has signi cantly increased (p<0.05) in the
groups administered with of chlorogenic acid (10, 50, and 100 mg/kg).
notransferase (AST) are evaluated. The enzymes levels
of ALT and AST are an indicator to assess the integrity
of liver cells and the enzyme level of ALP represents the
perfect synthesis of albumin and bile by the liver (Islam
et al., 2011). Our research has shown that the serum lev-
els of ALP, ALT, and AST have signi cantly increased
(p<0.05) in all groups administered with of chlorogenic
acid when compared to the control and negative control
groups. Antioxidants are including two major catego-
ries, enzymatic and non enzymatic. Enzymatic antioxi-
dants like catalase, glutathione peroxidase, and super-
oxide dismutase, which are produced by the body and
non enzymatic antioxidants like avonoids, tannins,
and carotenoids that obtained from plants (Lee et al.,
2004). Phenolic compounds as antioxidants in plants are
caused by the elimination of free radicals. The antioxi-
dant properties of phenolic compounds are related to
the characteristic of the redox (oxidation and reduction)
that reduce free radicals. Also, they are caused chelat-
ing of metals (Hsu, 2006). There is chlorogenic acid
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS THE PROTECTIVE EFFECT OF CHLOROGENIC ACID ON ARSENIC TRIOXIDE 171
Javad Ghahhari et al.
as a polyphenolic compound widely in various plants
(Šeruga and Tomac, 2014).
Studies, on the health bene ts of consuming chloro-
genic acid found in foods have shown that this antioxi-
dant to reduce diseases such as cancer, stroke, obesity,
diabetes, and Alzheimer’s disease (Zhao et al., 2012). The
serum levels of ALP, ALT, and AST have signi cantly
decreased (p<0.05) in the groups administered with of
chlorogenic acid (10, 50, and 100 mg/kg) when com-
pared to the positive control group (arsenic trioxide, 10
mg/kg). It sounds that the antioxidant property of chlo-
rogenic acid plays an important role in the protection of
cells against free radicals caused by arsenic trioxide. The
free radical is an atom or molecule that has an unpaired
electron. The cells use of oxygen for produce energy that
lead to the production of free radicals such as super-
oxide anion, hydroxyl, and peroxyl that are known as
reactive oxygen species (ROS) and mainly produced by
mitochondria. Free radicals can lead to a wide range of
toxic oxidative reactions like membrane lipid peroxi-
dation, the failure of enzymes and proteins that could
cause cell death (Umamaheswari and Chatterjee, 2008).
Free radicals cause oxidative stress that plays an impor-
tant role in the development of many diseases such as
diabetes, cardiovascular, and cancer (Bandyopadhyay et
al., 1999). The studies show that many metals such as
iron (Fe), lead (Pb), and copper (Cu) with modulating
the redox (oxidation and reduction) in cell are causing
the level change of thiols like glutathione in the cells
(Tchounwou et al., 2002). The glutathione (GSH) as a
thiol compound and important in cells have antioxidant
role and are caused destroy of free radicals. The glu-
tathione (GSH) is in various body organs such as liver,
kidney, brain, pancreas, and heart and highest levels of
that found in the liver (Aoyama and Nakaki, 2015).
In this study, the level of GSH has signi cantly
decreased (p<0.05) in the groups administered with of
chlorogenic acid (5, 10, 50, and 100 mg/kg) when com-
pared to the control and negative control groups that
was speci ed arsenic trioxide reduced GSH in the liver
but the level of GSH has signi cantly increased (p<0.05)
in the groups administered with of chlorogenic acid
(10, 50, and 100 mg/kg) when compared to the posi-
tive control group that was identi ed chlorogenic acid
as an antioxidant reduces free radicals and increases
the glutathione level of the liver. The glutathione (GSH)
with formation reversible disul de bonds between thiols
of protein can inhibit the oxidation of proteins during
oxidative stress (Giustarini et al., 2004). The glutath-
ione (GSH) as an antioxidant have an important role
in balancing of intracellular redox processes (Aoyama
and Nakaki, 2015). Overall, our results showed that chlo-
rogenic acid as a powerful and important antioxidant
had signi cant protective effects against the toxicity of
arsenic trioxide.
ACKNOWLEDGMENT
We appreciate Of all colleagues who have worked on this
project with us include the Laboratory of Toxicology in
the Faculty of Pharmacy of Mazandaran University of
Medical Sciences and Noor medical laboratory.
REFERENCES
Abe rnathy, C.O., Thomas, D.J., Calderon, R.L., 2003. Health
effects and risk assessment of arsenic. The Journal of nutrition
133, 1536S-1538S.
Aoy ama, K., Nakaki, T., 2015. Glutathione in cellular redox
homeostasis: association with the excitatory amino acid carrier
1 (EAAC1). Molecules 20, 8742-8758.
Ban dyopadhyay, U., Das, D., Banerjee, R.K., 1999. Reactive
oxygen species: oxidative damage and pathogenesis. Current
Science-Bangalore- 77, 658-666.
Bra lley, E.E., Greenspan, P., Hargrove, J.L., Wicker, L., Hartle,
D.K., 2008. Topical anti-in ammatory activity of Polygonum
cuspidatum extract in the TPA model of mouse ear in amma-
tion. Journal of In ammation 5, 1.
FIGURE 12. The level of GSH has signi cantly decreased (p<0.05) in the
groups administered with of chlorogenic acid (5, 10, 50, and 100 mg/kg).

172 THE PROTECTIVE EFFECT OF CHLOROGENIC ACID ON ARSENIC TRIOXIDE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Javad Ghahhari et al.
Cen teno, J.A., Mullick, F.G., Martinez, L., Page, N.P., Gibb, H.,
Longfellow, D., Thompson, C., Ladich, E.R., 2002. Pathology
related to chronic arsenic exposure. Environmental Health Per-
spectives 110, 883.
Das , N., Paul, S., Chatterjee, D., Banerjee, N., Majumder, N.S.,
Sarma, N., Sau, T.J., Basu, S., Banerjee, S., Majumder, P., 2012.
Arsenic exposure through drinking water increases the risk of
liver and cardiovascular diseases in the population of West
Bengal, India. BMC Public Health 12, 639.
De Azevedo, A., Mazzafera, P., Mohamed, R., Melo, S., Kieck-
busch, T.G., 2008. Extraction of caffeine, chlorogenic acids and
lipids from green coffee beans using supercritical carbon diox-
ide and co-solvents. Brazilian Journal of Chemical Engineering
25, 543-552.
Fan g, Y.-Z., Yang, S., Wu, G., 2002. Free radicals, antioxidants,
and nutrition. Nutrition 18, 872-879.
Flo ra, S., Bhadauria, S., Kannan, G., Singh, N., 2007. Arsenic
induced oxidative stress and the role of antioxidant supple-
mentation during chelation: a review. Journal of Environmen-
tal Biology 28, 333.
Giu starini, D., Rossi, R., Milzani, A., Colombo, R., Dalle‐Donne,
I., 2004. S‐Glutathionylation: from redox regulation of protein
functions to human diseases. Journal of cellular and molecular
medicine 8, 201-212.
Grade cka, D., Palus, J., Wasowicz, W., 2001. Selected mecha-
nisms of genotoxic effects of inorganic arsenic compounds.
International journal of occupational medicine and environ-
mental health 14, 317-328.
Halli well, B., Whiteman, M., 2004. Measuring reactive species
and oxidative damage in vivo and in cell culture: how should
you do it and what do the results mean? British journal of
pharmacology 142, 231-255.
Holt, E.M., Steffen, L.M., Moran, A., Basu, S., Steinberger, J.,
Ross, J.A., Hong, C.-P., Sinaiko, A.R., 2009. Fruit and vegeta-
ble consumption and its relation to markers of in ammation
and oxidative stress in adolescents. Journal of the American
Dietetic Association 109, 414-421.
Hsu, C.-Y., 2006. Antioxidant activity of extract from Poly-
gonum aviculare L. Biological research 39, 281-288.
Islam , K., Haque, A., Karim, R., Fajol, A., Hossain, E., Salam,
K.A., Ali, N., Saud, Z.A., Rahman, M., Rahman, M., 2011. Dose-
response relationship between arsenic exposure and the serum
enzymes for liver function tests in the individuals exposed to
arsenic: a cross sectional study in Bangladesh. Environmental
health 10, 64.
Lee, J., Koo, N., Min, D., 2004. Reactive oxygen species, aging,
and antioxidative nutraceuticals. Comprehensive reviews in
food science and food safety 3, 21-33.
Mitta l, D.K., Joshi, D., Shukla, S., 2012. Hepatoprotective Role
of Herbal Plants–A Review. International Journal of Pharma-
ceutical Sciences 3, 150-157.
Sabat h, E., Robles-Osorio, M.L., 2012. Renal health and the
environment: heavy metal nephrotoxicity. Nefrologia 32, 279-
286.
Saeed i Saravi, S., Shokrzadeh, M., 2008. Histopathological and
biochemical disorders following administration of Sambucus
ebulus extract on mice and rats and preventive effects of vita-
mins C and E on renal and hepatic disorders. Phcog Mag 5,
131-135.
Sarva nkumar, G., Lalitha, V., Sengottuvelu, S., SHARIF, S.H.,
SIVAKUMAR, T., 2011. Nephroprotective activity of Vitex
negundo Linn bark against chemical induced toxicity in exper-
imenal Rats. An International Journal of Advances in Pharma-
ceutical Sciences 2, 462-470.
Šerug a, M., Tomac, I., 2014. Electrochemical behaviour of
some chlorogenic acids and their characterization in coffee
by square-wave voltammetry. International journal of electro-
chemical science 9, 6134-6154.
Shokr zadeh, M., Chabra, A., Ahmadi, A., Naghshvar, F., Habibi,
E., Salehi, F., Assadpour, S., 2015. Hepatoprotective effects of
Zataria multi ora ethanolic extract on liver toxicity induced
by cyclophosphamide in mice. Drug research 65, 169-175.
Steve ns, J.J., Graham, B., Walker, A.M., Tchounwou, P.B., Rogers,
C., 2010. The effects of arsenic trioxide on DNA synthesis and
genotoxicity in human colon cancer cells. International journal
of environmental research and public health 7, 2018-2032.
Tapio , S., Grosche, B., 2006. Arsenic in the aetiology of cancer.
Mutation Research/Reviews in Mutation Research 612, 215-
246.
Tchou nwou, P., Wilson, B., Abdelghani, A., Ishaque, A., Pat-
lolla, A., 2002. Differential cytotoxicity and gene expression in
human liver carcinoma (HepG2) cells exposed to arsenic triox-
ide, and monosodium acid methanearsonate (MSMA). Interna-
tional Journal of Molecular Sciences 3, 1117-1132.
Tchou nwou, P.B., Centeno, J.A., Patlolla, A.K., 2004. Arsenic
toxicity, mutagenesis, and carcinogenesis–a health risk assess-
ment and management approach. Molecular and cellular bio-
chemistry 255, 47-55.
Um, M .-Y., Choi, W.-H., Aan, J.-Y., Kim, S.-R., Ha, T.-Y., 2006.
Protective effect of Polygonum multi orum Thunb on amyloid
-peptide 25-35 induced cognitive de cits in mice. Journal of
ethnopharmacology 104, 144-148.
Umamah eswari, M., Chatterjee, T., 2008. In vitro antioxidant
activities of the fractions of Coccinia grandis L. leaf extract.
African Journal of Traditional, Complementary and Alterna-
tive Medicines 5, 61-73.
Wu, G. , Fang, Y.-Z., Yang, S., Lupton, J.R., Turner, N.D., 2004.
Glutathione metabolism and its implications for health. The
Journal of nutrition 134, 489-492.
Zhao,
Y., Wang, J., Ballevre, O., Luo, H., Zhang, W., 2012. Anti-
hypertensive effects and mechanisms of chlorogenic acids.
Hypertension Research 35, 370-374.