
Microbiological
Communication
Biosci. Biotech. Res. Comm. 10(2): 155-159 (2017)
Antibiotic resistance pattern of Shiga-toxigenic
Escherichia coli
isolated from ready-to-eat food stuffs
Mohammad Hossein Sakhaie Shahreza
1
, Ebrahim Rahimi
2
* and Hassan Momtaz
3
1
Student of Veterinary Medicine, College of Veterinary Medicine, Shahrekord Branch, Islamic Azad
University, Shahrekord, Iran
2
Department of Food Hygiene and Public Health, College of Veterinary Medicine, Shahrekord Branch, Islamic
Azad University, Shahrekord, Iran
3
Department of Microbiology, College of Basic Sciences, Shahrekord Branch, Islamic Azad University,
Shahrekord, Iran
ABSTRACT
Shiga toxigenic Escherichia coli are the most important causes of food-borne diseases due to the consumption of
contaminated ready to eat foods. The present investigation was done to study the prevalence rate and antibiotic
resistance pattern of STEC strains recovered from various types of ready to eat foods. Seven-hundred and twenty
various types of food samples were collected and cultured. Isolated E. coli strains were approved another time using
PCR. Approved colonies were tested for antibiotic susceptibility using the disk diffusion. Twenty-six out of 720 food
samples (5.20%) were positive for E. coli strains. Prevalence of STEC strains were 1.52%. Salad (15%) had the highest
prevalence of bacteria, while hamburger (2.50%) had the lowest. STEC strains exhibited the highest levels of resist-
ance against tetracycline (100%), ampicillin (100%), gentamicin (81.81%) and cipro oxacin (72.72%), while exhibited
the lowest against chloramphenicol (27.27%) and cotrimoxazole (45.45%). High prevalence of resistant STEC strains
in ready to eat foods showed irregular prescription of antibiotic as well as lack of proper hygiene in restaurant and
fast food centers. Cautious prescription of antibiotics and attentions to the principles of food security can decrease
the risk of resistant STEC strains in ready to eat foods.
KEY WORDS: SHIGA TOXIGENIC
ESCHERICHIA COLI
, PREVALENCE, ANTIBIOTIC RESISTANCE, READY TO EAT FOODS
155
ARTICLE INFORMATION:
*Corresponding Author: Ebrahimrahimi55@yahoo.com
Received 15
th
May, 2017
Accepted after revision 17
th
June, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/

156 ANTIBIOTIC RESISTANCE PATTERN OF SHIGA-TOXIGENIC
ESCHERICHIA COLI
ISOLATED BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Shahreza, Rahimi and Momtaz
INTRODUCTION
Food hygiene in restaurants and fast-foods are one of
the most important critical issues in the society. Using
from low quality raw materials, cooking of foods more
than daily requirement and their storage in unsuitable
conditions and nally lack in the observation of proper
hygiene in cooking of foods are the main factors
causing enhancement of microbial spoilage and growth
of dangerous food-borne pathogens in foods (Isara and
Isah, 2009). Among all pathogenic agents causing food-
borne diseases and food poisoning, Escherichia coli
(E. coli) strains had a signi cant importance (Momtaz
et al. 2012; Momtaz et al. 2013). is a gram-negative,
non-sporulating, agellated, rod-shaped and faculta-
tive anaerobic bacterium which belongs to Enterobac-
teriaceae family. Shiga (vero) toxin (Stx)-producing E.
coli (STEC) is a subdivision of an important pathogenic
group of this bacterium named enterohemorrhagic E.
coli (EHEC) (Momtaz et al. 2012; Momtaz et al. 2013;
Dehkordi et al. 2014). STEC strains are responsible for
intensive clinical syndromes like lethal hemolytic ure-
mic syndrome (HUS), bloody and non-bloody diarrhea,
thrombotic thrombocytopenic purpura (TTP) and hemor-
rhagic colitis (HC) (Momtaz et al. 2012; Momtaz et al.
2013; Dehkordi et al. 2014 and Ranjbar et al. 2017).
High levels of resistance in STEC strains is another
important factor which increase the pathogenicity of
bacteria. Unfortunately, STEC strains recovered from
food stuffs and also cases of diarrhea and food poison-
ing harbored the high levels of resistance against com-
monly used groups of antibiotics including quinolones,
aminoglycosides, macrolides, cephalosporins, sulfona-
mides, uoroquinolones and tetracycline (Momtaz et
al. 2013b; Momtaz et al. 2013c; Stewardson et al.
2014; Amézquita-López et al. 2016). In the other hands,
STEC strains of food poisoning show a high incidence
of resistance (85-100%) against commonly used anti-
microbial agents (Momtaz et al. 2013b; Momtaz et al.
2013c; Stewardson et al. 2014; Amézquita-López et al.
2016).
MATERIALS AND METHODS
The study was approved by the Ethical Committee of
Islamic Azad University, Shahrekjord Branch (Consent
Ref Number IAU 2053). Veri cation of this research
project and the licenses related to sampling process were
approved by the Prof. EbrahimRahimi (Approval Ref
Number Food-Hygiene 95 2020). From September 2013
to September 2014, a total of 720 various types of ready-
to-eat foods including sausage (n=70), salami (n=70),
hamburger (n=60), roast mouthful (n=60), traditional
dressing (n=65),traditional salad (n=60), traditional
candy (n=60), traditional ice-cream (n=60), barbecue
(n=70), soup (n=75) and spices (n=70) were randomly
collected from various restaurant in the Isfahan prov-
ince, Iran. Samples were immediately transferred to lab-
oratory in cooler with ice-packs.
Totally, 10-g of crushed food samples were homog-
enized for 2 min in 90 ml of Peptone Water (PW,
Merck, Germany). Then the samples were cultured on
5% sheep blood and MacConkey agar (Merck, Ger-
many) and incubated for 18 to 24 h at 37
o
C. Colo-
nies with the typical color and appearance of E. coli
were picked and streaked again on blood agar plates
and re-streaked on EMB agar (Merck, Germany). All
plates were further incubated for 24 h at 37
o
C. The
green metallic sheen colonies were considered as E.
coli. The presumptive colonies were biochemically
tested for growth on triple sugar iron agar (TSI) and
lysine iron agar (LIA), oxidative/fermentative degrada-
tion of glucose, citrate utilization, urease production,
indol fermentation, tryptophan degradation, glucose
degradation (methyl red test) and motility. Bacterial
strains were sub-cultured overnight in Luria-Bertani
broth (Merck, Germany) and further incubated for 48
h at 37
o
C. Genomic DNA was extracted from bacterial
colonies using the DNA extraction kit (Fermentas, Ger-
many) according to manufacturer’s instruction. Bac-
terial colonies were further con rmed using the 16S
rRNA-based Polymerase Chain Reaction (PCR) (Woo
et al. 2001) Set of primers used for this purpose are
Forward: 5’-AGTTTGATCCTGGCTCAG-3’ and Reverse:
5’-AGGCCCGGGAACGTATTCAC-3’ (1343 bp). Preva-
lence of STEC strains was determined according to the
method described by SafarpoorDehkordi et al. (2014)
and Momtaz et al. (2013a).
Pattern of antimicrobial resistance was studied using
the simple disk diffusion technique on Mueller–Hinton
agar (Merck, Germany). Susceptibility of STECstrains
were tested against tetracycline (30 u/disk), ampicillin
(10 u/disk), cefotaxime (30 μg/disk), gentamycin (10 μg/
disk), cipro oxacin (5 μg/disk), cotrimoxazole(30 μg/
disk), enro oxacin(5 μg/disk), trimethoprim(5 μg/disk),
and chloramphenicol (30 μg/disk) antibiotic agents
(Oxoid, UK). All of the inoculated plates were aerobically
incubated at 37 °C for 18-24 h. Results were interpreted
based on the instruction provided by CLSI (2012) (Wayne
2012). E. coli ATCC 25922 was used as quality control.
Statistical analysis was performed using SPSS/16.0 soft-
ware for signi cant relationships. The incidence of anti-
biotics resistance of STEC isolated from various types
of ready-to-eat food samples were statistically analyzed.
Statistical signi cance was regarded at a value < 0.05.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTIBIOTIC RESISTANCE PATTERN OF SHIGA-TOXIGENIC
ESCHERICHIA COLI
ISOLATED 157
Shahreza, Rahimi and Momtaz
RESULTS AND DISCUSSION
The present investigation was done in order to assess
the prevalence of E. coli and STEC strains as well as
study the antibiotic resistance pattern of isolated bacte-
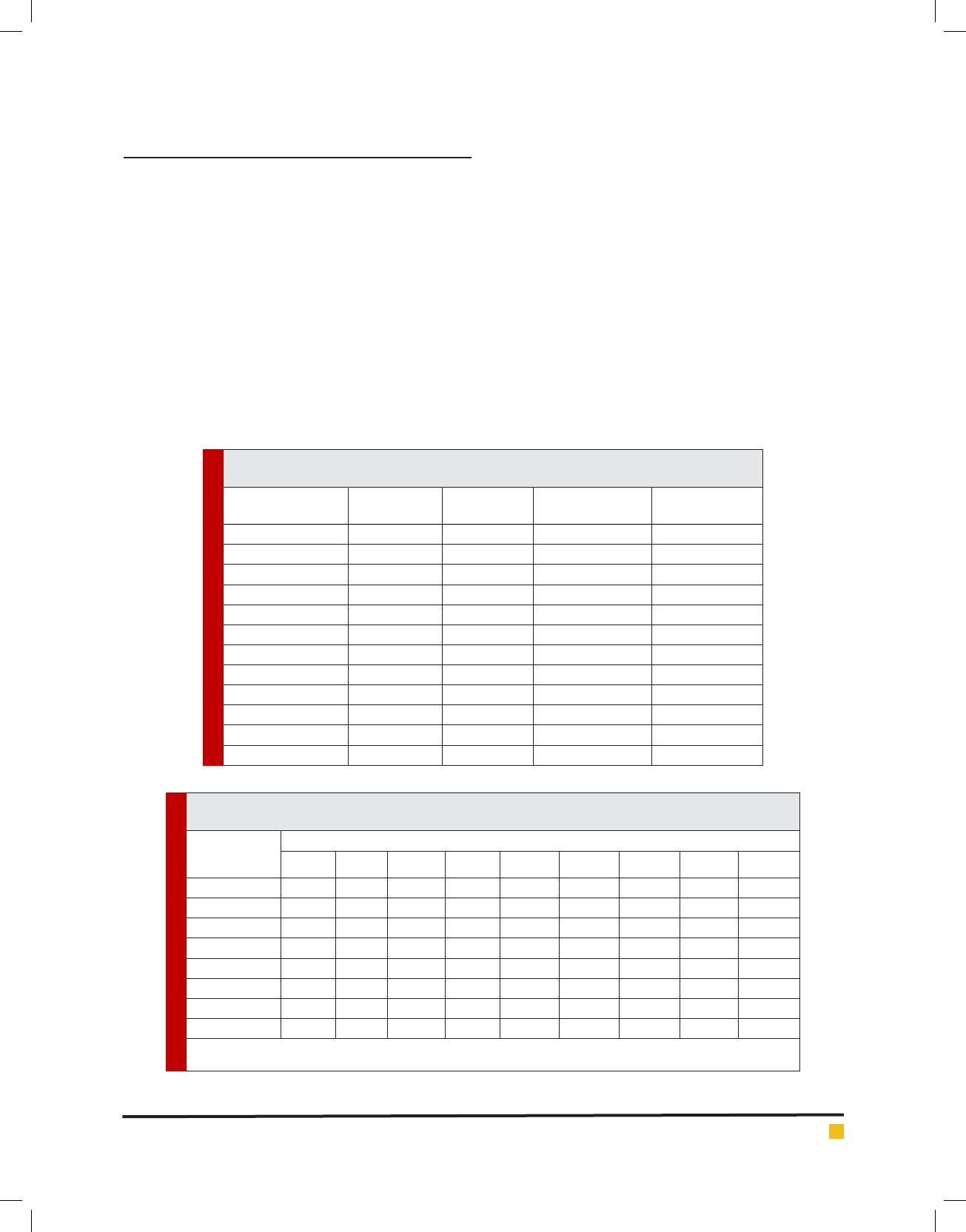
ria. Table 1 represents the total prevalence of E. coli and
STEC strains isolated from various types of ready to eat
foods. Twenty-six out of 720 food samples (5.20%) were
positive for E. coli strains. Among all 26 E. coli strains,
11 strains (42.30%) were considered as STEC strains.
Salad (15%), candy (12.50%) and barbecue (10%) were
the most commonly contaminated samples. There were
no positive results for Sausage, salami, roast mouthful
and soup samples. Statistically signi cant differences
were seen between the types of samples and prevalence
of STEC strains (<0.05).
Table 2 represents the antibiotic resistance pattern of
STEC strains isolated from various types of ready to eat
food samples. We found that STEC strains harbored the
highest levels of resistance against tetracycline (100%),
ampicillin (100%), gentamicin (81.81%) and cipro oxacin
(72.72%). Resistance against chloramphenicol (27.27%)
and cotrimoxazole (45.45%) were low. Statistically signif-
icant differences were seen between the types of samples
and prevalence of antibiotic resistance (p<0.05).
The present investigation showed that resistant STEC
strains had a considerable prevalence in various types of
ready to eat food samples. Total prevalence of E. coli and
also STEC strains among the food samples of our study
were 5.20% and 1.52%, respectively which emerged an
important public health issue regarding the consump-
tion of ready to eat foods.
Table 1. Total prevalence of Escherichia coli and also STEC strains in various types of ready
to eat food samples.
Types of samples
No. samples
collected
No positive
strains (%)
PCR con rmation
(%)
STEC strains (%)
Sausage 70 - - -
Salami 70 - - -
Hamburger 60 1 (2.50) 1 (2.50) 1 (100)
Roast mouthful 60 - - -
Dressing 65 4 (8) 4 (8) 1 (25)
Salad 60 6 (15) 6 (15) 2 (33.33)
Candy 60 5 (12.50) 5 (12.50) 1 (20)
Ice cream 60 2 (5) 2 (5) 2 (100)
Barbecue 70 5 (10) 5 (10) 2 (40)
Soup 75 - - -
Spices 70 3 (5) 3 (5) 2 (66.66)
Total 720 26 (5.20) 26 (5.20) 11 (42.30)
Table 2. Total distribution of antibiotic resistance pattern of STEC strainsisolated from various types of
ready to eat food samples.
Samples (No.
STEC strains)
Antibiotic resistance pattern (%)
Tet* Amp Cef Gen Cip Cot Enr Tri C30
Hamburger (1) 1 (100) 1 (100) 1 (100) 1 (100) 1 (100) 1 (100) 1 (100) 1 (100) 1 (100)
Dressing (1) 1 (100) 1 (100) 1 (100) 1 (100) 1 (100) - - - -
Salad (2) 2 (100) 2 (100) 1 (50) 1 (50) 1 (50) 1 (50) 1 (50) 1 (50) -
Candy (1) 1 (100) 1 (100) - 1 (100) 1 (100) - 1 (100) 1 (100) -
Ice cream (2) 2 (100) 2 (100) 1 (50) 2 (100) 1 (50) 1 (50) 1 (50) 1 (50) -
Barbecue (2) 2 (100) 2 (100) 2 (100) 2 (100) 2 (100) 1 (50) 1 (50) 1 (50) 2 (100)
Spices (2) 2 (100) 2 (100) 1 (50) 1 (50) 1 (50) 1 (50) 1 (50) 1 (50) -
Total (11) 11 (100) 11 (100) 7 (63.63) 9 (81.81) 8 (72.72) 5 (45.45) 6 (54.54) 6 (54.54) 3 (27.27)
*Tet: tetracycline (30 u/disk), Amp: ampicillin (10 u/disk), Cef: cefotaxime (30 μg/disk), Gen: gentamycin (10 μg/disk), Cip: cipro oxacin
(5 μg/disk), Cot: cotrimoxazole(30 μg/disk), Enr: enro oxacin(5 μg/disk), Tri: trimethoprim(5 μg/disk), C30: chloramphenicol (30 μg/disk).

158 ANTIBIOTIC RESISTANCE PATTERN OF SHIGA-TOXIGENIC
ESCHERICHIA COLI
ISOLATED BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Shahreza, Rahimi and Momtaz
There were some likelyexplanations for the high preva-
lence of E. coli and also STEC strains in the ready to eat
food samples. At rst. high-volume food production and
long process of catering caused several problems such as
lack of adequate precision and accuracy in the preparation
and washing of raw materials and even their well cook-
ing, lack of enough time and even temperature for cooking
of raw materials, cooling of foods during processing, lack
of reaching of suf cient heat to the center of meat and
other food materials, lack of enough time to withdraw
meat from the frozen state and nally cooking of meat
and its products more than the daily requirement and
then their storage at improper temperature and conditions.
These mentioned circumstances maybe lead to survival
and even growth of pathogenic microorganisms in ready
to eat foods. At second, using from unsanitary and also
contaminated equipment and dishes for production of
foods in restaurant and fast food centers. At third, presence
of infected staffs which maybe the sources of dangerous
pathogenic agentsin the food processing stage (Shahrani et
al. 2014; Hemmatinezhad et al. 2015; Ranjbar et al. 2017).
Sausage, salami and roast mouthful are mainly
produced in high temperature and also they have been
presented in a hygienic package. Therefore, it is not
surprising that all of these samples were free from E.
coli. High temperature using for cooking of soup make
it clean from any pathogenic agents like E. coli. Because
the large numbers of E. coli isolates recovered from raw
meat, proper preparation of the raw meat can eliminate
the distribution of bacteria. Unfortunately, principles of
meat inspections were not observed in Iranian slaughter-
houses. Therefore, close contact of animal carcasses with
each other and even slaughterhouse oor, blood, content
of the digestive tract and wool and skin of slaughtered
animal caused transmission and distribution of patho-
genic agents like E. coli to meat of slaughtered animals.
Besides, the role of possible colonizers such as meat
inspectors, butchers and miscellaneous people which
mainly have come into the slaughterhouse for buying of
meat and nally animals like rats, cats and birds which
have been entered from outside the slaughterhouse as a
sources of pathogenic E. coli should not be overlooked.
Survival of STEC strains of raw food samples even after
cooking procedure and occurrence of cross contamination
after cooking procedure are two important routes of
hospital foods contamination (Shahrani et al. 2014;
Hemmatinezhad et al. 2015; Ranjbar et al. 2017).
High prevalence of resistance against commonly used
antibiotic agents is another important nding of this
study. We found that STEC strains harbored the high
levels of resistance against tetracycline, ampicillin, cefo-
taxime, gentamycin, cipro oxacin, cotrimoxazole, enro-
oxacin, trimethoprim and chloramphenicol antibiotics.
Indiscriminate and unauthorized prescription of
antibiotics especially in the eld of veterinary caused
such high prevalence of antibiotic resistance in STEC
strains recovered from foods (Shahrani et al. 2014;
Hemmatinezhad et al. 2015; Ranjbar et al. 2017).
Several studies have been done in this eld in various
parts of the world. Miri et al. (2014) reported that from a
total of 190 food samples, four samples (2.1%) were con-
taminated withE. coli. All of theE. coli strains were iso-
lated from hamburger samples (3.3%). They found that
all isolates (100%) were resistant to one or more anti-
microbial agents and especially tetracycline and ampi-
cillin.Srinivasan et al. (2007) showed that all of the E.
coli strains of food samples exhibited resistance to ve
or more antimicrobial agents and especially ampicillin,
aztreonam, cefaclor, cephalothin, cinoxacin, and nalid-
ixic acid which was similar to us. Kalantar et al. (2013)
reported that a total of 87 E. coli strains were detected
from 466 rectal swabs from children with acute diarrhea
and 40 E. coli strains were detected from the 125 frozen
food samples of animal origin. They showed that con-
sumption of contaminated foods may play an impor-
tant role in occurrence of E. coli infections in children.
Stewardson et al. (2014) reported that the prevalence of
susceptibility of E. coli strains of food samples against
meropenem, gentamicin, cipro oxacin, cotrimoxazole,
and fosfomycinin Switzerland were 100%, 90%, 87%,
79%, and 98%, respectively.
CONCLUSION
In conclusions, we identi ed a large number of STEC
strains which were resistant against several types
of antibiotic agents. Resistance against ampicillin,
gentamycin andtetracycline and presence of multi-
drug resistant strains were the most commonly detected
characters in the STEC strains of ready to eat foods. It
seems that there were no severemanagements on the
princ iples of food hygiene in Iranian restaurant and fast
food centers. Due to the low levels of STEC resistance
against chloramphenicol and cotrimoxazole, occurrence
of food poisonings due to the STEC strains in tested
Iranian restaurant and fast food centers can be treated
with their regular prescription. Attentions to the results
of disk diffusion method and principles of hazard analy-
sis and critical control point (HACCP) system can reduce
the risk of STEC strains in food stuffs.
ACKNOWLEDGEMENTS
The authors would like to thank Prof. Amir Shakerian
at the Department of Food Hygiene, Shahrekord Branch,

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS ANTIBIOTIC RESISTANCE PATTERN OF SHIGA-TOXIGENIC
ESCHERICHIA COLI
ISOLATED 159
Shahreza, Rahimi and Momtaz
Isamic Azad University, Shahrekord, Iran for his impor-
tant technical support. This work was supported by
the Islamic Azad University, Shahrekjord Branch (Ref
Number IAU-Bud-177)
REFERENCES
Amézquita-López, B. A., Quiñones, B., Soto-Beltrán, M., Lee, B.
G., Yambao, J. C., Lugo-Melchor, O. Y. and Chaidez, C. (2016):
Antimicrobial resistance pro les of Shiga toxin-producing
Escherichia coli O157 and Non-O157 recovered from domestic
farm animals in rural communities in Northwestern Mexico.
Antimicrob Resist Infect Control. 5, 1.
Dehkordi, F. S., Yazdani, F., Mozafari, J. and Valizadeh, Y.
(2014): Virulence factors, serogroups and antimicrobial resist-
ance properties of Escherichia coli strains in fermented dairy
products. BMC Res Note. 7, 1.
Hemmatinezhad, B., Khamesipour, F., Mohammadi, M., Safa-
rpoor Dehkordi, F. and Mashak, Z. (2015): Microbiological
Investigation of O‐Serogroups, Virulence Factors and Anti-
microbial Resistance Properties of Shiga Toxin‐Producing
Escherichia coli Isolated from Ostrich, Turkey and Quail Meats.
J Food Safety. 35, 491-500.
Isara, A. R. and Isah, E. C.(2009): Knowledge and practice of food
hygiene and safety among food handlers in fast food restaurants
in Benin City, Edo State. Niger Postgrad Med J. 16, 207-12.
Kalantar, E., Alikhani,M. Y., Naseri, M. H. andTorabi, V. (2013):
Antibiotic resistance patterns of STEC and ETEC strains: A
study on frozen foods of animal origin and children with acute
diarrhea. J MicrobiolInfect Dis.3, 31-35.
Miri, A., Rahimi, E.,Mirlohi, M., Mahaki, B., Jalali, M. and-
GhasemianSafaei, H. (2014): Isolation of shiga toxin-producing
Escherichia coli O157:H7/ NM from hamburger and chicken
nugget. IntJ Environ Health Eng. 3, 19-23.
Momtaz, H., Dehkordi, F. S., Hosseini, M. J., Sarshar, M. and
Heidari, M. (2013a): Serogroups, virulence genes and antibiotic
resistance in Shiga toxin-producing Escherichia coli isolated
from diarrheic and non-diarrheic pediatric patients in Iran. Gut
Pathog. 5, 1.
Momtaz, H., Dehkordi, F. S., Rahimi, E., Ezadi, H. and Arab, R.
(2013b): Incidence of Shiga toxin-producing Escherichia coli
serogroups in ruminant’s meat. Meat Sci. 95, 381-8.
Momtaz, H., Farzan, R., Rahimi, E., Safarpoor Dehkordi, F. and
Souod, N. (2012): Molecular characterization of Shiga toxin-
producing Escherichia coli isolated from ruminant and donkey
raw milk samples and traditional dairy products in Iran. Sci
World J. 2012, 231342.
Momtaz, H., Karimian, A., Madani, M., Dehkordi, F. S., Ran-
jbar, R., Sarshar, M. and Souod, N. (2013c): Uropathogenic
Escherichia coli in Iran: serogroup distributions, virulence fac-
tors and antimicrobial resistance properties. Ann Clin Micro-
biol Antimicrob. 12, 1.
Ranjbar, R., Masoudimanesh, M., SafarpoorDehkordi, F.,
Jonaidi-Jafari, N. andRahimi, E. (2017): Shiga (Vero)-toxin
producing Escherichia coli isolated from the hospital foods;
virulence factors, o-serogroups and antimicrobial resistance
properties. Antimicrob Res Infect Control. 6, 4.
Shahrani, M., Dehkordi, F. S.and Momtaz, H.(2014): Charac-
terization of Escherichia coli virulence genes, pathotypes and
antibiotic resistance properties in diarrheic calves in Iran. Biol
Res. 47, 28.
Srinivasan, V., Nguyen, L. T., Headrick, S. I., Murinda, S. E. and
Oliver, S. P.(2007): Antimicrobial resistance patterns of Shiga
toxin-producing Escherichia coli O157:H7 and O157:H7- from
different origins. Microb Drug Resist. 13, 44-51.
Stewardson, A. J., Renzi, G., Maury, N., Vaudaux, C., Brassier,
C., Fritsch, E., Pittet, D., Heck, M., van der Zwaluw, K. and Reu-
land, E. A. (2014): Extended-Spectrum -Lactamase–Produc-
ing Enterobacteriaceae in Hospital Food: A Risk Assessment.
Infect Control Hosp Epidemiol. 35, 375-83.
Wayne, P. (2012): Clinical and Laboratory Standards Institute
(CLSI). Performance standards for antimicrobial susceptibility
testing. Twenty-second informational supplement M100-S21.
Wayne Pa.
Woo. P. C., Cheung, E. Y., Leung, K. W. and Yuen, K. Y. (2001):
Identi cation by 16S ribosomal RNA gene sequencing of an
Enterobacteriaceae species with ambiguous biochemical pro-
le from a renal transplant recipient. Diagn Microbiol Infect
Dis. 39, 85-93.