Medical
Communication
Biosci. Biotech. Res. Comm. 9(4): 795-803 (2016)
MTR
-A2756G and breast cancer risk: a study of
Iranian women with a meta-analysis
Mohammad Reza Sharif
1,2
and Mohammad Karimian
3
1
Autoimmune DiseasesResearch Center, Kashan University of Medical Sciences, Kashan, Iran
2
Department of Pediatrics, Kashan University of Medical Sciences, Kashan, Iran
3
Anatomical Sciences Research Center, Kashan University of Medical Sciences, Kashan, Iran
ABSTRACT
Polymorphisms in folate metabolizing genes have been revealed to be associated with the risk of breast cancer. One
of the key regulatory enzymes in the folate metabolism pathway is methionine synthase (MTR) enzyme. The aim of
this study was to investigate the association of A2756G polymorphism in MTR gene with breast cancer risk followed
by a meta-analysis. In a case-control study, 157 women with sporadic breast cancer and 188 healthy women were
included. MTR-A2756G genotyping was performed by using PCR-RFLP method. In our meta-analysis, a total of 22
studies re ecting 14037cases and 16621 healthy controls were included. Our case-control study revealed that GG
genotype is associated with breast cancer risk (OR: 2.89, 95%CI: 1.06-7.90, p=0.039). In meta-analysis, a signi cant
association was observed between MTR-A2756G and breast cancer risk within Asian population. While we observed
a protective association of MTR-A2756G polymorphism with breast cancer risk. Based on result, MTR-A2756G may
be a genetic risk factor and a protective factor for breast cancer in Asian and Caucasian populations, respectively.
This different effects of MTR-A2756G polymorphism in breast cancer risk may arise from ethnicity.
KEY WORDS: BREAST CANCER;
MTR
GENE; A2756G POLYMORPHISM; META-ANALYSIS
795
ARTICLE INFORMATION:
*Corresponding Author: mdkarimian@yahoo.com
Received 30
th
Nov, 2016
Accepted after revision 28
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Breast cancer is one of the main causes of cancer-related
mortality in women worldwide. Every year about 1.2
million women suffer from breast cancer in the world
and this number is increasing. The etiology of the breast
cancer is poorly understood. Some factors such as repro-
ductive history, age of menopause, changes in estrogen
level, diet, smoking, familiar history, and genetic factors
can be involved in this cancer. The greatest obstacle for
the treatment of this disease is a highly variable out-
come of breast cancer among patients, even women with
the same biological characteristics and stage. It seems
some genes may provide a possible explanation for
796 MTR GENE POLYMORPHISM AND BREAST CANCER RISK BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Reza Sharif and Karimian
breast cancer predisposition and could be essential for
treatment choices and improve patients’ survival. Genes
contributed to DNA repair, synthesize and methylation
such as genes involved in folate metabolizing pathway,
are good candidates for this purpose. Failures in folate
metabolizing genes, may be associated with the risk of
breast cancer ( Parkin, 2001; Stover, 2004, Ferlay et al.,
2004, Hankinson et al., 2004; Dumitrescu et al., 2005,
Babyshkina et al., 2013 and Wu et al., 2014).
Methionine synthase (MTR) which catalyzes the rem-
ethylation of homocysteine to methionine is a regula-
tory enzyme in folate metabolism (Fodinger et al., 2000).
Mutations or Single nucleotide polymorphisms (SNPs)
in coding sequence of MTR gene may lead to decrease
modi cation of DNA methylation pro les due to reduce
in the ef ciency of purine nucleotides and thymidylate
synthesis (Chen et al., 2001, Ma et al, 2009b; Suzuki et
al, 2008; Kakkoura et al, 2015; Lopez_Cortes et al, 2015).
There is a common SNP (A2756G; rs1805087) in
MTR sequence which result in substitution of aspartic
acid to glycine residue at position 919 (D919G) in the
protein sequence (Yu et al., 2007; Ma et al., 2009
a
). To
date, the association between MTR-A2756G and breast
cancer risk started to receive attention. A numerous
studies, investigate the association of this polymorphism
with breast cancer in different populations however the
results are inconsistent. Thus, in the present study we
investigate the association of MTR-A2756G with the risk
of breast cancer followed by a meta-analysis.
MATERIAL AND METHODS
SUBJECTS
A total of 345 women comprised of 157 women with
sporadic breast cancer as case group and 188 healthy
women as control group were included in this study. All
the participants were Iranian, and lived in the Kashan
city (Kashan, Iran). Patients (with mean age 54.40±10.12
years) were women referred to the Shahid Beheshti hos-
pital (Kashan, Iran) from 2011 to 2013. They were with
a histologically con rmed diagnosis of breast cancer.
Controls (with mean age 58.30±5.87) were women with
no history of oncological disease, who contributed in
the local mammography screening plan, and they had
no positive result. Finally from each subjects, 2mL blood
collected. All the participants’ informed written consent
and this study con rmed by the principles outlined in
the Declaration of Helsinki and approved by the Hospi-
tal’s Ethics Committee.
SNP GENOTYPING
Genomic DNA was isolated from blood samples by DNG-
plus buffer (Cinnagen Co., Iran). MTR-A2756G geno-
typing was performed by polymerase chain reaction-
restriction fragment length polymorphism (PCR-RFLP)
method. For PCR purpose the primer oligonucleotide were
designed by Oligo7 software. The forward and reverse
primer sequences were 5’-AAGCCCACTGAGTTTAC-
CTTTTC-3’ and 5’-ATCCAAAGCCTTTTACACTCCTC-3’,
respectively. The PCR reaction was done in a 20µl total
volume containing, 10µl PCR master mix buffer (Cinna-
gen Co., Iran), 0.35M each of forward and reverse prim-
ers, and 50ng genomic DNA. The thermal cycling program
for PCR porous were: initial denaturation for 5 min at
94ºC, followed by 35 repetitive cycles of 45s at 94ºC, 45s
at 63ºC, and 45s at 72ºC, with a nal extension of 5 min
at 72ºC. The ampli ed PCR fragments were digested by
HaeIII restriction enzyme (Fermentas, Lithuania) and vis-
ualized by ethidium bromide after electrophoresis in 1%
agarose gel. The accuracy of PCR-RFLP data was ensured
by DNA sequencing. For this purpose, three PCR products
with different genotypes were selected and sequenced by
Bioneer Company (Korea).
META-ANALYSIS
The PubMed, SienceDirect, and Google Scholar data-
bases were searched for published reports examining
associations between MTR-A2756G and breast cancer
risk by using the following keywords: breast cancer,
MTR, A2756G, and polymorphism. The literature search
was performed up to October 2015. Original articles
with suf cient information to compute odd ratio (OR)
with 95% Con dence Interval (CI) were selected. The
inclusion criteria for studies were as follows: (i) inves-
tigation of the MTR-A2756G polymorphism and breast
cancer risk (ii) studied on human beings; (iii) in a case-
control study design. The characteristics of included
studies in meta-analysis are introduced in Table 1. Also
the association between MTR-A2756G and breast cancer
was further strati ed by ethnicity.
STATISTICAL ANALYSIS
Odds ratio (OR) with 95% con dence interval (95%CI)
was calculated for various alleles and genotypes in case
and control groups. The Chi-square test was used to
compare the allele and genotype frequencies between
the case and control groups. Also, Hardy-Weinberg
equilibrium (HWE) in the case and control groups was
calculated. The p-value less than 0.05 (p<0.05) con-
sidered as statistically signi cant. These statistical
calculations were done by SPSS Statistical Package
version 19.
In meta-analysis a Chi square based ‘Q’ test was used
to evaluate the heterogeneity. When there was no het-
erogeneity among the studies (P
heterogeneity
>0.1), the pooled
ORs were calculated by a xed-effects model (the Man-
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS MTR GENE POLYMORPHISM AND BREAST CANCER RISK 797
Reza Sharif and Karimian
tel-Haenszel method). To sensitivity analysis, each study
was excluded at a time to determine the magnitude of
effect on the total summary assessment. Begg’s funnel
plot and Egger’s test were used to evaluate the publica-
tion bias (Begg and Mazumdar 1994; Egger et al., 1997).
These calculations were performed by using Comprehen-
sive Meta-Analysis software (version 2.0).
RESULTS
A2756G GENOTYPING
The MTR fragment containing A2756G with size of 381
bp was ampli ed by using the forward and reverse prim-
ers. After PCR-RFLP procedure, electrophoresis of the
products on agarose gel showed the AA, AG, and GG
genotypes with one band (381 bp), three bands (381 bp,
251 bp, and 130 bp), and two bands (251 bp, and 130
bp), respectively. PCR-RFLP results were con rmed by
DNA sequencing.
DISTRIBUTION OF A2756G
The MTR genotypes distribution for A2756G transition
was in Hardy-Weinberg equilibrium in the case and con-
trol groups. The genotypes and alleles frequencies for
the A2756G in case and control groups are presented
in Table 2. The AA, AG and GG genotypes frequencies
of cases were 55.41%, 36.31%, and 08.28%, while these
ratios in controls were 61.70%, 35.11%, and 03.19%,
respectively. The A and G allele frequencies for case
group were 73.57% and 26.43%, while these ratios in
controls were 79.26% and 20.74%, respectively. Geno-
type analysis showed a signi cant association of AG
genotype with breast cancer (OR= 2.89, 95%CI= 1.06-
7.90, p= 0.039). But, we observed no signi cant associa-
tion between A allele and breast cancer risk (OR= 1.37,
0.96-1.96, p=0.079).
META-ANALYSIS
After screening of the articles, a total of 21 eligible stud-
ies were included in our meta-analysis (Justenhoven et
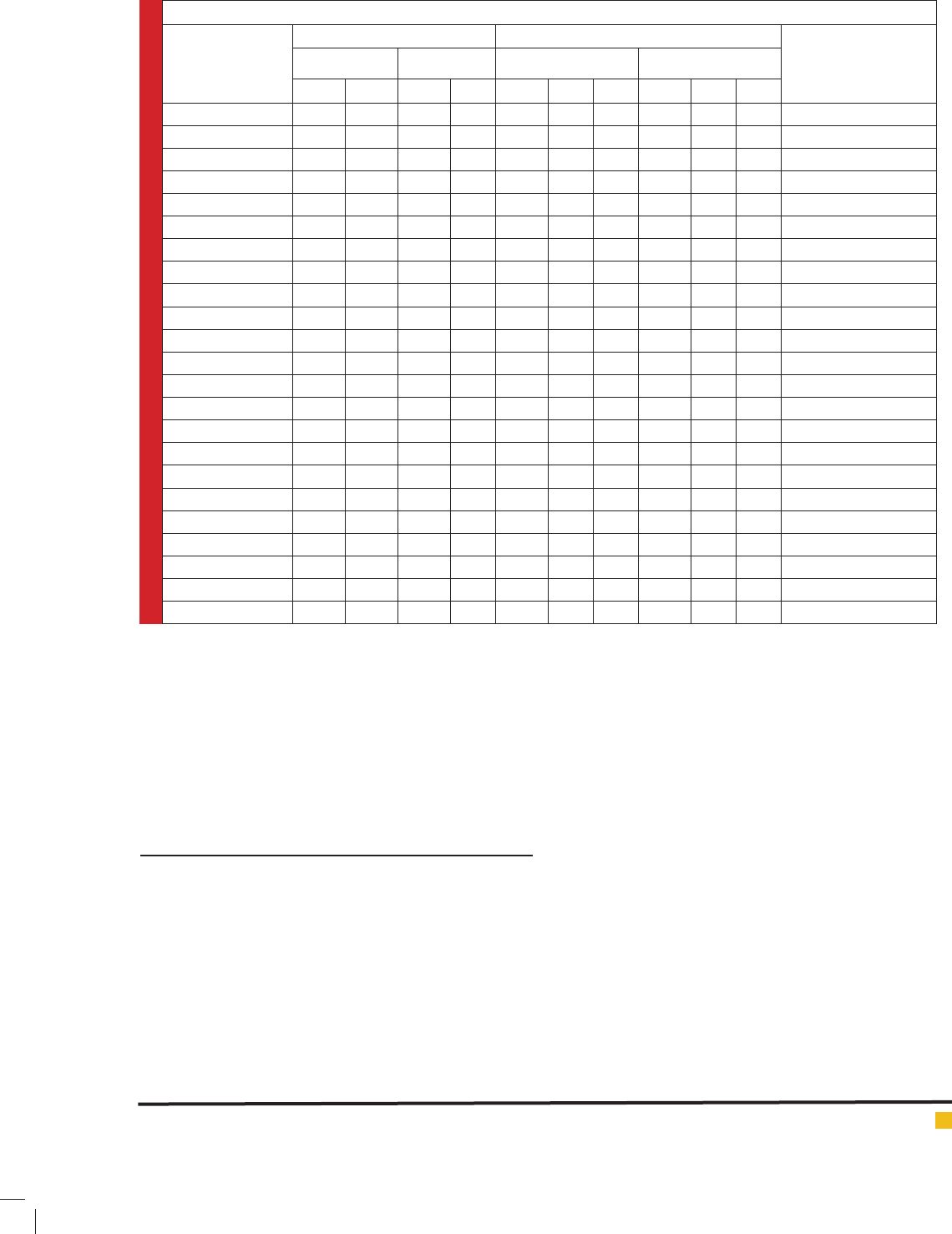
Table 1: Characteristics of included studies in meta-analysis
Country
(ethnicity)
Allele frequencies Genotype frequencies Author. Year
Control Case Control Case
A G A G AAAGGGAAAGGG
Germany 1095 247 929 241 451 193 27 366 197 22 Justenhoven et al. 2005
Chinese 2059 217 1935 197 932 195 11 877 181 8 Shrubsole et al. 2006
Polish 3534 1034 3150 820 1350 834 100 1244 662 79 Lissowka et al. 2007
Taiwan 740 96 192 24 324 92 2 85 22 1 Yu et al. 2007
USA 1776 428 1723 385 714 348 40 705 313 36 Xu et al. 2007
Canada 1230 320 1543 335 489 252 34 635 273 31 Kutsopoulous et al. 2008
Taiwan 895 171 586 118 384 127 22 246 94 12 Cheng et al. 2008
USA 2970 780 1673 425 1184 602 89 678 317 54 Platek et al. 2009
Japan 1501 319 737 173 616 269 25 301 135 19 Suzuki et al. 2008
Brazil 737 179 723 191 294 149 15 294 135 28 Ma et al. 2009 a
Japan 635 139 611 165 261 113 13 237 137 14 Ma et al. 2009 b
Brazil 282 66 255 93 109 64 1 82 91 1 Carvalho et al. 2012
Russian 1116 300 1293 357 443 230 35 505 283 37 Weiner et al. 2012
Chinese 421 341 325 295 127 167 87 97 131 82 He et al. 2014
Chinese 1089 257 808 262 479 131 63 344 120 71 Jiang-hua et al. 2014
Chinese 1494 206 1425 191 662 170 18 631 163 14 Xi et al. 2014
Chinese 455 177 408 184 176 103 37 149 110 37 Weiwei et al.2014
Chinese 151 19 153 39 69 13 3 59 35 2 Wu et al. 2014
Greek-Cypriot 1772 540 1708 440 684 404 68 679 350 45 Kakkoura et al.2015
Ecuadorian Mestizo 308 82 206 22 123 62 10 94 18 2 Lopez-cortes et al. 2015
European American 1007 263 1021 239 408 191 36 410 201 19 Gong et al. 2015
African American 1047 371 865 295 387 273 49 325 215 40 Gong et al. 2015
Iran 298 78 231 83 116 66 6 87 57 13 This study
798 MTR GENE POLYMORPHISM AND BREAST CANCER RISK BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Reza Sharif and Karimian
al., 2005; Shrubsole et al., 2006; Lissowka et al., 2007;
Yu et al., 2007; Xu et al., 2007; Kotsopolous et al., 2008;
Cheng et al., 2008; Suzuki et al., 2008; Platek et al.,
2009; Ma et al
a,b
., 2009; Carvalho et al., 2012; Weiner et
al., 2012; He et al., 2014; Jiang-hua et al., 2014. Xi et al.,
2014; Weiwie et al., 2014; Wu et al., 2014; Kakkoura et
al., 2015; Lopez-Cortes et al., 2015; Gong et al., 2015).
Also the data from our case-control study was added
to the meta-analysis. The studies selection procedure
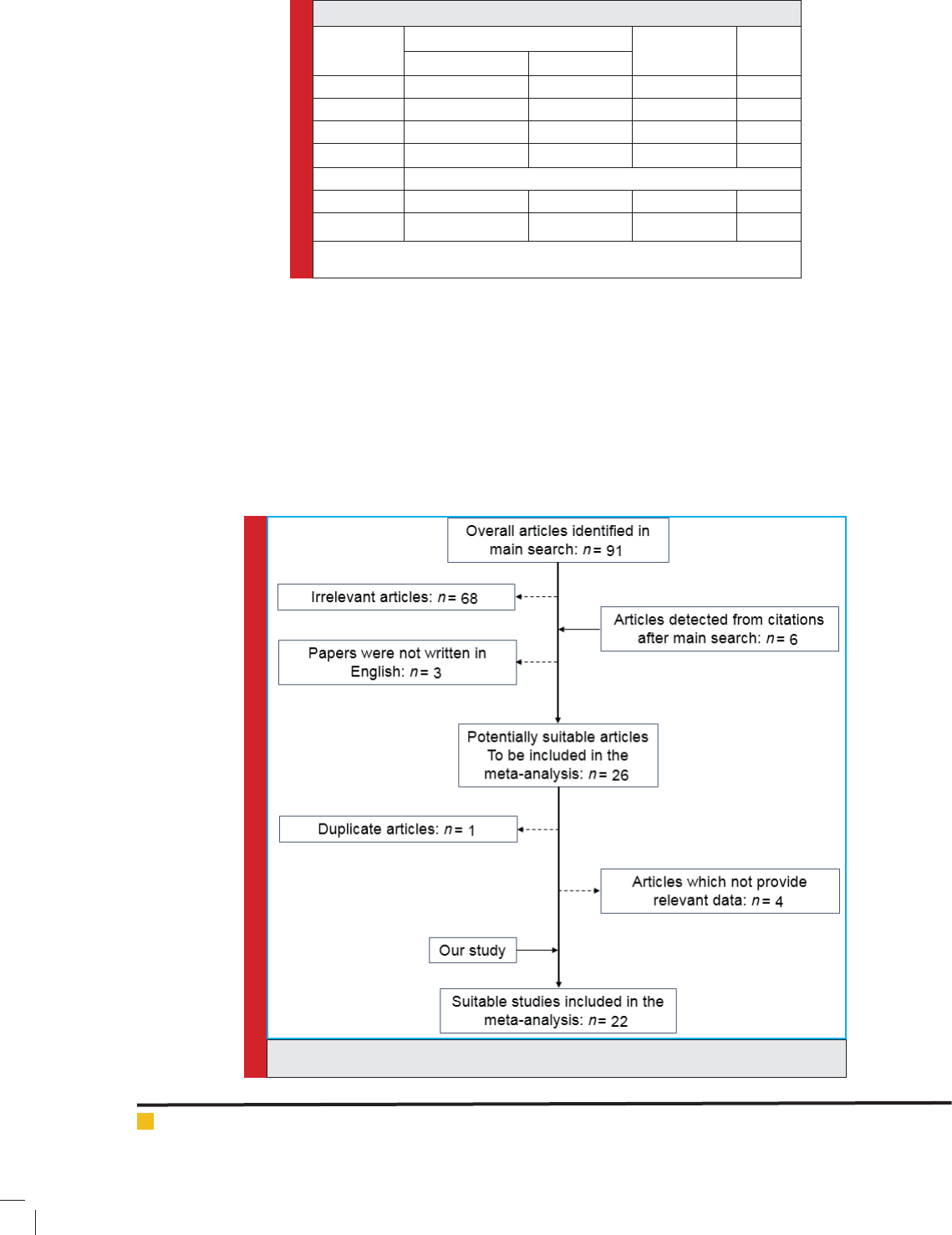
is introduced in Figure 1. As a result, 22 studies were
included in the meta-analysis, re ecting 14037cases
and 16621 healthy controls. There were 5 studies of
Caucasians, 11 studies of Asians and 6 studies of other
ethnicities. The total results of the meta-analysis are
represented in Table 3. When meta-analysis performed
for the 22 pooled studies, no signi cant association was
observed between MTR-A2756G and breast cancer risk
in any of genetic models. Results of meta-analysis for
Table 2: Genotype and allele frequencies of A2756G in cases and controls
Genotype No. and Percentage OR (95% CI) p-value
Control (n=188) Case (n=157)
AA 116 (61.70%) 87 (55.41%) - -
AG 66 (35.11%) 57 (36.31%) 1.15 (0.73-1.81) 0.539
GG 6 (03.19%) 13 (08.28%) 2.89 (1.06-7.90) 0.039
AG+GG 72 (38.30%) 70 (44.59%) 1.30 (0.84-1.99) 0.238
Allele
A 298 (79.26%) 231 (73.57%) - -
G 78 (20.74%) 83 (26.43%) 1.37 (0.96-1.96) 0.079
OR: odds ratio, CI: con dence interval
Signi cant differences between the case and control groups are bolded
FIGURE 1. Flow diagram of the study selection process.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS MTR GENE POLYMORPHISM AND BREAST CANCER RISK 799
Reza Sharif and Karimian
Table 3: Results of meta-analysis for MTR-A2756G polymorphism and breast cancer risk
ethnicity Genetic model Analysis
model
OR
(95%CI)
P-value tau2 Ph I2 PE
Total populations
G vs. A
(Allelic)
Random
effect
1.024
(0.951-1.103)
0.522 0.019 < 0.001 65% 0.052
GG vs. AA
(Codominant)
Random
effect
0.999
(0.862-1.159)
0.992 0.040 0.053 35% 0.915
AG vs. AA
(Codominant)
Random
effect
1.025
(0.941-1.117)
0.571 0.023 < 0.001 60% 0.022
AG+GG vs. AA
(Dominant)
Random
effect
1.057
(0.962-1.161)
0.245 0.033 < 0.001 70% 0.045
GG vs. AA+AG
(Recessive)
Fixed
effect
1.000
(0.898-1.114)
1.000 - 0.114 27% 0.813
Caucasian population
G vs. A
(Allelic)
Random
effect
0.929
(0.837-1.032)
0.170 0.008 0.051 58% 0.336
GG vs. AA
(Codominant)
Fixed
effect
0.809
(0.672-0.976)
0.026 - 0.693 0% 0.814
AG vs. AA
(Codominant)
Random
effect
0.952
(0.829-1.092)
0.481 0.015 0.030 63% 0.193
AG+GG vs.AA
(Dominant)
Random
effect
0.994
(0.814-1.215)
0.955 0.043 < 0.001 84% 0.295
GG vs. AA+AG
(Recessive)
Fixed
effect
0.832
(0.692-1.001)
0.051 - 0.821 0% 0.872
Asian populations
G vs. A
(Allelic)
Fixed
effect
1.133
(1.053-1.220)
< 0.001 - 0.159 30% 0.099
GG vs. AA
(Codominant)
Fixed
effect
1.270
(1.054-1.530)
0.012 - 0.571 0% 0.392
AG vs. AA
(Codominant)
Fixed
effect
1.126
(1.024-1.238)
0.014 - 0.158 31% 0.006
AG+GG vs.AA
(Dominant)
Fixed
effect
1.139
(1.043-1.244)
0.004 - 0.160 30% 0.014
GG vs. AA+AG
(Recessive)
Fixed
effect
1.216
(1.018-1.452)
0.031 - 0.554 0% 0.351
OR, odds ratio; CI, con dence interval; Ph, P-values for heterogeneity from Q test; PE, PEgger
Asian and Caucasian subgroups are presented in Table
3. The results showed that the MTR-A2756G was associ-
ated with the breast cancer risk within Asian population
in G vs. A (OR: 1.133, 95%CI: 1.053-1.220, p< 0.001), GG
vs. AA (OR: 1.270, 95%CI: 1.054-1.530, p=0.012), AG vs.
AA (OR: 1.126, 95%CI: 1.024-1.238, p=0.014), AG+GG
vs. AA (OR: 1.139, 95%CI: 1.043-1.244, p=0.004), and
GG vs. AA+AG (OR: 1.216, 95%CI: 1.018-1.452, p=0.031)
genetic models (Figure 2). This despite the fact that we
observed a protective association between MTR-A2756G
and breast cancer risk in GG vs. AA (OR: 0.809, 95%CI:
0.672-0.976, p=0.026) genetic model (Figure 2). Also
we observed a protective association in GG vs. AA+AG
model, but this association was not statistically signi -
cant (OR: 0.832, 95%CI: 0.692-1.001, p=0.051).
As depicted in Table 3 in overall meta-analysis, there
was a high heterogeneity for G vs. A, AG vs. AA, and
AG+GG vs. AA genetic model (P
heterogeneity
< 0.001). In
Caucasian population, a high heterogeneity was found
in AG+GG vs. AA genetic model with P
heterogeneity
< 0.001
(Table 3). While, we don’t observed a true heterogeneity
within Asian population in any of ve genetic models
(Table 3). Egger’s test and Funnel plot were used to eval-
uate the publication bias in meta-analysis. The Egger’s
test suggested a publication bias for the G vs. A (PEg-
ger= 0.052), AG vs. AA (PEgger= 0.022), and AG+GG
vs. AA (PEgger= 0.045) genetic models. In subgroup
analysis, also we observed a publication bias in AG vs.
AA (PEgger= 0.006) and AG+GG vs. AA (PEgger= 0.014)
models within Asian population (Table3). Whereas, the
shapes of funnel plot for other genetic models in Asian
subgroup and for all of the ve genetic models in Cau-
casian subgroup seemed approximately symmetrical,
suggesting the lack of publication bias (Figure 3). The
lack of publication bias was con rmed by Egger’s test
(Table 3). Sensitivity analysis was done by excluding

Reza Sharif and Karimian
800 MTR GENE POLYMORPHISM AND BREAST CANCER RISK BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
FIGURE 2. Forest plot for the association of MTR-A2756G with
breast cancer. Findings of quantitative data synthesis under G vs. A
(A); GG vs. AA (B); GG vs. AA+AG (C) models in Asian population;
and GG vs. AA model in Caucasian population (D).
a study at one time. The results of sensitivity analysis
showed that the estimates before and after the omission
of every study were similar, suggesting this meta-analy-
sis were stable (datanotshown).
DISCUSSION
Genetic polymorphisms in folate metabolizing enzymes
may be involved in the susceptibility breast cancer pre-
disposition. MTR is one of key regulatory enzymes in
folate metabolism. The A2756G transition in the MTR
gene lead to aspartic acid to glycine residue substitution
at position 919 near the cobalamin-binding domain of
the MTR enzyme (Chen et al, 1997; van der Put et al,
1998). In the present study we investigated the associa-
tion of MTR-A2756G polymorphism with breast cancer
risk in Iranian population followed by a meta-analysis.
Our case-control study revealed that GG genotype is
associated with breast cancer risk (OR: 2.89, 95%CI: 1.06-
7.90, p= 0.039). Some studies reported a similar asso-
ciation between this variety and breast cancer risk (Yu
et al., 2007; Ma et al., 2009
a
). While some other studies
reported no signi cant association between this variety
and breast cancer risk (Justenhoven et al., 2005; Platek
et al., 2009). To help clarify the inconsistent ndings,

Reza Sharif and Karimian
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS MTR GENE POLYMORPHISM AND BREAST CANCER RISK 801
we performed a meta-analysis to obtain a competitive
result via combining more eligible studies, enlarging the
sample size, and performing a subgroup analysis. Results
of overall meta-analysis, revealed no signi cant associa-
tion between MTR-A2756G and breast cancer risk in any
of ve genetic models. But the meta-analysis in ethni-
cal subgroups revealed different results. We observed a
signi cant association between MTR-A2756G and breast
cancer risk within Asian population in G vs. A, GG vs. AA,
AG vs. AA, AG+GG vs. AA, and GG vs. AA+AG genetic
models. While we observed a protective association of
MTR-A2756G with breast cancer risk in GG vs. AA (OR:
0.809, 95%CI: 0.672-0.976, p=0.026) genetic model. The
geographic and ethnic variations could explain the con-
icting data between different studies.
DNA methylation is a key procedure for regulation of
genome integrity and gene expression. Role of abnormal
DNA methylation in carcinogenesis is complex. Hyper-
methylation of special genes and hypomethylation of
global DNA are two most common mechanism detected
in several tumors (Pufulete et al., 2003). In the human
genome, cytosines are mostly methylated in CpG islands
(Jones and Takai, 2001; Takai and Jones, 2002). The CpG
dinucleotide are frequently located around and in the
start sites of transcription of nearly half of the human
genes. The CpG islands in a several genes, which are gen-
erally unmethylated in normal tissues, are methylated
in human cancers including breast cancer, with variable
degrees (Yanget al., 2001a). Methionine synthase with
vitamin B12 as a cofactor catalyzes the remethylation
of homocysteine to methionine. Methionine synthase
play a crucial role in maintaining suitable intracellular
methionine, normal homocysteine and folate concentra-
tions. Methionine as a crucial amino acid and a precur-
sor of S-adenosylmethionine is a common methyl group
donor involved in reactions of methylation including
DNA methylation (Ma et al., 1999).
Missense mutations are responsible for several cred-
ited to single gene disorders. Some studies stated that
non-synonymous SNPs (nsSNP) are dangerous for struc-
ture of the proteins (Karimian and Hosseinzadeh Colagar,
2014; Nikzad et al., 2015). Wang and Moult reported that
some nsSNPs disrupt the function of protein by alteration
of the protein hydrophobicity (Wang and Moult, 2001) or
by affecting the three-dimensional structure of the pro-
tein depending on the location of nsSNP (Sunyaev et al.,
2001). A2756G as an nsSNPs may alter the MTR func-
tion, therefor we suggests that future studies focus on it.
There are three similar meta-analyses about the asso-
ciation of the MTR-A2756G polymorphism and the
breast cancer risk (Lu et al., 2010; Weiner et al., 2012,
Zhong et al., 2013). Lu et al. (2012) reported that there is
no signi cant association between MTR-A2756G gene
polymorphism and risk of breast cancer, overall. But, in
the strati ed analysis, they found signi cantly decreased
risk of breast cancer in Europeans (Lu et al., 2010). Simi-
larly, Zhong et al. reported a protective association in
Caucasian population (Zhong et al., 2013). Weiner et
al. (2012) represented a deferent results. They observed
no signi cant association between MTR-A2756G poly-
FIGURE 3. Funnel plot of breast cancer risk associated with A2756G. The plots under G vs. A (A); GG vs. AA (B);
GG vs. AA+AG (C) models in Asian population; and GG vs. AA model in Caucasian population (D).

802 MTR GENE POLYMORPHISM AND BREAST CANCER RISK BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Reza Sharif and Karimian
morphism and breast cancer risk in any ethnical groups
(Weiner et al., 2012). The data which reported Naushad
et al. were wrong (Naushad et al. 2011), but Zhong et al.
included this study in their meta-analysis (Zhong et al.,
2013).
There are some limitations in our meta-analysis that
must be considered. First, the lack of original data from
the studies restricted our more assessment of the possible
interactions such as gene-gene and gene-environment
which may modulate cancer risk. Second, restriction
of the study to English language articles may poten-
tially lead to language bias. Third, this meta-analysis
is absence of adequate data from African populations.
Last, as the MTR gene has some polymorphisms except
the A2756G, our analysis cannot state the role of other
polymorphisms in the breast cancer risk.
CONCLUSION
Our genetic association study suggests that the MTR-
A2756G polymorphism is associated with the risk of
breast cancer. Supplementary studies with large sample
size are necessary to con rm our ndings. Future studies
must include homogeneous breast cancer patients with
well-matched controls. Furthermore, other MTR gene
polymorphisms and speci c haplotypes may contribute
to the risk of breast cancer. More studies investigating
gene-environment and gene-gene interactions should be
performed to better understand the role of MTR-A2756G
transition in breast cancer predisposition.
CONFLICT OF INTEREST
The authors do not have any con icts of interest in this
article.
REFERENCES
Babyshkina, N., Malinovskaya, E., Nazarenko, M.(2013) The
effect of folate-related SNPs on clinicopathological features,
response to neoadjuvant treatment and survival in pre- and
postmenopausal breast cancer patients. Gene 518, 397-404.
Begg, C.B., Mazumdar, M. (1994) Operating characteristics of a
rank correlation test for publication bias. Biometrics 50, 1088-
1101.
Carvalho Barbosa Rde, C., Menezes, D.C., Freire, T.F.V.(2012)
Associations of polymorphisms of folate cycle enzymes and
risk of breast cancer in a Brazilian population are age depend-
ent. Molecular Biology Reports 39, 4899-4907.
Chen, J., Stampfer, M. J., Ma, J.(2001) In uence of a methio-
nine synthase (D919G) polymorphism on plasma homocysteine
and folate levels and relation to risk of myocardial infarction.
Atherosclerosis 154, 667–672.
Chen, L.H., Liu, M.L., Hwang, H.Y. (1997) Human methionine
synthase. cDNA cloning, gene localization, and expression.
Journal of Biological Chemistry 272, 3628-3634.
Cheng, C.W., Yu, J.C., Huang, C.S.(2008) Polymorphism of
cytosolic serine hydroxymethyltransferase, estrogen and breast
cancer risk among Chinesewomen in Taiwan. Breast Cancer
Research and Treatment 111, 145-155.
Dumitrescu, R.G., Cotarla, I. (2005) Understanding breast can-
cer risk-where do we stand in 2005? Journal of Cellular and
Molecular Medicine 9, 208-221.
Egger, M., Davey Smith, G., Schneider M., et al. (1997) Bias in
meta-analysis detected by a simple, graphical test. BMJ 315,
629-634.
Fodinger, M., Horl W.H., Sunder-Plassmann G. (2000) Molec-
ular biology of 5, 10 methylene tetrahydrofolate reductase.
Journal of Nephrology 13, 20-33.
Gong, Z., Yao, S., Zirpoli, G.(2015) Genetic variants in one
carbon metabolism genes and breast cancer risk in European
American and African American women. International Journal
of Cancer 137, 666-677.
Hankinson, S.E., Colditz, G.A., Willett, W.C. (2004) Towards an
integrated mod breast cancer etiology. The lifelong interplay
of genes, lifestyle, and hormones. Breast Cancer Research 6,
213-218.
He, J.M., Pu, Y.D., Wu, Y.J. (2014) Association between dietary
intake of folate and MTHFR and MTR genotype with risk of
breast cancer. Genetics and Molecular Research 13, 8925-8931.
Jamali, S., Karimian, M., Nikzad, H. (2016) The c.− 190 C>
A transversion in promoter region of protamine1 gene as a
genetic risk factor for idiopathic oligozoospermia. Molecular
Biology Reports 43, 795-802.
Jiang-hua, Q., De-chuang, J., Zhen-duo, L.(2014) Association
of methylenetetrahydrofolate reductase and methionine syn-
thase polymorphisms with breast cancer risk and interaction
with folate, vitamin B6, and vitamin B 12 intakes. Tumour
Biology 35, 11895-11901.
Justenhoven, C., Hamann, U., Pierl, C. B. (2005) One-carbon
metabolism and breast cancer risk: no association of MTHFR,
MTR, and TYMS polymorphisms in the GENICA study from
Germany. Cancer Epidemiology, Biomarkers & Prevention 14,
3015-3018.
Kakkoura, M. G., Demetriou, C. A., Loizidou, M. A. (2015)
Single-nucleotide polymorphisms in one carbon metabolism
genes, Mediterranean diet and breast cancer risk: a case-con-
trol study in the Greek-Cypriot female population. Genes &
Nutrition 10, 453.
Karimian, M., Colagar, A.H. (2016a) Methionine synthase
A2756G transition might be a risk factor for male infertility:
Evidences from seven case-control studies. Molecular and Cel-
lular Endocrinology 425, 1-10.
Karimian, M., Colagar, A.H. (2016b) Association of C677T
transition of the human methylenetetrahydrofolate reductase
(MTHFR) gene with male infertility. Reproduction Fertility and
Development 28, 785-794.
Reza Sharif and Karimian
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS MTR GENE POLYMORPHISM AND BREAST CANCER RISK 803
Karimian, M., Nikzad, H., Azami-Tameh, A.(2015) SPO11-
C631T gene polymorphism: association with male infertility
and an in silico-analysis. Journal of Family and Reproductive
Health 9, 155-163.
Kotsopoulos, J., Zhang, W. W., Zhang, S. (2008) Polymorphism
in folate metabolizing enzyme and transport proteins and the
risk of breast cancer. Breast Cancer Research and Treatment
112, 585-593.
Lissowska, J., Gaudet, M. M., Brinton, L. A.(2007) Genetic poly-
morphism in the one-carbon metabolism pathway and breast
cancer risk: a population –based case-control study and meta-
analyses. International Journal of Cancer 120, 2696-2703.
López-Cortés, A., Echeverría, C., Oña-Cisneros, F. (2015) Breast
cancer risk associated with gene expression and genotype pol-
ymorphism of the folate-metabolizing MTHFR gene: A case-
control study in high altitude Ecuadorian mestizo population.
Tumour Biology 36, 6451-6461.
Lu, M., Wang, F., Qiu, J. (2010) Methionine synthase A2756G
polymorphism and breast cancer risk: a meta-analysis involv-
ing 18,953 subjects. Breast Cancer Research and Treatment
123, 213-217.
Ma, J., Stampfer, M. J., Christensen, B. (1999) A polymorphism
of the methionine synthase gene: association with plasma
folate, vitamin B12, homocyst(e)ine, and colorectal cancer risk.
Cancer Epidemiology, Biomarkers & Prevention 8, 825-829.
Ma, E., Iwasaki, M., Junko, I. (2009a) Dietary intake of folate,
vitamin B6, and vitamin B12, genetic polymorphism of related
enzymes, and risk of breast cancer: a case-control study in
Brazilian women. BMC Cancer 9, 122.
Ma, E., Iwasaki, M., Kobayashi, M. (2009b) Dietary intake of
folate, vitamin B2, vitamin B6, vitamin B12, genetic polymor-
phism of related enzyme, and risk of breast cancer: a case-
control study in Japan. Nutrition and Cancer 61, 447-456.
Naushad, S. M., Pavani, A., Rupasree, Y. (2012) Association of
aberrations in onecarbon metabolism with molecular pheno-
type and grade of breast cancer. Molecular Carcinogenesis 51,
E32-E41.
Nikzad, H., Karimian, M., Sareban, K. (2015) MTHFR-Ala222Val
and male infertility: a study in Iranian men, an updated meta-
analysis and an in silico-analysis. Reproductive BioMedicine
Online 31, 668-680.
Ottaviano, Y.L., Issa, J.P., Parl, F.F (1994) Methylation of the
estrogen receptor gene CpG island marks loss of estrogen
receptor expression in human breast cancer cells. Cancer
Research 54, 2552-2555.
Parkin, D.M. (2001) Global cancer statistics in the year 2000.
The Lancet Oncology 2, 533-543.
Platek, M.E., Shields, P.G., Marian, C. (2009) Alcohol consump-
tion and genetic variation in methylenetetrahydrofolate reduc-
tase and 5 methyltetrahydrofolate-homocysteine methyltrans-
ferase in relation to breast cancer risk. Cancer Epidemiology,
Biomarkers & Prevention 18, 2453-2459.
Pufulete, M., Al-Ghnaniem, R., Leather (2003) Folate status,
genomic DNA hypomethylation, and risk of colorectal ade-
noma and cancer: a case control study. Gastroenterology 124,
1240-1248.
Raygan, F., Karimian, M., Rezaeian, A. (2016) Angiotensino-
gen-M235T as a risk factor for myocardial infarction in Asian
populations: a genetic association study and a bioinformatics
approach. Croatian Medical Journal 57, 351-362.
Shrubsole, M.J., Gao, Y.T., Cai, Q. (2006) MTR and MTRR poly-
morphisms, dietary intake, and breast cancer risk. Cancer Epi-
demiology, Biomarkers & Prevention 15, 586-588.
Stover, P.J. (2004) Physiology of folate and vitamin B12 in
health and disease. Nutrition Reviews 62, S3-S12 (discussion
S13).
Sunyaev, S., Ramensky, V., Koch, I.(2001) Prediction of delete-
rious human alleles. Human Molecular Genetics 10, 591-597.
Suzuki, T., Matsuo, K., Hirose, K. (2008) One-carbon metabo-
lism related gene polymorphisms and risk of breast cancer.
Carcinogenesis 29, 356-362.
van der Put, N. M., Gabreëls, F., Stevens, E. M.(1998) A second
common mutation in the methylenetetrahydrofolate reductase
gene: an additional risk factor for neural-tube defects? The
American Journal of Human Genetics 62, 1044-1051.
Wang, Z., Moult, J. (2001) SNPs, protein structure, and disease.
Human Mutation 17, 263-270.
Weber, M., Hellmann, I., Stadler, M. B.(2007) Distribution,
silencing potential and evolutionary impact of promoter DNA
methylation in the human genome. Nature Genetics 39, 457-
466.
Weiner, A.S., Boyarskikh, U.A., Voronina, E.N., et al. (2012)
Polymorphisms in the folate-metabolizing genes MTR, MTRR,
and CBS and breast cancer risk. Cancer Epidemiology 36, e95-
e100.
Weiwei, Z., Liping, C., Li, D. (2014) Association between dietary
intake of folate, vitamin B6, B12 & MTHFR, MTR Genotype
and breast cancer risk. Pakistan Journal of Medical Sciences
30, 106-110.
Wu, X., Zou, T., Cao, N., (2014) Plasma homocysteine levels
and genetic polymorphisms in folate metabolism are associ-
ated with breast cancer risk in Chinese women. Hereditary
Cancer in Clinical Practice 12, 2.
Xi, J., Su, Y., Fadiel, A. B. (2014) Association of physical activ-
ity and polymorphism in FGFR2 and DNA methylation related
genes with breast cancer risk. Cancer Epidemiology 38, 708-
714.
Xu, X., Gammon, M.D., Zhang, H. (2007) Polymorphism of
one-carbon-metabolizing genes and risk of breast cancer in a
population-based study. Carcinogenesis 28, 1504-1509.
Yu, C.P., Wu, M.H., Chou, Y.C.(2007) Breast cancer risk associ-
ated with multi genotypic polymorphisms in folate-metaboliz-
ing genes: a nested case-control study in Taiwan. Anticancer
Research 27, 1727-1732.
Zhong, S., Xu, J., Li, W. (2013) Methionine synthase A2756G
polymorphism and breast cancer risk: an up-to-date meta-
analysis. Gene 527, 510-515.