
Pathological
Communication
Biosci. Biotech. Res. Comm. 10(2): 88-92 (2017)
Relationship between stress, anxiety, depression and
salivary IgA with periodontal disease
Mohammadreza Karimi
1
, Shahryay Elyahoo
2
*, Leyla Golchin
3
and Tahereh Kermani
4
1
Assistant Professor, Periodontics Department, Dental Branch, Islamic Azad University, Tehran, Iran
2
Post graduate student, Periodontics Department, Dental Branch, Islamic Azad University, Tehran, Iran
3
General Dentist
4
Psychiatrist
ABSTRACT
Psychological stress, if sustained over an extended period of time can have deleterious effects on the body. Psycho-
logical stress has been implicated as risk indicators for periodontal disease. So, the aim of the current study was to
determine the relationship of stress, anxiety and depression level and salivary IgA with periodontal disease. A total
30 patients who referred to periodontology included to the study and divided into 2 experimental groups. Group
control patients do not suffer from any periodontal disease and patients CAL up 3 millimeters or more and BOP in
upper teeth (case group). All cases were evaluated for stress, anxiety and depression level by DASS42 test. Salivary
samples were obtained using spitting method and IgA level is determined with ELISA. Data was analyzed by 2 and
Mendle-hazel tests. The patients suffering from periodontal disease were not encountering higher level of stress and
anxiety (P=0.3). People suffering periodontal disease (86.7%) were depressed while 60% of people neither periodontal
disease nor depressed (P=0.1). A signi cant difference detected in salivary IgA level in control group (312.66+107.3)
compared to case group (207.95+57.21) (P=0.001). Conclusion: these results suggested a correlation exists between
incidence of the periodontal disease and immunity.
KEY WORDS: PERIODONTAL DISEASE, STRESS, ANXIETY, DEPRESSION, IGA
88
ARTICLE INFORMATION:
*Corresponding Author:
Received 10
th
Jan, 2017
Accepted after revision 10
th
May, 2017
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007 CODEN: USA BBRCBA
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2017: 4.31 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2017. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
Mohammadreza Karimi et al.
INTRODUCTION
Periodontal disease is a multifactorial disease. Dental
plaque which harbours speci c periodontal pathogens is
its main aetiologic factor. Numerous factors have been
associated with periodontitis, such as systemic diseases,
genetic polymorphisms, socio-economic and/or educa-
tional status, smoking and psychological stress. Stress is
considered as one of the essential factors with etiology
of the periodontal disease (Boyapati and Wang, 2007).
Psychological stress down regulates the cellular immune
response (Castro et al. 2006). There is interconnection
among the central nervous system and the immune sys-
tem which happens via a complex network of bidirec-
tional signals linking the nervous, endocrine, and immune
systems (Gundala et al. 2012 Shende et al. (2016).
Chronic stress has a net negative effect on the
immune response which leads to an imbalance between
host and parasites and consequently resulting in perio-
dontal break down (Radafshar et al. 2012). The potential
relationship between stress and oral in ammatory infec-
tious diseases is known for many years. A wide range of
biomarkers is measurable in saliva, including hormones
and their metabolites, enzymes, immunoglobulins (IgA),
other proteins (eosinophil cationic protein) and DNA
(Koh et al. 2007). Anxiety, depression, burnout and staff
turnover are correlated with several salivary biomarkers.
Chronic stress is associated with the activation of the
hypothalamic–pituitary–adrenal (HPA) axis, as well as
with the depression of immune function, mainly salivary
IgA and lysozyme (Heinrichs et al. 2005).
Periodontal disease and its progression associated with
psychosocial, nancial stress and depression (Rosania et al.
2009). However, there are reports no association between
stress and periodontal disease. Therefore, more research is
needed to get a clearer understanding of this relationship
(Rai et al. 2011). So, the aim of the current study was to
determine the relationship of stress, anxiety and depres-
sion level and salivary IgA with periodontal disease.
MATERIAL AND METHODS
PATIENTS
For this case-control study, 30 patients (15 male and 15
female) referred to periodontology department, Islamic
Azad University, Dental Branch, Tehran, Iran during
2015 were included. The average age for the patients
was 42-44 years old. All patients were informed about
the study and signed the agreement form.
STUDY PROTOCOL
Two types of the patients were included into the study.
The criteria for control group were the patients who not
being periodontal disease and the case group were the
patients who suffer periodontal disease. Then patients
were divided into 2 experimental groups (n=15 in each).
Group control patients do not suffer from any periodon-
tal disease and patients CAL up 3 millimeters or more
and BOP in upper teeth (case group). The probing pocket
depth (PPD), clinical attachment level (CAL), plaque
index (PI), Gingival Index (GI) and bleeding on probing
(BOP) examinations were done. All cases were evaluated
for stress, anxiety and depression level by DASS42 test.
Salivary samples were obtained using spitting method.
Brie y, patients collected the oral cavity saliva for 5
minutes; collected into a sterile container ant -20˚C and
then IgA level is determined with enzyme-linked immu-
nosorbent assays (ELISA) detecting kits. To minimize
experimental error, all experimental procedure was done
at 11:00 AM until 13:00 PM.
STATISTICAL ANALYSIS
Data analyzed by repeated measure two-way analysis of
variance (ANOVA) using SPSS 16.0 for Windows (SPSS,
Inc., Chicago, IL, USA). For treatment showing a main
effect by ANOVA, means were compared by 2 and
Mendle-hazel tests. Data is presented as mean ± stand-
ard deviation. P<0.05 was considered as signi cant dif-
ferences between treatments.
RESULTS
The demographic information of the patients included
into the study is presented in the table 1. According to
the results, there was no signi cant difference for gen-
der, age and educational level among the control (with-
out periodontal disease) and case (with periodontal dis-
ease) groups.
As seen in table 2, no signi cant difference detected
for PDD and PI indexes in control compared to the
Table 1. the demographic information of the patients included into the study
Gender Age (years) Education level
Periodontal disease Male Female Diploma < Diploma University
Control (n=15) 9 6 42.4±5.4 8 6 1
Case (n=15) 9 6 44.53±8.4 3 10 2
P value 0.09 0.4 0.8
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS RELATIONSHIP BETWEEN STRESS, ANXIETY, DEPRESSION AND SALIVARY IGA WITH PERIODONTAL DISEASE 89

Mohammadreza Karimi et al.
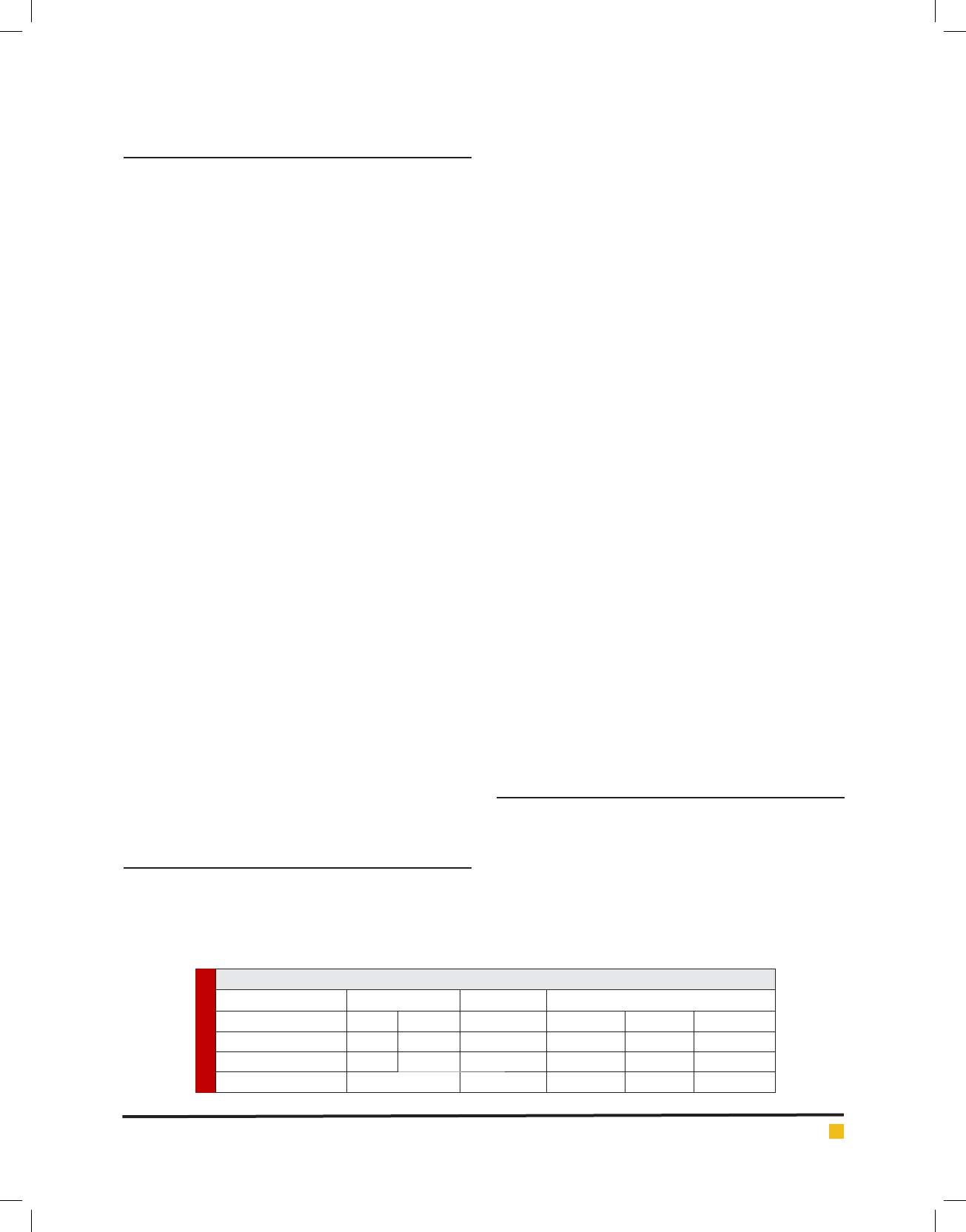
Table 2. The frequency of the Periodontal disease index
among patients
Experimental groups
Control (n=15) Case (n=15) P value
PPD (mm) 1.8 ± 0.7 2.7 ± 0.4 P=0.05
CAL (mm) 0 3.002 ± 0.6 P=0.001
PI (%) 61.8 ± 27.2 75.6 ± 22.9 P=0.4
GI 1.02 ± 0.5 2.1 ± 0.7 P=0.001
BOP (%) 10.3 ± 10.5 20.5 ± 10.4 P=0.001
PPD: probing pocket depth; CAL: clinical attachment level; PI: plaque
index; GI: Gingival Index; BOP: bleeding on probing.
Table 3. The correlation between stress, depression and salivary IgA levels with periodontal disease
Experimental groups
Factors Control (n=15) Case (n=15) P value
Stress
No 5 (33.3) 4 (26.6)
P=0.9
Yes 10 (66.7) 11 (73.4)
Anxiety
No 6 (40) 3 (20)
P=0.3
Yes 9 (60) 12 (80)
Depression
No 6 (40) 2 (13.3)
P=0.1
Yes 9 (60) 13 (86.6)
Salivary IgA - 312.66+107.3 207.95+57.21 P=0.001
case groups, P=0.05 and P=0.4, respectively. However,
a signi cant difference detected for CAL (P=0.001),
GI (P=0.001) and BOP (P=0.001) indexes between two
groups.
The correlation between stress, anxiety, depression
and salivary IgA levels with periodontal disease is pre-
sented in table 3. According to the results, there was no
signi cant difference on stress (P=0.9), anxiety (P=0.3)
and depression (P=0.1) in control compared to the case
groups. However, a signi cant difference detected in
salivary IgA levels in control compared to the case
groups (P=0.001).
DISCUSSION
Periodontitis is an in ammatory disease caused by peri-
odontopathic bacteria in the dental bio lm, leading to
destruction of the tooth supporting tissues. Systemic dis-
eases, habits, social factors, and psychological stress are
considered risk factors in uencing disease incidence and
progression. Although psychological stress was found to
be an important risk factor for periodontitis, the biologic
mechanisms of its implication for disease progression is
not fully elicited (Haririan et al. 2012).
In this study patients suffering from periodontal dis-
ease were not encountering higher level of stress and
anxiety. People suffering periodontal disease (86.7%)
were depressed while 60% of people neither periodontal
disease nor depressed. There are several factors included
age, smoking, systemic diseases and psychological stress
which are important risk factors for periodontitis (Ishi-
saka et al. 2007). In this regard, De Marco (1976) coined
the term “Periodontal Emotional Stress Syndrome” for
individuals with severe periodontitis who had emotional
stress associated with active service in Vietnam suggest-
ing a role of occupational stress in the progression of
periodontitis.
Periodontitis patients with inadequate stress behav-
iors strategies were suggested to be at higher risk for
severe periodontal diseases (Wimmer et al. 2002). Lit-
erature reported the relationship between stress, depres-
sion and periodontal disease are signi cant because they
address critical areas of interest surrounding an impor-
tant perio–systemic connection (Peruzzo et al. 2007).
During stress, the HPA axis and sympathetic nervous
system interact which leads to up-surge of the glucocor-
ticoid and increases the susceptibility of the periodon-
tal diseases (Bansal et al. 2014). The mediating effect
of chronic stress on periodontium can be explained by
diminished immune response which causes release of
local neuropeptides such as substance P and neurokinin
A, may provide a mechanism for neural modi cation
of in ammatory changes in the periodontium (Linden
et al. 1998).
In the current study, no signi cant difference detected
for the PDD and PI indexes in control compared to the
case group while a signi cant difference detected for
CAL, GI and BOP indexes between two groups. Shende et
al. (2016) no signi cant differences showed on PI, prob-
ing depth, CAL among the subjects with varying levels
of stress. There was no statistical signi cance for stress
to be contributing toward the periodontal disease. Saliva
testing is a novel, quick, painless, non-invasive diag-
nostic method for various diseases, in particular for oral
diseases (Giannobile et al. 2009). There are numerous
speci c and nonspeci c biomarkers affecting the bio lm
which is a plaque formed by aggregates of proteins and
90 RELATIONSHIP BETWEEN STRESS, ANXIETY, DEPRESSION AND SALIVARY IGA WITH PERIODONTAL DISEASE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS

Mohammadreza Karimi et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS RELATIONSHIP BETWEEN STRESS, ANXIETY, DEPRESSION AND SALIVARY IGA WITH PERIODONTAL DISEASE 91
micro bacterium. The major speci c defense factors of
saliva are the IgA, IgG, and IgM (Sindhu and Jagan-
nathan, 2014).
The adherence of bacteria and bacterial metabolism
interfered in presence of the immunoglobulins, particu-
larly with the IgA type. Many biomarkers have been
found in saliva and some of these were shown to be
involved in periodontal disease (Giannobile et al. 2009).
The salivary concentrations of these immunoglobulins
increase in periodontitis which decreases following peri-
odontal therapies (Sindhu and Jagannathan, 2014). As
seen in this study, salivary IgA decreased in periodontal
patients compared to the normal people (without peri-
odontal disease). These results suggested a correlation
exists between incidence of the periodontal disease and
immunity. In condition pain, anxiety and stress, many
metabolic and endocrine changes occur in the body,
the most common effect of which is increased cortisol
level in the blood. It plays a role in the regulation of the
immune system and vascular reactions. Also known as
the stress hormone, cortisol is a decisive index in stress-
ful situations (Ismail et al. 2007). One of the likely mech-
anisms of such relationship is activation of the HPA axis
following stressful life events, which leads to elevation
of cortisol concentrations in gingival crevicular uid,
serum, and other body uids. Leukocytic, chemotactic,
polymorphonuclear leukocytes, IgG production and sali-
vary IgA secretion are considerably declined by contin-
ued elevation in cortisol concentrations, placing the host
in an immunosuppressive status, hence more vulnerable
to periodontal infection and breakdown (Wong, 2008).
The IgA level measurement is a reliable method to
determine the immune system function (Shah et al. 2009).
Chronic stress decreases the immune system performance
and suppresses the immunoglobulin production. So, the
IgA levels play a role in the pathogenesis of oral mucosa
and its associated clinical changes (Rabiei et al. 2012).
The IgA and IgG levels Increased in patients with lichen
planus (Sistig et al. 2002) and lichenoid reaction Lesions
(Ghalayani et al. 2009). Also, Sato et al. (1991) studies
compared the salivary IgA level in patients with differ-
ent oral diseases using the ELISA method. An increase
in the salivary IgA level was observed in patients with
oral leukoplakia, oral lichen planus and carcinoma of
the oral cavity. Higher IgA levels facilitate the antigen
supply by Langerhans cells, make changes in the basal
layer destruction and dispatch immune cells to the area
(Nosratzehi et al. 2014). Furthermore, Salivary IgA lev-
els in uenced by mental stress and several studies have
suggested a negative correlation between the levels of
salivary IgA and stress (Fukui et al. 2010). Despite the
mechanism for how Salivary IgA affects by mental stress
is not fully elicited, it is reported Chromogranin A is
an acidic glycoprotein which is released along with cat-
echolamines from the adrenal medulla and the sympa-
thetic nerve endings, and has been receiving attention
as a novel stress marker in the saliva (Fukui et al. 2010).
In conclusion these results suggested a correlation
exists between incidence of the periodontal disease and
immunity. We think obtained results can use as informa-
tion for clinical applications in human therapies. Also,
we think further researches is needed to determine the
direct molecular and cellular mechanism for observed
data.
REFERENCES
Bansal, J., Bansal, A., Shahi, M., Kedige, S. and Narula, R. 2014
Periodontal Emotional Stress Syndrome:Review of Basic Con-
cepts, Mechanism and Management. Open Journal of Medical
Psychology, 3, 250-261.
Castro GDC, Oppermann RV, Haas AN, Winter R, Alchieri JC:
2006. Association between Psychosocial factors and Periodon-
titis. J Clin Periodontol 33:109-114.
De Marco, T. (1996) Periodontal Emotional Stress Syndrome.
Journal of Periodontology, 47, 67-68.
Fukui M, Hinode D, Yokoyama M, Yoshioka M, Kataoka K,
Ito HO. 2010 Levels of salivary stress markers in patients
with anxiety about halitosis. Archives of oral biology 55
842-847.
Ghalayani P, Razavi SM, Gholami D. 2009. Comparative
study of number and distribution of IgG+ cells in oral lichen
planus and oral lichenoid lesions. Dent Res J (Isfahan). 6(1):
1-5.
Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli
T, Wong DT.2009 Saliva as a diagnostic tool for periodontal
disease: Current state and future directions. Periodontol 2000
50:52-64.
Gundala R, Chava VK, Ramesh RBV.2012 Role of Stress in Peri-
odontal Disease. Indian J Dent Adv 4(1): 763-771
Heinrichs M, Wagner D, Schoch W, et al.2005. Predicting post-
traumatic stress symptoms from pretraumatic risk factors: a
2-year prospective follow-up study in re ghters. Am J Psy-
chiatry 162:2276–86.
Ishisaka, A., Ansai, T., Soh, I., Inenaga, K., Yoshida, A., Shigey-
ama, C., et al. (2007) Association of Salivary Levels
Ismail SB, Kumar SK, Zain RB.2007.Oral licheplanus and
lichenoid reactions: Etiopathogenesis, diagnosis, management
and malignantn transformation. J Oral Sci. 49(2): 89-106.
Koh DSQ, Koh G Ch H. 2007. The use of salivary biomarkers
in occupational and environmental medicine. Occup Environ
Med 64:202–210.
Lakshmi Boyapati and Hom-lay Wang.2007. The role of
stress in periodontal disease and wound healing. Periodontol;
44:195-210.
Linden, G.J., McKinnell, J., Shaw, C. and Lundy, F.T. (1998)
Substance P and Neurokinin A in Gingival Crevicular Fluid in

Mohammadreza Karimi et al.
92 RELATIONSHIP BETWEEN STRESS, ANXIETY, DEPRESSION AND SALIVARY IGA WITH PERIODONTAL DISEASE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Periodontal Health and Disease. Journal of Clinical Periodon-
tology, 24, 799-803.
Nosratzehi T, Arbabi-Kalati F, Salimi S, Honarmand E. 2014.
The evaluation of psychological factor and salivary cortisol
and IgA levels in patients with oral lichen planus. ZJRMS
16(7): 31-34
of Cortisol and Dehydroepiandrosterone with Periodontitis in
Older Japanese Adults. Journal of Periodontology, 78, 1773-
1767.
Peruzzo, D.C., Benatti, B.B., Ambrosano, G.M., Nogueira-Filho,
G.R., Sallum, E.A., Casati, M.Z. and Nociti, F.H.(2007) A Sys-
tematic Review of Stress and Psychological Factors as Possible
Risk Factors for Periodontal Disease. Journal of Periodontol-
ogy, 78, 1491-1504.
Rabiei M, Sadegh-Kanjani M, Kazemnezhad-Leili E and
Kohanghadam S. 2012. The comparsion between anxiety, level
of salivary cortisol SIgA in oral lichen planus. J Res Dent Sci.
9(3): 125-131.
Radafshar G., Zarrabi H., Jalayer S. 2012. Relationships of
Stress and Coping Styles to Periodontal Disease: A Case-Con-
trol Study Journal of Dentistry Shiraz University of Medical
Sciences 13(4): 169-175.
Rai B, Kaur J, Anand SC, Jacobs R. 2011. Salivary stress mark-
ers, stress, and periodontitis: a pilot study. J Periodontol 82:
287-292.
Rosania AE, Low KG, McCormick CM, Rosania DA. 2009.
Stress, depression, cortisol, and periodontal disease. J Peri-
odontol 80: 260-266.
Sato K.1991. Enzyme-linked immunosorbent assay of SIgA in
whole saliva of healthy subjects and patients with oral dis-
eases. Bull Tokyo Med Dent Univ. 38(2): 9-18.
Shah B, Ashok L, Sujatha GP. Evalution of salivary cortisol and
psychological factors in patients with lichen planus. Indian J
Dent Res. 20(3): 288-92.
Shende AS, Bhatsange AG, Waghmare AS, Shiggaon LB,
Mehetre VN, Meshram EP.2016. Determining the association
between stress and periodontal disease: A pilot study. J Int Clin
Dent Res Organ 8:111-4.
Sindhu S, Jagannathan N. Saliva: 2014 A Cutting Edge in
Diagnostic Procedures. Journal of Oral Diseases. Volume Arti-
cle ID 168584, 8 pages. http://dx.doi.org/10.1155/2014/168584
Sistig S, Vucicevic-Boras V, Lukac J and Kusic Z.2002. Salivary
IgA and IgG subclasses in oral mucosal diseases. Oral Dis. 8(6):
282-6.
Wimmer, G., Janda, M., Wieselmann-Penkner, K., Jakse, N.,
Polansky, R. and Pertl, C. (2002) Coping with Stress: Its In uence
on Periodontal Disease. Journal of Periodontology, 73, 1343-351.
Wong D.2008. Salivary diagnostics. 1st ed. New Jersey: Wiley-
Blackwell; 2008: 37-59.