Microbiological
Communication
Biosci. Biotech. Res. Comm. 9(4): 856-864 (2016)
Evaluation of phyto constituent and synergistic
antibacterial activity of
Ocimum sanctum
extract
against some gram-positive and gram-negative species
Fatema Shah*
1
, Ziaul Hasan
1
and Kamal Uddin Zaidi
2
1
Department of Microbiology, Sai a College of Science, Bhopal 462001, India
2
Biotechnology Pharmacology Laboratory, Centre for Scienti c Research & Development, People’s University,
Bhopal 462037, India
ABSTRACT
Plant and plant extracts have been used in traditional medicine since time immemorial. O.sanctum has often been
cited as one of the main pillars of herbal medicine as it possesses greater medicinal value. It has been proved to be
effective against gram positive and gram negative bacteria. This study aimed to determine the in vitro antibacterial
activity of the medicinal plants O. sanctum against the bacterial strains associated with infectious diseases. Extracts
of O. sanctum were tested for their antibacterial activity against three bacterial species includes Staphylococcus
aureus, Escherichia coliandPseudomonas aeruginosa using the microdilution method. In the present study we found
that methanolic extract of leaves of O. sanctum was used in combination with ampicillin against E. coli, S. aureus
and P. aeruginosa showed fraction inhibitory concentration index (FICI ≤ 0.5). The synergistic antibacterial activ-
ity (FICI ≤ 0.5 ) was observed with combination of methanolic extract of stem and root of O. sanctum when used in
combination with antibiotic ampicillin against E. coli, S. aureus and P. aeruginosa showed indifferent antibacterial
activity (FICI = 1.0 - 4.0). Benzene extract of leaves, stem and root of O. sanctum when used in combination with
ampicillin against E. coli, S. aureus and P. aeruginosa showed partial synergistic antibacterial activity (FICI = 0.5 -
1.0, 0.5 - 4.0, 1.0 - 4.0) respectively. The synergistic antibacterial activity (FICI ≤ 0.5) was observed with combination
of aqueous extract of leaves of O.sanctum with ampicillin. While, the aqueous extract of stems and roots of O. sanc-
tum when used in combination with antibiotic ampicillin against E. coli, S. aureus and P. aeruginosa showed partial
synergistic antibacterial activity and indifferent antibacterial activity (FICI = 0.5 - 4.0, 1.0 - 4.0) respectively. present
investigation indicates clear evidence supporting the traditional use of O. sanctum in treating infectious diseases.
KEY WORDS: TULSI, SYNERGISTIC EFFECT, DISEASES, EXTRACTION, FRACTION INHIBITORY CONCENTRATION
856
ARTICLE INFORMATION:
*Corresponding Author: me_fatema3@yahoo.com
Received 7
th
Nov, 2016
Accepted after revision 24
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/

Shah, Hasan and Zaidi
INTRODUCTION
In modern complementary and alternative medical prac-
tice, plants are the primary source of therapeutics and
each part of the plant, including the seeds, root, stem,
leaves, and fruit, potentially contains bioactive com-
ponents. The search for alternative antimicrobial com-
pounds is an urgent area of biomedical research and
extracts derived from plants have long held interest as
potential sources of new therapeutic agents. The medici-
nal use of plants is probably as old as mankind. Plants
have continued to be a valuable source of natural prod-
ucts for maintaining human health. Various medicinal
plants have been used for years in daily life to treat
disease all over the world. One of the remotest works in
traditional herbal medicine is Viriksh Ayurveda, com-
piled even before the beginning of Christian era (Himal
et al., 2008, Mandave et al., 2014. Jiang et al., 2015 and
Sarah et al., 2016).
In the ancient Ayurvedic text, the Charaka Samhita,
Tulsi has been documented to be of immense use in
the treatment of headaches, rhinitis, stomach disorders,
in ammation, heart diseases, various forms of poison-
ing and malaria (Gupta et al., 2002). Each part of the
plant has proven to offer protection against various
diseases; the aqueous and alcoholic extract from the
leaves have various pharmacological activities such as
anti-in ammatory, antipyretic, analgesic, antiasthmatic,
antiemetic, antidiabetic, hepatoprotective, hypotensive,
hypolipidemic, and antistress agents. Further, distilla-
tion of the leaves yields oil of the plant which is known
to possess antibacterial, antioxidant, and anti-in am-
matory properties and is used extensively in the phar-
maceutical industry mainly for skin cream preparations
(Watson et al., 2012). Different parts of Ocimum sanc-
tum Linn (known as Tulsi), a small herb seen throughout
India, have been used for various medicinal purposes.
The main bioactive components in medicinal plants are
considered to be combinations of secondary metabolites
(Wu et al., 2016).
Recently Hanaa et al (2016) have been demonstrated
that antimicrobial activity of Ocimum tenui orum essen-
tial oil and their major constituents against three species
of bacteria. This study was designed at authenticated
the traditional use of Ocimum sanctum medicinal plants
against human pathogenic bacteria, causing a number of
human disease includingEscherichia coli,Pseudomonas
aeruginosa andStaphylococcus aureus by assess their in
vitro antibacterial activity.Due to insuf cient screening
of the natural compounds and the limited understanding
of their mechanism of action against the microorganism
the need of the hour is to identify more and more natural
compounds which exhibit synergistic behavior with the
antibiotics.
MATERIAL AND METHODS
Plant materials Ocimum sanctum (Tulsi) were purchased
from local market and were authenticated by Dr. S.S.
Khan of Botany, Department Sai a Science College Bho-
pal. The voucher specimen no (J/R201) was deposited at
the Herbarium of the Faculty of Botany Department, Sai-
a Science College Bhopal (M.P.) India. Human disease
causing bacteria; Escherichia coli (MTCC 739), Staphy-
lococcus aureus (MTCC 96) Pseudomonas aeruginosa
(MTCC 74) were procured from Institute of Microbial
Technology, Chandigarh (IMTECH), India. These bacte-
rial strains were then used for studying the antimicrobial
ef ciency.
The phytochemical constituents of the Ocimum sanc-
tum plant parts (leaf, stem, and root) were extracted in
soxhlet apparatus using various solvents Kokate, 1991;
Trease and Evans, 1989). Soxhlet extraction is used for
separating components based on the difference in the
solubility in the solvent. The powdered plant material
(50 gm) was placed in the soxhlet extractor ask. 500
ml of the organic solvent was taken in the round bot-
tom ask. The soxhlet extraction was carried out con-
tinuously at an appropriate temperature for 6-8 hrs, till
colorless extract is collected in the extractor ask. The
extract thus obtained was collected in collection bottles
and was further subjected to concentration using Rotary
vacuum evaporator. After soxhlet extraction the extracts
obtained were ltered and then each of the extract was
concentrated using rotary vacuum evaporator. The indi-
vidual extracts were taken in round bottom ask which
was heated at appropriate temperature on a water bath.
The vapors of the solvent rise in the condenser and after
condensation the solvent droplets was collected in the
collecting ask.
The resultant sticky mass was collected in the cruci-
ble. It was dried at a low temperature in the oven. The
solid mass obtained was stored in a suitable volume of
10% dimethyl sulphoxide (DMSO) with a drop of Tween-
20. Aqueous extract of the individual plant parts was
prepared by decoction method. Filter paper packets of
50 gm of the individual plant parts were prepared. These
packets were place separately in 200 ml of hot water
contained in bottles. The extraction was carried out for
24
o
C with intermittent shaking. The extracts obtained
were concentrated and dried. The dried mass obtained
was stored in a suitable volume of 10% dimethyl sul-
phate (DMSO) with a drop of tween-20.
Phytochemical analysis
Chemical test were carried out to identify various con-
stituents using standard method of (Trease and Evans
1989; Harbone, 1973). Mayer reagent was prepared by
dissolving 1.36 grams of mercuric chloride in 60 ml of
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF PHYTO CONSTITUENT AND SYNERGISTIC ANTIBACTERIAL ACTIVITY 857
Shah, Hasan and Zaidi
distilled water and 5 grams of potassium iodide in 20 ml
of distilled water. Both the above solution was mixed
and volume of the reagent adjusted to 100 ml by dis-
tilled water. 1 ml of the plant extract was taken and few
drops of mayer reagents were added. Formation of cream
colour precipitate was con rms the presence of alkaloid.
Fehling solution was prepared by dissolving 4.36 gram
of copper sulphate in 50 ml of distilled water and by
dissolving 17.3 grams of sodium potassium tartarate and
5 gram of sodium hydroxide in 50 ml of distilled water.
Both the solution were mixed prior to use.1 ml of the
extract were taken and few drops of fehling solution
was added. Formation of red precipitate con rms the
presence of carbohydrates and glycosides. Ferric chlo-
ride solution was prepared by dissolving 5 grams of fer-
ric chloride in 100 ml of 90% ethanol. 1 ml of extract
was taken and few drops of ferric chloride solution were
added. Formation of bluish black precipitate con rms
the presence of phenolic compounds and tannins.
Ninhydrin solution was prepared by dissolving 0.3
grams of ninhydrin in 100 ml of ethanol. 1 ml of extract
was taken and few drops of ninhydrin solution were
added and purplish pink colour con rms the presence of
proteins and amino acids in extracts. Alkaline reagent
was prepared by dissolving 10 grams of sodium hydrox-
ide in 100 ml of distilled water. 1 ml of extract was taken
and few drops of sodium hydroxide solution were added.
Intense yellow colour con rms the presence of avo-
noids. 1 ml of the extract was taken and mixed with few
drops of chloroform and few drops of sulphuric acids.
A reddish brown colour con rms the presence of ter-
penoids.1 ml of the extract was taken and diluted with
distilled water to 10 ml. Formation of stable foam con-
rms the presence of saponins.1 ml of the extract was
mixed with 5 ml of distilled water mixture was heated
and to it was added 5 ml of 1% HCl. Formation of red
precipitate con rms the presence of phlobatanins.1 ml
of the extract was taken and to it was added in 2 ml
of chloroform and 2 ml of concentrated sulphuric acid.
Formation of reddish brown layer at the interface con-
rms the presence of steroids.
Assay of antimicrobial activity using disc diffusion
method
Disc diffusion method for antimicrobial susceptibil-
ity testing was carried out according to the standard
method by Bauer et al., 1996 to assess the presence of
antibacterial activities of the various samples. A bacte-
rial suspension was prepared for each of bacteria used
for the study. 1 ml of the bacterial suspension was taken
in sterile petriplate. To it was added molten nutrient agar
media under aseptic conditions and mixed well. It was
allowed to solidify for 1 hour to allow the bacteria to
grow. These plates were used for sensitivity test. What-
man lter paper disc were impregnated with the samples
and were placed on nutrient agar surface. Positive con-
trol plate was also prepared with standard antibiotic disc
and negative control plate was prepared using DMSO.
The plates were then incubated at 37
o
C for 24 hrs. After
the incubation the plates were examine for zone of inhi-
bition. The inhibition zones were measured using antibi-
otic zone reader scale
Determination of Minimum Inhibitory Concentration (MIC)
The minimum inhibitory concentration of the plant
extracts, antibiotics and combination of plant extracts
and antibiotics was determined by diluting the extracts
in Nutrient broth to give concentration of 1024, 512,
256, 128, 64, 32, 16, 8, 4 and 2 μg /ml. 2 ml of plant
extracts, antibiotics and combination of plant extracts
and antibiotics was added to the rst tube containing
2 ml of broth. The tube was shaken and 2 ml trans-
ferred aseptically to the next tube containing the same
quantity of broth. This was done until serial dilution was
achieved in the last tube that is the tenth tube. Then 0.1
ml of the MTCC bacterial culture suspension was inocu-
lated into each test tube and they were incubated at 37
o
C for 24 hours. The absorbance of the tubes was taken
in UV-VIS spectrophotometer. The minimum inhibitory
concentration was regarded as the lowest concentration
of the extract that did not permit any visible growth
when compared with the control tube.
Calculate FIC and FIC Index
A widely accepted method, to measure the effect of com-
bination of plant extract and antibiotic is the fractional
index. The fractional index is used to identify whether a
combination therapy is synergistic, additive or antago-
nistic. The Inhibitory Concentration is determined using
MIC measurements. The fractional Inhibitory Concentra-
tion Index (∑FIC) is the sum of the FICS of each of the
plant extract and antibiotics (
Rakholiya et al., 2015).
Calculations
The FIC was calculated for plant extract and antibiotic
as follows:
MIC of plant extractin combination
FIC for Plant extract =
MIC of plant extract
The FIC was calculated for plant extract and antibiotic
as follows:
MIC of plant extractin combination
FIC for Antibiotic =
MIC of plant extract
Calculated the summation of FIC (FIC) index for each
combination as follows:-
FIC = FIC of Plant Extracts + FIC of Antibiotics
858 EVALUATION OF PHYTO CONSTITUENT AND SYNERGISTIC ANTIBACTERIAL ACTIVITY BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Shah, Hasan and Zaidi
RESULTS
Antibacterial activity of methanolic extracts of
O.
sanctum
with ampicillin
The zone of inhibition of the leaf extract against E. coli,
P. aeruginosa and S. aureus was 18mm, 15mm and 23mm
respectively. The ZOI of the combination of leaf extract of
O. sanctum and ampicillin were 29mm, 27mm and 30mm.
The MIC of the leaf extract was 32μg/ml, 16μg/ml and
32μg/ml against E. coli, P. aeruginosa and S. aureus. The
MIC of the combination of leaf extract of O. sanctum and
ampicillin were 4 μg/ml, 4μg/ml and 4μg/ml respectively.
The ZOI of the stem extract against E. coli, P. aeruginosa
and S. aureus were 15mm, 10mm and 14mm respectively.
The ZOI of the combination of stem extract of O. sanctum
and ampicillin were 28mm, 21mm and 26mm (Table 1).
The minimum inhibitory concentration values of
the stem extract were 16μg/ml, 32μg/ml and 64μg/
ml against E. coli, P. aeruginosa and S. aureus. The
MIC of the combination of stem extract of O. sanc-
tum and ampicillin were 4μg/ml, 16μg/ml and 16μg/ml
respectively. The ZOI of the root extract against E. coli,
P. aeruginosa and S. aureus were 0mm, 0mm and 10mm
respectively. The ZOI of the combination of root extract
of O. sanctum and ampicillin were 24mm, 22mm and
23mm. The MIC of the root extract was 64μg/ml, 256μg/
ml and 128μg/ml against E. coli, P. aeruginosa and S.
aureus. The MIC of the combination of root extract of
O. sanctum and ampicillin were 32μg/ml, 32μg/ml and
16μg/ml respectively (Table 1).
Antibacterial activity of combined effect of
benzene extracts of
O. sanctum
with ampicillin
The zone of inhibition of the leaf extract against E. coli,
P. aeruginosa and S. aureus was 13mm, 8mm and 15mm
respectively. The ZOI of the combination of leaf extract
of O. sanctum and ampicillin were 26mm, 21mm and
28mm. The MIC of the leaf extract was 32μg/ml, 16μg/
ml and 32μg/ml against E. coli, P. aeruginosa and S.
aureus. The MIC of the combination of leaf extract of
O. sanctum and ampicillin were 8μg/ml. The ZOI of the
stem extract against E. coli, P. aeruginosa and S. aureus
was 11 mm, 0 mm and 10 mm respectively. The ZOI
of the combination of stem extract of O. sanctum and
ampicillin were 25 mm, 20 mm and 22mm (Table 2).The
minimum inhibitory concentration values of the stem
extract were 64μg/ml, 128μg/ml and 32μg/ml against
E. coli, P. aeruginosa and S. aureus. The MIC of the
combination of stem extract of O. sanctum and ampicil-
lin were 8μg/ml, 16μg/ml and 16μg/ml respectively. The
ZOI of the root extract against E. coli, P. aeruginosa and
S. aureus was 0mm, 0mm and 8mm respectively.The ZOI
of the combination of root extract of O. sanctum and
ampicillin were 24 mm, 22 mm and 23 mm. The MIC of
the root extract was 64 μg/ml, 32μg/ml and 64 μg/ml
against E. coli, P. aeruginosa and S. aureus. The MIC of
the combination of root extract of O. sanctum and ampi-
cillin were 16μg/ml, 16μg/ml and 32μg/ml respectively
(Table 2).
Antibacterial activity of combined effect of aqueous
extracts of
O. sanctum
with ampicillin
The zone of inhibition of the leaf extract against E. coli,
P. aeruginosa and S. aureus was 17mm, 14mm and
20mm respectively. The ZOI of the combination of leaf
extract of O. sanctum and ampicillin were 28mm, 26mm
and 29mm. The MIC of the leaf extract was 16 μg/ml,
64μg/ml and 32μg/ml against E. coli, P. aeruginosa and
S. aureus. The MIC of the combination of leaf extract of
O. sanctum and ampicillin were 2μg/ml, 16μg/ml and 8
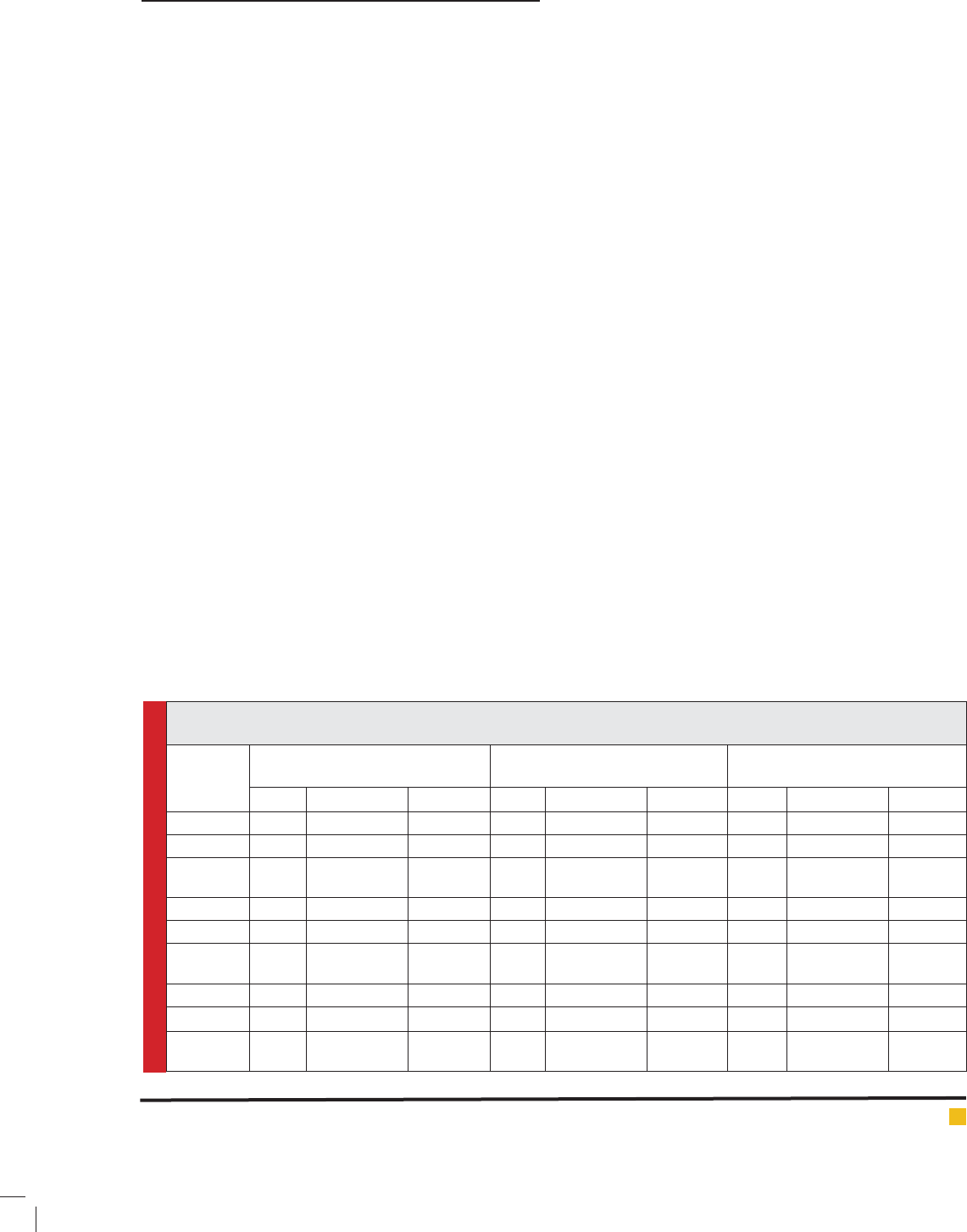
Table 1: Zone of Inhibition, Minimum Inhibitory Concentration and Fractional Inhibitory Concentration of methanolic
extract of leaf, stem and root of O. sanctum.
Plants
material
Zone of Inhibition (mm) Minimum Inhibitory
Concentration in μg/ml (MIC)
Fractional Inhibitory Conc.
FIC & FIC
E. coli P. aeruginosa S. aureus E. coli P. aeruginosa S. aureus E. coli P. aeruginosa S. aureus
Leaf 18 15 23 32 16 32 0.125 0.250 0.125
Ampicillin 23 21 22 16 16 32 0.250 0.250 0.125
Leaf with
Ampicillin
29 27 30 4 4 4 0.375 0.500 0.250
Stem 15 10 14 16 32 64 0.250 0.500 0.250
Ampicillin 23 21 22 16 32 32 0.250 0.500 0.500
Stem with
Ampicillin
28 21 26 4 16 16 0.500 1.000 0.750
Root - - 10 64 256 128 0.500 1.000 1.500
Ampicillin 23 21 22 32 32 16 0.125 1.000 1.125
Root with
Ampicillin
24 22 23 32 32 16 0.125 1.000 1.125
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF PHYTO CONSTITUENT AND SYNERGISTIC ANTIBACTERIAL ACTIVITY 859
Shah, Hasan and Zaidi
μg/ml respectively. The ZOI of the stem extract against
E. coli, P. aeruginosa and S. aureus was 12 mm, 0 mm
and 16 mm respectively. The ZOI of the combination of
stem extract of O. sanctum and ampicillin were 25mm,
22mm and 28mm (Table 3).
The minimum inhibitory concentration values of the
stem extract was 32μg/ml, 32μg/ml and 32μg/ml against
E. coli, P. aeruginosa and S. aureus. The MIC of the
combination of stem extract of O. sanctum and ampicil-
lin were 8μg/ml, 16μg/ml and 8μg/ml respectively. The
ZOI of the root extract against E. coli, P. aeruginosa and
S. aureus was 9mm, 0mm and 8mm respectively. The
ZOI of the combination of root extract of O. sanctum
and ampicillin were 24mm, 22mm and 23mm. The MIC
of the root extract was 32μg/ml, 32μg/ml and 256μg/ml
against E. coli, P. aeruginosa and S. aureus. The MIC of
the combination of root extract of O. sanctum and ampi-
cillin were 16μg/ml, 16μg/ml and 32 μg/ml respectively
(Table 3).
Analysis of the Plant Extracts
O. sanctum
Pytochemical screening of the leaf, stem and root of Oci-
mum sanctum L. were conducted and its results showed
presence of different constituents in methanolic, ben-
zene and aqueous extracts. (Table 4). Methanolic extracts
showed the presence of alkaloids, carbohydrate, glyco-
sides, phenolic compounds, tannins, proteins, amino
acids, avonoids, terpenoids, saponins and steroids. In
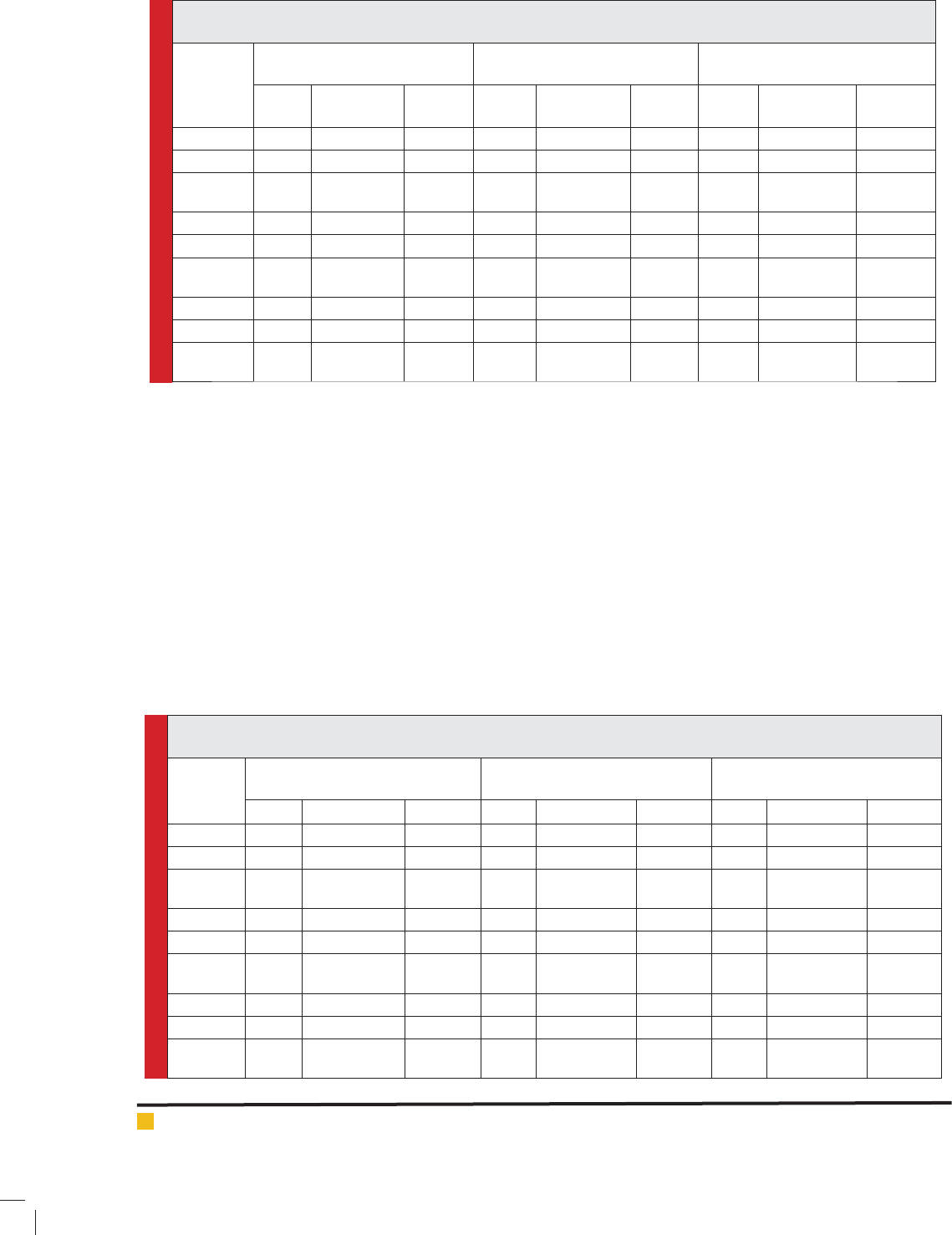
Table 2: Zone of Inhibition, Minimum Inhibitory Concentration and Fractional Inhibitory Concentration of Benzene
extract of leaf, stem and root of O. sanctum.
Plants
material
Zone of Inhibition (mm)
Minimum Inhibitory
Concentration in μg/ml (MIC)
Fractional Inhibitory Conc.
FIC & FIC
E. coli
P.
aeruginosa
S.
aureus E. coli
P.
aeruginosa
S.
aureus E. coli
P.
aeruginosa S. aureus
Leaf 13 8 15 32 16 32 0.250 0.500 0.250
Ampicillin 23 21 22 16 16 32 0.500 0.500 0.250
Leaf with
Ampicillin
26 21 28 8 8 8 0.750 0.1000 0.500
Stem 11 - 10 64 128 32 0.125 0.125 0.500
Ampicillin 23 21 22 16 16 32 0.500 0.1000 0.500
Stem with
Ampicillin
25 20 22 8 16 16 0.625 1.125 0.1000
Root - - 8 64 32 64 0.250 0.500 0.500
Ampicillin 23 21 22 16 16 32 1.000 1.000 1.000
Root with
Ampicillin
24 22 23 16 16 32 1.250 1.500 1.500
Table 3: Zone of Inhibition, Minimum Inhibitory Concentration and Fractional Inhibitory Concentration of Aqueous
extract of leaf, stem and root of O. sanctum.
Plants
material
Zone of Inhibition ( mm ) Minimum Inhibitory
Concentration in μg/ml ( MIC )
Fractional Inhibitory Conc.
FIC & FIC
E. coli P. aeruginosa S. aureus E. coli P. aeruginosa S. aureus E. coli P. aeruginosa S. aureus
Leaf 17 14 20 16 64 32 0.125 0.250 0.250
Ampicillin 23 21 22 16 32 32 0.125 0.500 0.250
Leaf with
Ampicillin
28 26 29 2 16 8 0.250 0.750 0.500
Stem 12 - 16 32 32 32 0.250 0.500 0.250
Ampicillin 23 21 22 16 32 32 0.500 0.500 0.250
Stem with
Ampicillin
25 22 28 8 16 8 0.750 0.1000 0.500
Root 9 - 8 32 32 256 0.500 0.500 0.125
Ampicillin 23 21 22 16 32 32 1.000 0.500 1.000
Root with
Ampicillin
24 22 23 16 16 32 1.500 1.000 1.125
860 EVALUATION OF PHYTO CONSTITUENT AND SYNERGISTIC ANTIBACTERIAL ACTIVITY BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Shah, Hasan and Zaidi
benzene extract carbohydrate, glycosides, phenols, tan-
nins, proteins and amino acids were present. In aqueous
extract along with above components terpenoids and
saponins were also present. Stem extract of O. sanctum
in methanol showed presence of alkaloids, phenols, tan-
nins, avonoids, terpenoids, saponins and phlobatannin
while extraction with benzene solvent showed presence
of avonoids only. In aqueous extract phenols, tan-
nins, avonoids, terpenoids, saponins and phlobatan-
nins were present. Methanolic extracts of root showed
the presence of the different phytochemical constituents
viz. alkaloids, carbohydrates, glycosides, phenols, tan-
nins, saponins, phlobatannins and steroids. Extraction
with benzene showed presence of carbohydrate, gly-
cosides, phenols and tannins. Aqueous extracts of the
roots showed the presence of alkaloids, carbohydrates,
glycosides, phenols, taninns,saponins and phlobatanins
(Table 4).
DISCUSSION
In this study, we attempted to obtain information on
the antimicrobial ef cacy of Ocimum sanc, particularly
against pathogens namely E. coli, S. aureus and P. aerug-
inosa, as these microbes are more commonly associated
with initiation and progression of various pathogenic
diseases, especially aggressive periodontitis. Synergism
was found when methanolic extract of leaves of Oci-
mum sanctum was used in combination with antibiotic
ampicillin against E. coli, S. aureus and P. aeruginosa
bacterial species ( FICI ≤ 0.5 ). The results showed that
ocimum leaves extract showed good inhibition against
the bacterial strains. This observed antimicrobial activ-
ity could be explained by the fact that plant extract may
attach to the surface of the cell membrane disturbing
permeability and respiration functions of the cell. The
interaction of plant extract with microbial cytoplasmic
components and nucleic acids can inhibit the respiratory
chain enzymes and interferes with the membrane per-
meability limiting the development of bacteria. It is also
possible that extract not only interact with the surface
of membrane but can also penetrate inside the bacte-
ria. The synergistic antibacterial activity ( FICI ≤ 0.5 )
was observed with combination of methanolic extract of
stem of Ocimum sanctum with ampicillin showed par-
tial synergistic antibacterial activity (FICI = 0.5 - 1.0) .
Methanolic extract of root of Ocimum sanctum when
used in combination with antibiotic ampicillin, against
E. coli , S. aureus and P. aeruginosa showed indifferent
antibacterial activity (FICI = 1.0 - 4.0).
Our ndings are in agreement with Mishra and
Mishra, (2011) who studied antibacterial activity of
the aqueous, alcoholic, chloroform extract and oil
obtained from leaves of Ocimum sanctum against E.coli,
P.aeruginosa, S. typhimurium and S.aureus. Extract
obtained from Ocimum sanctum were observed equally
effective against pathogenic gram positive and gram
negative bacteria. Benzene extract of leaves of Oci-
mum sanctum when used in combination with antibiotic
ampicillin against E. coli, S. aureus and P. aeruginosa
showed partial synergistic antibacterial activity (FICI =
0.5-1.0). Benzene extract of stems of Ocimum sanctum
when used in combination with antibiotic ampicillin,
against E. coli , S. aureus and P. aeruginosa showed
partial synergistic antibacterial activity and indifferent
antibacterial activity (FICI = 0.5 - 4.0). Benzene extract
Table 4: Phytochemical constituents present in methanolic, benzene and aqueous extracts of leaf, stem and root of
O. sanctum.
Plant
Constituents
Leaf Stem Root
Methanol Benzene Aqueous Methanol Benzene Aqueous Methanol Benzene Aqueous
Alkaloids + - + + - - + - +
Carbohydrates
Glycosides
+ ++++++-+
Phenolics
compounds
Tannins
+-+------
Proteins
Amino acids
+ +++++ - -+
Flavonoids + - + - - - - - -
Terpenoids + - + - - - + - +
Saponins + - - + - + + - +
Phlobatannins - - - - - - - - -
Steroids + - - + - - + - -
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF PHYTO CONSTITUENT AND SYNERGISTIC ANTIBACTERIAL ACTIVITY 861
Shah, Hasan and Zaidi
of roots of Ocimum sanctum when used in combination
with antibiotic ampicillin against E. coli , S. aureus and
P. aeruginosa showed indifferent antibacterial activity
(FICI = 1.0 - 4.0) These results are broadly similar to
those of studies that used disk diffusion or optical den-
sity reduction methods; however, there are differences in
reported activity toward Gram positive and Gram nega-
tive bacteria, ( Helen et al 2011; Poole et al 2011 and
Hanaa et al 2016).
The synergistic antibacterial activity (FICI ≤ 0.5) was
observed with combination of aqueous extract of leaves
of Ocimum sanctum with ampicillin. Aqueous extract
of stems of Ocimum sanctum when used in combina-
tion with antibiotic ampicillin against E. coli, S. aureus
and P. aeruginosa showed partial synergistic antibacte-
rial activity and indifferent antibacterial activity (FICI
= 0.5 - 4.0). Aqueous extract of roots of Ocimum sanc-
tum when used in combination with antibiotic ampicil-
lin against E. coli, S. aureus and P. aeruginosa showed
indifferent antibacterial activity (FICI = 1.0 - 4.0). This is
in agreement with many literatures reporting of differ-
ences in the activities of extracts obtained from the same
morphological part of a plant using different solvents.
Similar ndings have been reported by Ahmad and
Aqil, (2007) who found synergistic interaction between
crude extracts of Indian medicinal plants and antibiot-
ics against extended spectrum lactamase producing
multidrug-resistant enteric bacteria. Similar observation
was found by Sajjanshetty et al., (2016) who reported
the antimicrobial ef cacy of Ocimum sanctum leaf
extract on periodontal pathogens it was observed Oci-
mum sanctum i extracts showed antimicrobial activity
against A. actinomycetemcomitans, similar to doxycy-
cline with similar inhibition zones (P > 0.05). P. gingi-
valis and P. intermedia, however, exhibited resistance to
Tulsi extract that showed signi cantly smaller inhibition
zones (P < 0.05).
Methanolic extracts of the leaf showed the pres-
ence of alkaloids, carbohydrate, glycosides, phenolic
compounds, tannins, proteins, amino acids, avonoids,
terpenoids, saponins and steroids. In benzene extract
carbohydrate, glycosides, phenols, tannins, proteins
and amino acids were present. In aqueous extract along
with above components terpenoids and saponins were
also present. Phytochemical constituents such as ster-
oids, alkaloids, avonoids, tannins, phenol, and several
other aromatic compounds are secondary metabolites of
plants that serve a defense mechanism against predic-
tion by many microorganisms, insects and other herbi-
vores (Bonjar et al., 2004). These secondary metabolites
exert antimicrobial activity through different mecha-
nisms. Secondary metabolite alkaloids are one of the
largest groups of phytochemicals in plants found in all
of extract of Ocimum sanctum. One of the most common
biological properties of alkaloids is their toxicity against
cells of foreign organisms like bacteria, tannins have
been found to form irreversible complexes with proli-
nerich protein resulting in the inhibition of cell protein
synthesis (Sibanda and Okoh 2007).
Stem extract of O. sanctum in methanol showed pres-
ence of phenols, tannins, avonoids, terpenoids, sapo-
nins and phlobatannins while extraction with benzene
solvent showed presence of avonoids only. In aqueous
extract phenols, tannins, avonoids, terpenoids, saponins
and phlobatannins were present. Methanolic extracts of
root showed the presence of the different phytochemical
constituents viz. Alkaloids, carbohydrates , glycosides,
phenols, tannins, saponins, phlobatannins and steroids.
Extraction with benzene showed presence of carbohy-
drate, glycosides, phenols and tannins. Aqueous extracts
of the roots showed the presence of alkaloids, carbo-
hydrates, glycosides, phenols , tannins , saponins and
phlobatannins .
Eugenol (l-hydroxy-2-methoxy-4-al-
lylbenzene) the active constituent present in O. sanctum,
is mainly responsible for the therapeutic potential of the
plant (Prakash and Gupta, 2005) and the other important
constituents include carvacrol, linalool, methyl euge-
nol ,
caryophyllene , methyl chavicol and ursolic acid
as lead compounds in the composition of O. sanctum,
(Mohan et al., 2011).
As Ocimum is widespread in India, it can be recom-
mended as an easily available and renewal source of
antimicrobial agent instead of synthetic chemicals. The
present ndings indicate that Ocimum possesses com-
pounds with antimicrobial properties against patho-
genic microorganisms. It is quit safer to use as an herbal
medicine as compare to chemically synthesized drug.
Prostanthera species, like many other australian plants,
have been shown to have essential oils with potent anti-
microbial activity. Essential oils from the desert species
P. centralis have been shown to be effective against
gram-positive bacteria with MICs against S. aureus of
approximately 0.1 mg/ml (Collins et al., 2014).
On the other hand, streptomycin sulfate and chlo-
ramphenicol used as positive controls showed strong
antibacterial activities against both Gram-positive and
Gram-negative bacteria like as the results of previous
studies (Khan et al., 2014). All the plants parts were
extracted with methanol, benzene and aqueous because
these considered as the best solvent for the extraction
of antimicrobial substances and may contain diverse
chemical compounds with biological activity (Robles
et al., 2013; Tekwu et al., 2012).
The alcoholic extract
has greater effect as compared to Benzene and aque-
ous extract which may be due to the fact that alcohol is
comparatively a better solvent as compared with water
and benzene for extraction of phytochemical (Levy and
Marshall, 2004). Plants antimicrobials have been found
862 EVALUATION OF PHYTO CONSTITUENT AND SYNERGISTIC ANTIBACTERIAL ACTIVITY BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Shah, Hasan and Zaidi
to be synergistic enhancer in that they have little anti-
microbial property alone but when they are taken con-
currently with standard drug enhances the effect of anti-
biotics (Chanda and Rakholiya 2011).
CONCLUSION
In ancient and modern era, aerial parts of herbs have
been generally used for the cure of crucial health care
and variety of ailment across the world depends on
geographical cultivation. Leaves of O. sanctum play a
vital role in health care system due to containing of
certain phytochemical. Overall results of current study
re ect that highest antimicrobial activities were deter-
mined againstEscherichia coli,Pseudomonas aeruginosa
and Staphylococcus aureus. Among selected studied
medicinal plant material, O. sanctum leaf showed more
antibacterial activity.
ACKNOWLEDGEMENTS
The authors are grateful to Sai a College of Science
Bhopal, for providing laboratory facilities and for grant-
ing nancial assistance to carry out this work, and Prin-
cipal and Secretary, Sai a College of Science, Bhopal,
for encouragement.
REFERENCES
Ahmad, I., Aqil, F. (2007) In vitro ef cacy of bioactive extracts
of 15 medicinal plants against ESbetaL-producing multidrug-
resistant enteric bacteria. Microbiol. Res.162 (3);264-75.
Bauer, R., Kirby, M., Sherris, J., Turck, M. (1996). Antibiotic
susceptibility testing by standard disk diffusion method. Am.
J. Clin. Pathology. 45; 493-496.
Bonjar, G., Nik, A., Aghighi, S. (2004). J. Biological Sci.4;
405–412.
Chanda, S., Rakholiya, K. (2011). Combination therapy: Syn-
ergism between natural plant extracts and antibiotics against
infectious diseases. A. Méndez-Vilas (Ed.).
Collins. TL., Jones, GL., Sadgrove, N.J. (2014) Volatiles from
the Rare Australian Desert Plant Prostanthera centralis B. J.
Conn (Lamiaceae): Chemical Composition and Antimicrobial
Activity. Agriculture. 4(4);308-16.
Gupta, SK., Prakash, J., Srivastava, S. (2002). Validation of tra-
ditional claim of Tulsi, Ocimum sanctum Linn as a medicinal
plant. Ind. J. Exp. Biol. 40;765-73.
Hanaa, A., Yamani-Edwin, C., Pang-Nitin, M., and Margaret,
A. (2016). Deighton Antimicrobial Activity of Tulsi (Ocimum
tenui orum) Essential Oil and Their Major Constituents against
Three Species of Bacteria. Front Microbiol. 7;681.
Harborne, J.B. (1973). Phytochemicals Methods. Chapman and
Hall Ltd., London, 49-188.
Helen, MP., Raju, V., Gomathy, SK., Nizzy, SK., Sree, SJ. (2011).
Essential oil analysis in Ocimum sps. Herbal Technol. Industry
8; 12–15.
Himal, P., Nisha, S., Jyoti, S., Anupa, K., Mansoor, S., Panna, T.
(2008). Phytochemical and Antimicrobial Evaluations of some
medicinal plants of Nepal. J. Sci, Eng Technol. 1(5);49-54.
Jiang, B., Mantri, N., Hu, Y., Lu, J., Jiang, W., Lu, H. (2015).
Evaluation of bioactive compounds of black mulberry juice
after thermal, microwave, ultrasonic processing, and storage
at different temperatures. Food Sci. Technol. Int. 21(5);392-9.
Khan, N., Abbasi, AM., Dastagir, G., Nazir, A., Shah, GM., Shah,
M.M. (2014). Ethnobotanical and antimicrobial study of some
selected medicinal plants used in Khyber Pakhtunkhwa (KPK)
as potential source to cure infectious diseases.BMC Comple-
ment Altern. Med.14;122.
Kokate, C. (1991). Practical Pharmacognosy. Vallabh
Prakashan, New Delhi. 107-111.
Levy, S., Marshall, B. (2004). Antibacterial resistance world-
wide: causes, challenges and responses. Nat. Med. 10;122-127.
Mandave, P., Pawar, P., Ranjekar, P., Mantri, N., Kuvalekar, A.
(2014). Comprehensive evaluation of in vitro antioxidant activ-
ity, total phenols and chemical pro les of two commercially
important strawberry varieties. Sci. Hortic. 172; 124–134.
Mishra, P., Mishra, S. (2011). Study of antibacterial activity
of Ocimum sanctum extract against Gram Positive and Gram
negative bacteria. American J. Food Tech. 6;336 - 341.
Mohan, L., Amberkar, M., Kumari, M. (2011). Ocimum sanctum
linn (Tulsi - An overview . Int. J. Pharm. Sci Rev. Res. 7;51-53.
Poole, K.(2011) Pseudomonas aeruginosa: resistance to the
max. Front Microbiol.2;65.
Prakash, P., Gupta, N. (2005). Therapeutic uses of Ocimum
sanctum linn (Tulsi) with a note on eugenol and its pharma-
cological actions : A short review. Ind.J. Physiol. Pharma-
col.49;125-131.
Rakholiya, K., Kaneria, M., Chanda, S. (2015). In vitro assess-
ment of novel antimicrobials from methanol extracts of mature
seed kernel and leaf of Magnifera indica L. for inhibition of
Pseudomonas spp. and their synergistic potential. Am. J. Drug
Discovery Development. 5(1);13-23.
Robles-Zepeda, RE., Coronado-Aceves, EW., Velazquez-
Contreras, CA., Ruiz-Bustos, E., Navarro-Navarro, M., Gar-
ibay-Escobar, A. (2013). In vitro anti-mycobacterial activity
of nine medicinal plants used by ethnic groups in Sonora,
Mexico.BMC Complement Altern Med.13;329.
Mallikarjun, S., Rao, A., Rajesh, G., Shenoy, R., Mithun P.
(2016). Antimicrobial ef cacy of Tulsi leaf (Ocimum sanctum)
extract on periodontal pathogens: An in vitro study J. Ind. Soc.
Periodontol. 20(2);145-150.
Sarah, M., Wigmore, M., Naiker, D,, Bean, C. (2016). Antimi-
crobial Activity of Extracts from Native Plants of Temperate.
Australia Pharmacogn. Commn. 6(2);80-84.
Sibanda, T., Okoh, AI. (2007). The challenges of overcoming
antibiotic resistance: Plant extracts as potential sources of
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EVALUATION OF PHYTO CONSTITUENT AND SYNERGISTIC ANTIBACTERIAL ACTIVITY 863
Shah, Hasan and Zaidi
antimicrobial and resistance modifying agents. Afr. J. Biotech-
nol. 6(25); 2886-2896.
Tekwu, EM., Pieme, AC., Beng, V.P. (2012). Investigations of
antimicrobial activity of some Cameroonian medicinal plant
extracts against bacteria and yeast with gastrointestinal rel-
evance.J. Ethnopharmacol. 142;265-73.
Trease, G., Evans, W. (1989). Pharmacognosy. Bailliere Tindall,
London : 45-50.
Watson, RR., Preedy, VR. Bioactive Foods and Extracts. Cancer
Treatment and prevention. 1
st
ed. United States of America:
CRS Press; 2011
Wu, Z., Jiang, W., Mantri, W., Bao, N., Q., X., Chen, SL., Tao
Z.M. (2016). Characterizing diversity based on nutritional and
bioactive compositions of yam germplasm (Dioscorea spp.)
commonly cultivated in China. J. Food Drug Anal. 24; 367–
375.
864 EVALUATION OF PHYTO CONSTITUENT AND SYNERGISTIC ANTIBACTERIAL ACTIVITY BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS