Biomedical
Communication
Biosci. Biotech. Res. Comm. 9(4): 821-827 (2016)
Effects of melatonin on repair of DNA double strand
breaks caused by ionizing radiation in rat peripheral
blood
Majid Valizadeh
1
, Alireza Shirazi*
2
, Pantea Izadi
3
and Javad Tavakkoli Bazzaz
4
,
Hamed Rezaeejam
5
and Ghasem Azizi Tabesh
6
1,2,5
Department of Medical, Physics and Biomedical Engineering, Tehran University of Medical Sciences,
Tehran, Iran
3,4,6
Department of Medical Genetics, Tehran University of Medical Sciences, Tehran, Iran
ABSTRACT
The present study aimed to evaluate the effect of melatonin on DNA Double-Strand Breaks repair using gene expres-
sion change of Ku70 and Xrcc4 in rat peripheral blood. One hundred eight male rats were randomly divided in six
different groups of control, vehicle-only, melatonin alone, irradiation-only, vehicle plus irradiation, and melatonin
plus irradiation. Rats were given an intraperitoneal (IP) injection of melatonin (100 mg/kg) 1 hr prior to irradiation.
Irradiation was done with 2Gy whole-body radiation by linear accelerator. Peripheral blood samples were collected
at 8, 24 and 48 h after irradiation and then RNA was immediately extracted from the peripheral blood lymphocytes
and the mRNA transcriptional changes of Ku70 and XRCC4 were evaluated by real-time quantitative polymerase
chain reaction (Real-time PCR). Irradiation-only group showed an increase in gene expression change of Ku70 and
XRCC4, which was signi cantly different from control group (P<0.01) in 24 h post-irradiation, while in melatonin
plus irradiation group, Ku70 and XRCC4 genes were upregulated signi cantly compared to control group (P<0.01) at
all three post-irradiation times. It is concluded that melatonin may provide modulation of Ku70 and XRCC4 expres-
sion to protect rat peripheral blood lymphocytes against ionizing radiation.
KEY WORDS: IONIZING RADIATION, DOUBLE-STRAND BREAK, MELATONIN, DNA REPAIR
821
ARTICLE INFORMATION:
*Corresponding Author: shirazia@tums.ac.ir
Received 30
th
Oct, 2016
Accepted after revision 10
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/

822 EFFECTS OF MELATONIN ON REPAIR OF DNA DOUBLE STRAND BREAKS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Majid Valizadeh et al.
INTRODUCTION
Ionizing radiation (IR) is applied constructively in a wide
variety of elds such as medicine, research, manufac-
turing, construction especially in medical diagnostic for
cancer treatment (Jobert et al. 2011; Thoms and Bristow,
2010), but presents a health hazard if proper measures
against undesired exposure aren’t followed. Exposure to
ionizing radiation causes damage to living tissue, and
can result in mutation, radiation sickness, cancer, and
death. The absorption of ionizing radiation by living
cells can directly disrupt atomic structures, producing
chemical and biological changes. It can also act indi-
rectly through radiolysis of water, thereby generating
reactive chemical species that may damage nucleic acids,
proteins and lipids. DNA damage of exposed tumour tis-
sue leading to cell death is one of the detrimental effects
of ionizing radiations, (Hall and Giaccia, 2006, Azzam
et al.2012; Lomax et al. 2013 an d Rezaeejam et al 2015).
There are many different forms of IR-induced DNA
damages such as single-strand breaks (SSBs), double-
strand breaks (DSBs), base and sugar damage, DNA-
DNA cross-links and DNA-protein cross-links. DSBs are
lesions formed when both strands of the DNA duplex are
broken. DSBs are highly toxic and are the most impor-
tant IR-induced DNA damages in chromosomes after
exposure which leads to cells death, mutation or car-
cinogen. They must be repaired to protect the genome
and cells survival, (Brandsma and Gent, 2012; Ciccia and
Elledge, 2010 Chapman et al. 2012).
These kinds of damages are mainly repaired by
homologous recombination (HR) and non-homologous
end joining (NHEJ) pathways (Lieber, 2010). HR leads to
accurate repair, while NHEJ is intrinsically mutagenic.
Mao et al (2008) suggested that NHEJ is a faster and
more ef cient DSB repair pathway than HR in human
cells. According to Guirouilh-Barbat et al. (2004), NHEJ
is the predominant DSB repair pathway in mammalian
cells. NHEJ is the major DSB repair pathway in eukary-
otes and is utilized in the cellular response of mamma-
lian cells to the repair of IR-induced DSBs. NHEJ can
take place throughout the cell cycle. NHEJ modi es the
broken DNA ends, and ligates them together with no
regard for homology, generating deletions or insertions
(Lieber, 2008), while HR uses an undamaged DNA tem-
plate to repair the break, leading to the reconstitution of
the original sequence (Thompson and Schild, 2001).
The proteins that participate in NHEJ pathway include
XRCC4, XRCC5 (Ku80), XRCC6 (Ku70), DNA-PKcs,
DNA ligase IV, Artemis, and XLF (Bassing et al. 2002;
Shrivastav et al. 2008). First, both ends of the break are
joined by the Ku70/80 heterodimer which protects the
DNA ends from degradation. Then Ku70/80 recruits the
catalytic subunit of the DNA dependent protein kinase
(DNA-PKcs) to DNA ends to form the active DNA-PK;
the ends can be trimmed or lled in by nucleases and
polymerases. Finally the DNA-PKcs complex stimulates
the end processing for subsequent ligation by XRCC4/
DNA ligase IV (Ahnesorg et al. 2006; San Filippo et
al.2008; Schulte-Uentrop et al. 2008; Zhang et al. 2013).
The radio protective agents protects against the dam-
aging effects of IR with various mechanisms. Many
studies have reported the ability of melatonin (N-acetyl-
5-methoxytryptamine), a pineal gland hormone, to pro-
tect against IR-induced damages (Reiter, 1991; Undeger
et al. 2004; Shirazi et al. 2011; Shirazi, 2011). It scaven-
gers free radicals, directly and indirectly, especially the
highly toxic hydroxyl radicals. Melatonin is also an anti-
oxidant agent by increasing antioxidant enzyme activ-
ity and inhibiting pro-oxidative enzyme activity (Koc
et al. 2003; Rodriguez et al. 2004; Parihar et al. 2007).
Many in vitro and in vivo investigations have con rmed
that melatonin protects mammalian cells from the toxic
effects of ionizing radiation. Furthermore, several clini-
cal reports indicate that melatonin administration, either
alone or in combination with traditional radiotherapy,
results in a favorable ef cacy (toxicity ratio) during the
treatment of human cancers (Vijayalaxmi et al. 2004).
There are many studies which have examined mela-
tonin as a radio protector in radiobiology but there is
a serious lack of information about its impact on the
DNA repair with NHEJ pathway. In this regard, in this
study we have investigated the effect of melatonin on
the repair of IR-induced DNA DSB in the peripheral
blood of rat when NHEJ pathway is used. We studied
the expression change of Ku70 and XRCC4 genes under
2Gy whole-body gamma irradiations to show the radio-
protective effect of 100 mg/kg administered melatonin
on DNA DSB repair.
MATERIALS AND METHOD
In this in vivo study, all experiments were in accord-
ance with the guidelines for care and use of labora-
tory animals as adopted by the Ethics Committee of the
School of Medicine, Tehran University of Medical Sci-
ences (TUMS), Tehran, Iran. One hundred eight 70-day
old male Wistar rats with a body weight range of 180 to
220 g were used for the study from pharmacy faulty of
TUMS. They were kept in a room temperature and main-
tained at 20-22° C and light-controlled environment
with a 12-hour light/dark cycle. All rats were given
standard diet with no additives.
EXPERIMENTAL DESIGN AND IRRADIATION
After one week acclimatization period, animals were
randomly divided into six different groups:
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EFFECTS OF MELATONIN ON REPAIR OF DNA DOUBLE STRAND BREAKS 823
Majid Valizadeh et al.
Group 1. Control (CON): In this group, rates
received no melatonin or irradiation
but received both an intraperitoneal (IP)
injection of 500 μl of phosphate-buff-
ered saline (PBS) and sham-irradiation;
Group 2. Melatonin (MEL): In this group, one hour
before irradiation, all anesthetized rats
received 100 mg/kg melatonin with IP
injection of 500 μl PBS and nal ethanol
concentration 5% and then they went
under and sham-irradiation. It should
be noted that all rats were anesthetized
with an IP injection of ketamin (100 mg/
kg) and xylazin (5 mg/kg);
Group 3. Irradiation (IR): In this group rats went
under 2 Gy whole body gamma radia-
tion and received the same volume of
PBS 1 h prior to irradiation;
Group 4. Vehicle (VEH): In this group rats received
5% absolute ethanol with IP injection of
500 μl PBS;
Group 5. Vehicle + irradiation (VEH+IR): In this
group rats received 5% absolute ethanol
with IP injection of 500 μl PBS plus 2 Gy
whole body gamma radiation;
Group 6. Melatonin + irradiation (MEL+IR): In
this group rats received 100 mg/kg mel-
atonin with 5% absolute ethanol and
an IP injection of 500 μl PBS plus 2 Gy
whole body gamma radiation.
It should be mentioned that melatonin rst was dissolved
in a small amount of absolute ethanol (25μl) and then
diluted by PBS (475μl) in nal ethanol concentration 5%
based on previous studies like Cassatt et al. (2002). Also,
all rats were anesthetized with ketamin (100 mg/kg) and
xylazin (5 mg/kg) by an IP injection before any inter-
vention based on previous studies like Prasad (1995).
Rats were irradiated with a 6 MV X-ray linear accelera-
tor machine (Elekta Compact 6 MV, China) with a xed
eld size of 35cm×35cm at room temperature (22 ± 2ºC).
Before irradiation, to ensure the output of the accelera-
tor, dosimetry and calibration were performed by using
an ionizing chamber based on International Atomic
Energy Agency (IAEA) TRS-398 standard.
BLOOD SAMPLE PREPARATION, RNA
ISOLATION AND CDNA SYNTHESIS
Each study group includes 18 rats and divided in three
subgroups containing six rats. From all of these sub-
groups peripheral blood sample was taken on EDTA ster-
ile tubes at 8, 24 and 48 h after irradiation. Total RNA
was isolated from whole blood by Hybrid-R blood RNA
mini 315-150 kit (GeneAll Biotechnology, Seoul, South
Korea) according to the manufacturer’s instructions. The
extracted RNA was quanti ed and its purity quali ed
by using a Nanodrop-2000 spectrophotometer (Thermo
Scienti c, Wilmington, USA) respectively at 260/280
nm and 260/230 nm ratios. The integrity of isolated
RNA was con rmed with Agarose gel electrophoresis.
The representative samples were stained with ethidium
bromide to visualize the 18S and 28S RNA subunits by
band size discrimination under UV transillumination.
For cDNA synthesis, a 2-μg aliquot of the total RNA was
reverse transcribed in a total volume of 20μl by using
the Hyperscript TM rst strand synthesis Kit (GeneAll
Biotechnology, Seoul, South Korea).
QUANTITATIVE REAL TIME RT-PCR
Quantitative real time PCR was used to measure the
expression of Ku70 and XRCC4 genes. After RNA isola-
tion and cDNA synthesis, Ku70 and XRCC4 primers were
designed by Gene Runner software and their expression
were determined by using HPRT as an internal control.
The sequences of forward and reverse primers were as
follows: Ku70, forward primer: GCT TGT CTT CCT CCC
TTA CG, reverse primer: CGA AAC TGT CGC TCC TGT
ATG; XRCC4, forward primer: CTG AGG AGG ATG GGC
TTT ATG AT, reverse primer: CAA GAT TTG TCT GCA
TTC GGT GT; and HPRT, forward primer: CCA GTC AAC
GGG GGA CAT AAA, reverse primer: GGG GCT GTA
CTG CTT GAC CAA. Basic Local Alignment Search Tool
(BLAST) searches were also conducted to verify primer
speci city in the absence of DNA ampli cation. The
primers were synthesized by Takapouzist laboratory in
Tehran, Iran.
The real time polymerase chain reactions (real time
PCR) were carried out by the Rotor-gene Q system
(QIAGEN), based on the SYBR green method using the
SYBR Premix Ex Taq No. RR820L (TaKaRa) following
the manufacturer’s instructions. The PCR reaction mix-
ture contained all reactions running in duplicate and the
real time PCR cycling conditions were as follows: ini-
tial denaturation at 95ºc for 10 min, followed by cycles
of denaturation at 95ºc for 10s, annealing at 60ºc for
20s. In real-time PCR studies, relative quanti cation or
relative gene expression is the parameter used for rela-
tive fold changes in expression of target genes (Ku70
and XRCC4), normalized to an internal reference (HPRT
gene) and a relevant untreated and unirradiated control.
This parameter is calculated according to
2
-
ΔΔCT
formula.
ΔΔCT is the difference between the mean ΔCT (treatment
group) and mean ΔCT (control group) and ΔCT is the
difference between the mean CT gene of interest and the
mean CT
of internal control gene in each sample. CT is
the threshold cycle, i.e. the cycle number at which the
824 EFFECTS OF MELATONIN ON REPAIR OF DNA DOUBLE STRAND BREAKS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Majid Valizadeh et al.
PCR product crosses the threshold of detection. For each
group at 8, 24 and 48 h post-irradiation, six independent
blood samples were assessed. Assays were performed in
duplicate for each sample.
STATISTICAL ANALYSIS
For analyzing data the mean ± SEM of experiments per
group were presented and one-way analysis of variance
(ANOVA) was performed in order to compare the differ-
ences among groups, followed by Tukey’s test for mul-
tiple comparison. The signi cance level was set at 0.05.
RESULTS
The expression change of Ku70 and XRCC4 genes in the
rat peripheral blood at 8, 24 and 48 h after irradiation of
sublethal dose of 2Gy whole body radiation were ana-
lyzed by real-time quantitative PCR. The results have
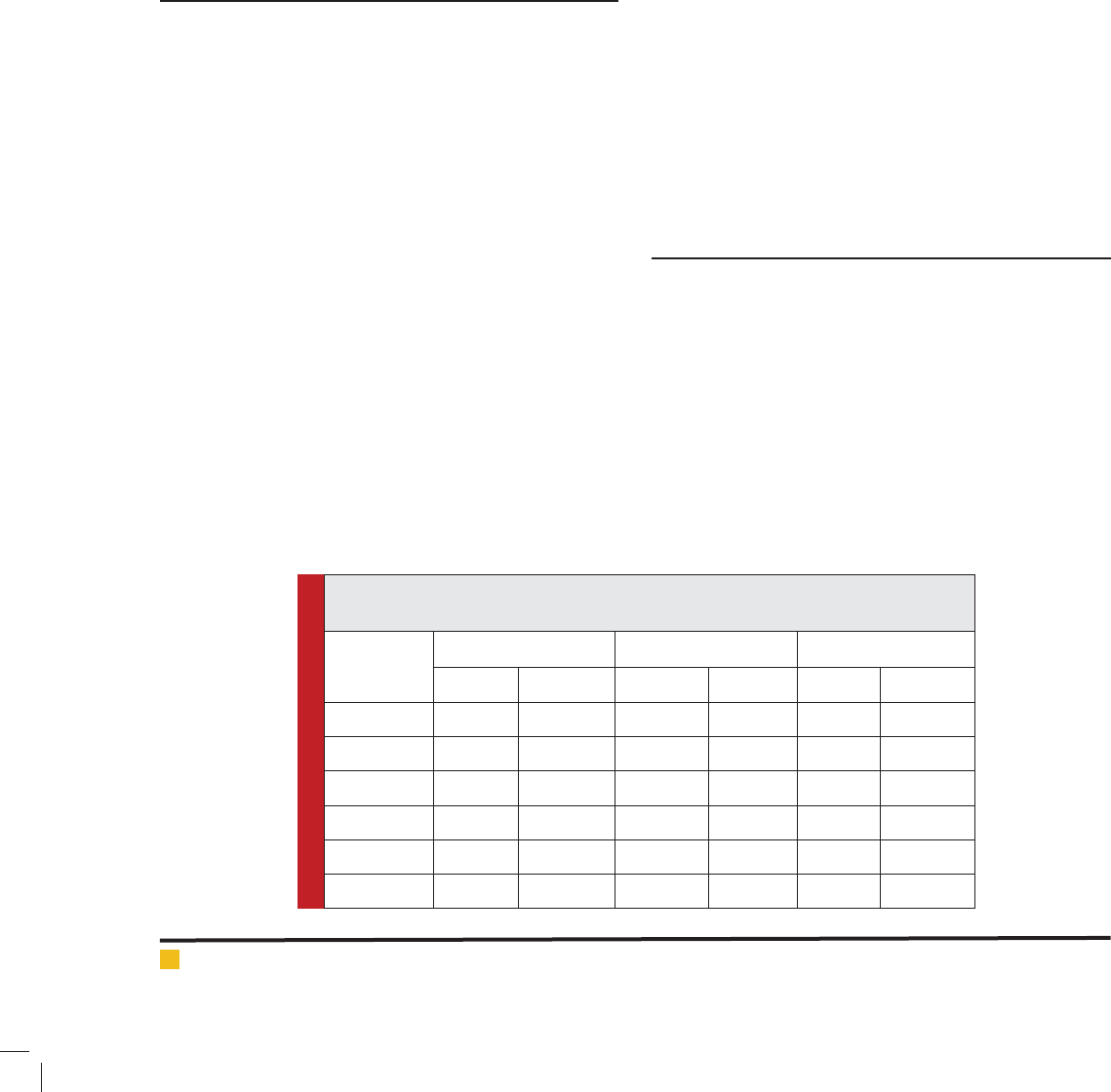
been summarized in Table 1.
The obtained results show that in comparison with
the control group, the mRNA levels of Ku70 and XRCC4
genes (normalized against HPRT) have signi cant
change in some groups. Gene expression increased sig-
ni cantly in IR and VEH + IR groups only at 24 h after
irradiation compared to control group or both genes.
In IR group these changes were 5.9 (P<0.01) and 7.67
fold (P<0.05) for Ku70 and XRCC4, respectively; and in
VEH + IR group the fold changes were 5.39 (P<0.01) and
7.01 (P<0.05) for Ku70 and XRCC4, respectively. Expres-
sion of these two genes also increased signi cantly in
MEL+IR group at all three times after irradiation in com-
parison with control group (Fig. 1). Expression changes
for XRCC4 gene at 8, 24 and 48 h were 14.42, 41.93, and
5.39 fold respectively (P<0.01), whiles these results for
Ku70 gene were 7.36, 29.65, and 4.47 fold, respectively
(P<0.01). We found no signi cant differences in the gene
expression of Ku70 and XRCC4 in other groups com-
pared to control group.
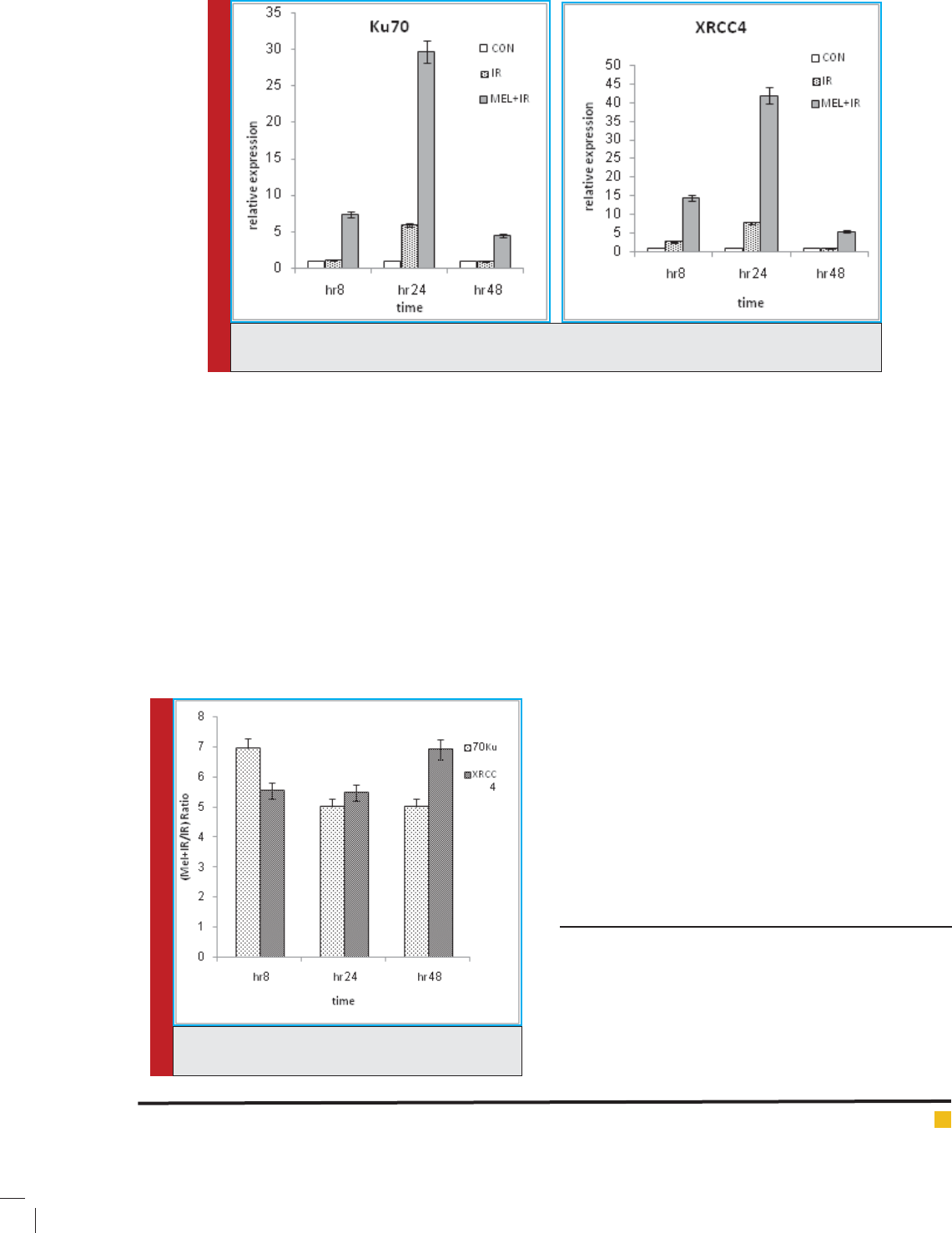
Results showed that regulation changes of Ku70 and
XRCC4 genes were signi cantly different in MEL + IR
group at three times after irradiation compared to IR
group as well. We calculated a ratio (fraction) in order to
understand the effect of melatonin on the NHEJ repair
pathway in the current study. Thus relative expression
of MEL+ IR group was divided by relative expression of
IR group. This ratio is going to show the role of mela-
tonin in regulating Ku70 and XRCC4 genes. Based on
the results for Ku70 and XRCC4 genes, it can be said
that melatonin upregulates Ku70 gene as 6.94, 5.02 and
5.02 fold higher and XRCC4 gene as 5.55, 5.47 and 6.91
fold higher at 8, 24 and 48 post- irradiation, respectively
(Fig.2). It can be seen that melatonin upregulates Ku70
more than XRCC4 gene at 8 h and upregulates XRCC4
gene more than Ku70 gene at 48 h after irradiation.
In other words, with the increase of post- irradiation
time, melatonin decreases gene expression for Ku70 and
increases it for XRCC4. Results indicate that melatonin
not only prevents cell death by apoptosis but also can
repair damaged cells in NHEJ pathway.
DISCUSSION
Some studies have been conducted on the protective
role of melatonin against oxidative DNA damage and
the effects of melatonin on cell cycle and apoptosis.
Before conducting our study, it was very important to
know if damaged cells which must be killed have been
previously repaired or not. The effect of melatonin on
apoptosis has been studied previously by Mohseni et
al. (2012), Shirazi et al. (2010), and Rezaeejam et al.
(2015) whose results showed that melatonin decreases
relative expression of pro-apoptotic BAX and increases
anti-apoptotic Bcl-2 genes. In this basis, current study
Table 1: Real-time quantitative RT-PCR analysis of the fold change of Ku70 and
XRCC4 at various time points after irradiation relative to control group.
Group
8 h 24 h 48 h
Ku70 XRCC4 Ku70 XRCC4 Ku70 XRCC4
CON 1 1 1 1 1 1
VEH 0.97 0.98 0.93 0.93 0.93 0.95
MEL 1.02 1.07 1.03 1.07 1.04 1.10
IR 1.06 2.60 5.90 7.67 0.89 0.78
VEH + IR 0.97 2.46 5.39 7.01 0.95 0.75
MEL + IR 7.36 14.42 29.65 41.93 4.47 5.39
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EFFECTS OF MELATONIN ON REPAIR OF DNA DOUBLE STRAND BREAKS 825
Majid Valizadeh et al.
FIGURE 1. Temporal response of Ku70 and XRCC4 genes expression at untreated and pretreated
groups. Points denote the mean of responses in six different rats.
FIGURE 2. Manifold effect of melatonin on the
regulation of genes.
investigated the in uence of melatonin on DNA DSBs
repair-related important genes because of sublethal dose
of ionizing radiation in the NHEJ pathway in peripheral
blood rat. According to many previous studies, XRCC4,
XRCC5 and XRCC6 (KU70) genes are the core of DNA
DSBs repair-related genes involved in NHEJ pathway. In
this study, the gene expression of Ku70 and XRCC4 were
examined using real-time quantitative PCR.
We used 2Gy of 6MV irradiation because it is a stand-
ard fraction that is administrated to patients daily in
a fractionation regimen and the highest sensitivity for
human DNA is obtained for this dose of radiation. The
selection of one hour interval between melatonin injec-
tion and exposure to gamma radiation was largely based
on previous studies (Vijayalaxmi et al. 1999; Hussein
et al. 2005). The melatonin concentration was selected
based on the experience from the performed studies by
other researchers (e.g. Yurtcu et al. 2007) and our pre-
vious studies (Mohseni et al. 2012; Shirazi et al. 2010;
Rezaeejam et al. 2015) where it was found out this con-
centration doesn’t have any toxicity.
Based on the results in this study, since VEH and MEL
groups showed no signi cant difference with control
group, we found out that ethanol and melatonin do not
have any effects on the expression change of Ku70 and
XRCC4 genes alone. On the other hand, IR group had
no signi cant difference with VEH + IR group, so we
can say that the injection of ethanol before irradiation
doesn’t affect the regulation of Ku70 and XRCC4 genes.
Furthermore, results illustrated that DBS damages was
repaired at 24 h and there was no repair at 8 and 48 h
after irradiation in the NHEJ pathway, while the DSBs
was repaired at 8, 24 and 48 h post-irradiation when
melatonin was injected before irradiation. The repair
process was also accelerated and strengthened in this
condition at 24 h post-irradiation.
CONCLUSION
In this study we concluded that melatonin injection
before ionizing radiation can increase the expres-
sion levels of Ku70 and XRCC4 genes, and melatonin
has positive effect on repair in NHEJ pathway. Further
inestigation is recommended on the other doses, post-
irradiation times and genes related to HR and NHEJ
pathways.

Majid Valizadeh et al.
826 EFFECTS OF MELATONIN ON REPAIR OF DNA DOUBLE STRAND BREAKS BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
ACKNOWLEDGEMENT
This research was supported by Tehran University of
Medical Sciences in Iran with health services grant
No.28688.
REFERENCES
Ahnesorg P., Smith P., Jackson S.P. (2006). XLF interacts with
the XRCC4-DNA ligase IV complex to promote DNA nonho-
mologous end-joining. Cell, 124(2):301-13.
Azzam E., Jay-Gerin J.P., Pain, D. (2012). Ionizing radiation-
induced metabolic oxidative stress and prolonged cell injury.
Cancer Letters, 327(0): 48–60.
Bassing C.H., Chua K.F., Sekiguchi J. (2002). Increased ionizing
radiation sensitivity and genomic instability in the absence of
histone H2AX. Proceedings of the National Academy of Sci-
ences of the United States of America, 99(12):8173-8.
Brandsma I., Gent D.C. (2012). Pathway choice in DNA double
strand break repair: observations of a balancing act. Genome
Integrity, 3(1):9.
Cassatt D.R., Fazenbaker C.A., Bachy C.M., Hanson M.S. (2002).
Preclinical modeling of improved amifostine (Ethyol) use in
radiation therapy. Seminars in Radiation Oncology, 12(1 Suppl
1):97-102.
Ciccia A., Elledge S.J. (2010). The DNA damage response:
making it safe to play with knives. Molecular Cell, 40(2):179-
204
Chapman J.R., Taylor M.R., Boulton S.J. (2012). Playing the
end game: DNA double-strand break repair pathway choice.
Molecular Cell, 47(4):497-510.
Guirouilh-Barbat J., Huck S., Bertrand P. (2004). Impact of the
KU80 pathway on NHEJ-induced genome rearrangements in
mammalian cells. Molecular Cell, 14 (5): 611–23.
Hall E.J., Giaccia A.J. (2006). Radiobiology for the Radiologist.
6th. Lippincott Williams & Wilkins, Philadelphia, PA.
Hussein M.R. , Abu-Dief E.E. , Abd El-Reheem M.H. , Abd-
Elrahman, A. (2005). Ultrastructural evaluation of the radio-
protective effects of melatonin against X-ray-induced skin
damage in Albino rats. International Journal of Experimental
Pathology, 86(1):45–55.
Jobert A., Vogin G., Devic C., Granzotto A., Viau M., Maalouf
M. (2011). Radiation biology: major advances and perspectives
for radiotherapy]. Cancer radiotherapie: Journal De La Societe
Francaise De Radiotherapie Oncologique, 15(5):348-54 . (Arti-
cle in French)
Koc M., Taysi S., Buyukokuroglu M.E., Bakan N. (2003). Mela-
tonin protects rat liver against irradiation-induced oxidative
injury. Journal Of Radiation Research, 44(3):211-5.
Lieber M.R. (2010). The mechanism of double-strand DNA
break repair by the nonhomologous DNA end joining pathway.
Annual Review Of Biochemistry, 79:181-211
Lieber M.R. (2008). The mechanism of human nonhomologous
DNA end joining. Journal of Biological Chemistry, 283:1–5.
Lomax M.E., Folkes L.K., O’Neill P. (2013). Biological Conse-
quences of Radiation-induced DNA Damage: Relevance to
Radiotherapy. Clinical Oncology,25(10):578-85.
Mao Z., Bozzella M., Seluanov A., Gorbunova V. (2008). Com-
parison of nonhomologous end joining and homologous recom-
bination in human cells. DNA Repair (Amst), 7(10):1765–1771.
Mohseni M., Mihandoost E., Shirazi A., Sepehrizadeh Z., Baz-
zaz J.T., Ghazi-khansari M. (2012). Melatonin may play a role
in modulation of bax and bcl-2 expression levels to protect rat
peripheral blood lymphocytes from gamma irradiation-induced
apoptosis. Mutation Research/Fundamental and Molecular
Mechanisms of Mutagenesis, 738(2):19-27.
Parihar V.K., Dhawan J., Kumar S., Manjula S., Subramanian
G., Unnikrishnan M. et al. (2007). Free radical scavenging and
radioprotective activity of dehydrozingerone against whole
body gamma irradiation in Swiss albino mice. Chemico-bio-
logicalinteractions, 170(1):49-58.
Prasad KN. (1995). Handbook of radiobiology (2nd Ed.), CRC
Press, Boca Raton, FL.
Reiter R.J. (1991). Pineal melatonin: cell biology of its synthe-
sis and of its physiological interactions. Endocrine Reviews,
12(2):151-80.
Rezaeejam H., Shirazi A., Valizadeh M., Izadi A. (2015). Candi-
date gene biodosimeters of mice and human exposure to ioniz-
ing radiation by quantitative reverse transcription polymerase
chain reaction. Journal of Cancer Research and Therapeutics,
11(3): 549-557.
Rodriguez C., Mayo J.C., Sainz R.M., Antolin I., Herrera F.,
Martín V. (2004). Regulation of antioxidant enzymes: a sig-
ni cant role for melatonin. Journal Of Pineal Research, 36(1):
1-9.
San Filippo J., Sung P., Klein H. (2008). Mechanism of eukary-
otic homologous recombination. Annual Review Of Biochem-
istry,77:229-57.
Schulte-Uentrop L., El-Awady R., Schliecker L., Willers H.,
Dahm-Daphi J. (2008). Distinct roles of XRCC4 and Ku80 in
non-homologous end-joining of endonuclease-and ionizing
radiation-induced DNA double-strand breaks. Nucleic Acids
Research, 36(8):2561-9.
Shirazi A., Mihandoost E., Mohseni M., Ghazi-Khansari M.,
Rabie Mahdavi S. (2011). Radio-protective effects of melatonin
against irradiation-induced oxidative damage in rat peripheral
blood. Physica Medica, 29(1):65-74.
Shirazi A. (2011). Radioprotective effect of melatonin in reduc-
ing oxidative stress in rat lenses. Cell Journal, 13(2):79.
Shirazi A., Haddadi G., Minaee B., Sepehrizadeh Z., Mahdavi
S., Jaberi E. (2010). Evaluation of melatonin for modulation
of apoptosis-related genes in irradiated cervical spinal cord.
International Journal of Low Radiation,7(6):436-45.
Shrivastav M., De Haro L.P., Nickoloff J.A. (2008). Regula-
tion of DNA double-strand break repair pathway choice. Cell
Research, 18(1):134-47.
Thompson L.H., Schild D. (2001). Homologous recombinational
repair of DNA ensures mammalian chromosome stability.
Majid Valizadeh et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS EFFECTS OF MELATONIN ON REPAIR OF DNA DOUBLE STRAND BREAKS 827
Mutation Research/Fundamental and Molecular Mechanisms
of Mutagenesis, 477:131–153.
Thoms J., Bristow R.G. (2010). DNA repair targeting and radio-
therapy: a focus on the therapeutic ratio. Seminars In Radia-
tion Oncology , 20(4):217-22.
Undeger Ü., Giray B., Zorlu A.F., Öge K., Baçaran N. (2004).
Protective effects of melatonin on the ionizing radiation
induced DNA damage in the rat brain. Experimental and Toxi-
cologic Pathology, 55(5):379-84.
Vijayalaxmi M., Reiter R., Herman T., Kumar K. (1999). Mela-
tonin and protection from whole-body irradiation: survival
studies in mice. Mutation Research/Fundamental and Molecu-
lar Mechanisms of Mutagenesis, 425(1):21-7.
Vijayalaxmi M., Reiter R.J., Tan D.X., Herman T.S., Thomas
C.R. Jr. (2004). Melatonin as a radioprotective agent: a review.
International Journal of Radiation Oncology, 59(3):639-
53.
Zhang L.Y., Chen L.S., Sun R., Sheng-Jun J., Ding Y.Y., Wu
J. et al. (2013). Effects of expression level of DNA repair-
related genes involved in the NHEJ pathway on radiation-
induced cognitive impairment. Journal Of Radiation Research,
54(2):235-42.