Toxicological
Communication
Biosci. Biotech. Res. Comm. 9(4): 790-794 (2016)
Individual and combined effect of mercuric chloride,
magnesium sulphate and selenium on testis of
Heteropneustes fossilis
Kiran Bansibal (Maheshwari),* M. M. Prakash* and M. S. Parihar**
*Postgraduate Department of Zoology, Government Model Autonomous Holkar Science College, Indore (MP)
**School of Studies in Zoology, Vikram University, Ujjain (MP) India
ABSTRACT
The present study deals with the individual and combined effects of mercuric chloride, magnesium sulphate and
selenium on testis of Heteropneustes fossilis. Individually all these three chemicals decreased the Na +/K+-ATPase
activity in the experimental shes upto 57.35 %, 34.22 % and 38.40 % respectively. However, in the combined effect
the Na +/K+-ATPase activity decreased up to 47.28 %. These ndings suggest that loss of Na +/K+-ATPase is due to
mercuric chloride which could be recovered up to 10.07% by supplementation of magnesium sulphate and selenium
in H. fossilis testis .
KEY WORDS: MERCURIC CHLORIDE, MAGNESIUM SULPHATE SELENIUM TOXICITY, NA +/K+-ATPASE ACTIVITY HETEROPNEUSTES
FOSSILIS TESTIS
790
ARTICLE INFORMATION:
*Corresponding Author:
Received 20
th
Nov, 2016
Accepted after revision 27
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Na+/K+-ATPase is an important energizer for ion trans-
port in epithelial tissue (Tipsmark and Madsen, 2003).
This enzyme is also important in determining the milieu
of cerbro-microvascular and Neurons (Caspers et al.,
1993). Maintenance of cation gradient by Na+/K+-
ATPase and Ca+ ATPase has fundamental importance in
the control of hydration volume, Nutrient uptake and
uidity of cells. It is also essential for contractibility
and excitability properties of muscles and nervous tis-
sues (Mohandas and Shohet, 1978). Mercuric chloride is
one of the most toxic forms of mercury and is primarily
nephro-toxic (Moraes-Silva, 2014). It is well known as
hematotoxic (Durak et al., 2010), hapatotoxic (Joshi et
al., 2014; Othman et al., 2014), neurotoxic (Moraes-Silva
et al., 2014) and genotoxic (Rozaqai et al., 2005) and
exert negative effect on the reproductive system in male
Maheshwari, Prakash and Parihar
rat (Kalander et al., 2013). Selenium has ability to reduce
the toxicity of several xenobiotics including heavy met-
als (Agha et al., 2014). Considerable data available on
sh Na+/K+-ATPase activity induced by Mercury chlo-
ride, Selenium and Magnesium sulphate individually
and collectively on testis is very meagre, present study
was attempted in Heteropneustes fossilis.
MATERIAL AND METHODS
Heteropneustes fossilis (Weight 50-60 gm.) were used
as experimental animals which were obtained live from
Nolakkha sh market, Indore (M.P.). Following chemi-
cals were used and their doses: Mercuric chloride-1.0
ppm (E-Merck India Ltd., Mumbai) of molecular weight
275.52 Dalton. Selenium -0.9 ppm (Loba Chem India
Ltd.) of molecular weight 246.47 Dalton. Magnesium
sulphate-0.3 ppm (Loba Chem India Ltd).Ten experimen-
tal shes were placed in separate glass aquarium having
10,000 cc of tap water free from chlorine.Experimen-
tal shes were divided into following groups: Group I
(Control group): Contained only chlorine free tap water.
Group II (Experimental group 1): Contained 1.0 ppm
10,000 cc aqueous mercuric chloride solution. Group
III (Experimental group 2): Contained 0.3 ppm 10,000
cc aqueous solution of Magnesium sulphate. Group
IV (Experimental group 3): Contained 0.9 ppm 10,000
cc aqueous selenium solution. Group V (Experimental
group 4): Contained 1.0 ppm aqueous mercuric chloride
solution +.3 ppm aqueous Magnesium sulphate solution
+0.9 ppm aqueous selenium solution inTotal volume of
10000 cc distilled water. Two shes were removed from
each group after 0 hrs, 24 hrs, 48 hrs, 72 hrs and 96 hrs.
Na+/K+-ATPase activity of test organ Testis was deter-
mined by the method given by Tipsmark and Madsan
(2003).
RESULTS AND DISCUSSION
It was observed that, 96 hrs exposure of 0.1 ppm mercu-
ric chloride reduced the Na+/K+-ATPase activity in the
testis of H.fossilis up to 57.35 per cent. The decrease
in activity at 24, 48, 72 1nd 96 hrs respectively was
47.25, 50.96, 54.25 and 57.35 per cent respectively
(Table 1and 2). 96 hrs exposure of 0.9 ppm selenium
also reduced the Na+/K+-ATPase activity in the testis
of exposed shes up to 34.22 per cent, the decrease in
activity at 24, 48, 72 1nd 96 hrs respectively was 19.66,
23.71, 29.54 and 34.22 per cent respectively (Table 3 and
4). Similarly, 96 hrs exposure of 0.3 ppm of magnesium
sulphate reduced the Na+/K+-ATPase activity in testis
up to 38.4o per cent. The decrease in activity at 24, 48,
72 1nd 96 hrs was 26.18, 33.27, 33.71 and 38.40 per cent
respectively (Table 5 and 6). Combination of mercuric
chloride, magnesium sulphate and selenium reduced the
Na+/K+-ATPase activity up to 47.28 per cent in 96 hrs.
The reduction in enzymatic activity in combined expo-
sure after 24, 48, 72 and 96 hrs were 38.42, 43.66, 47.23
and 47.28 per cent respectively (Table 7 and 8).
It is evident that mercury released in the environ-
ment affects the reproductive system of several animals.
Mercuric salts elicited direct toxic action on steroid pro-
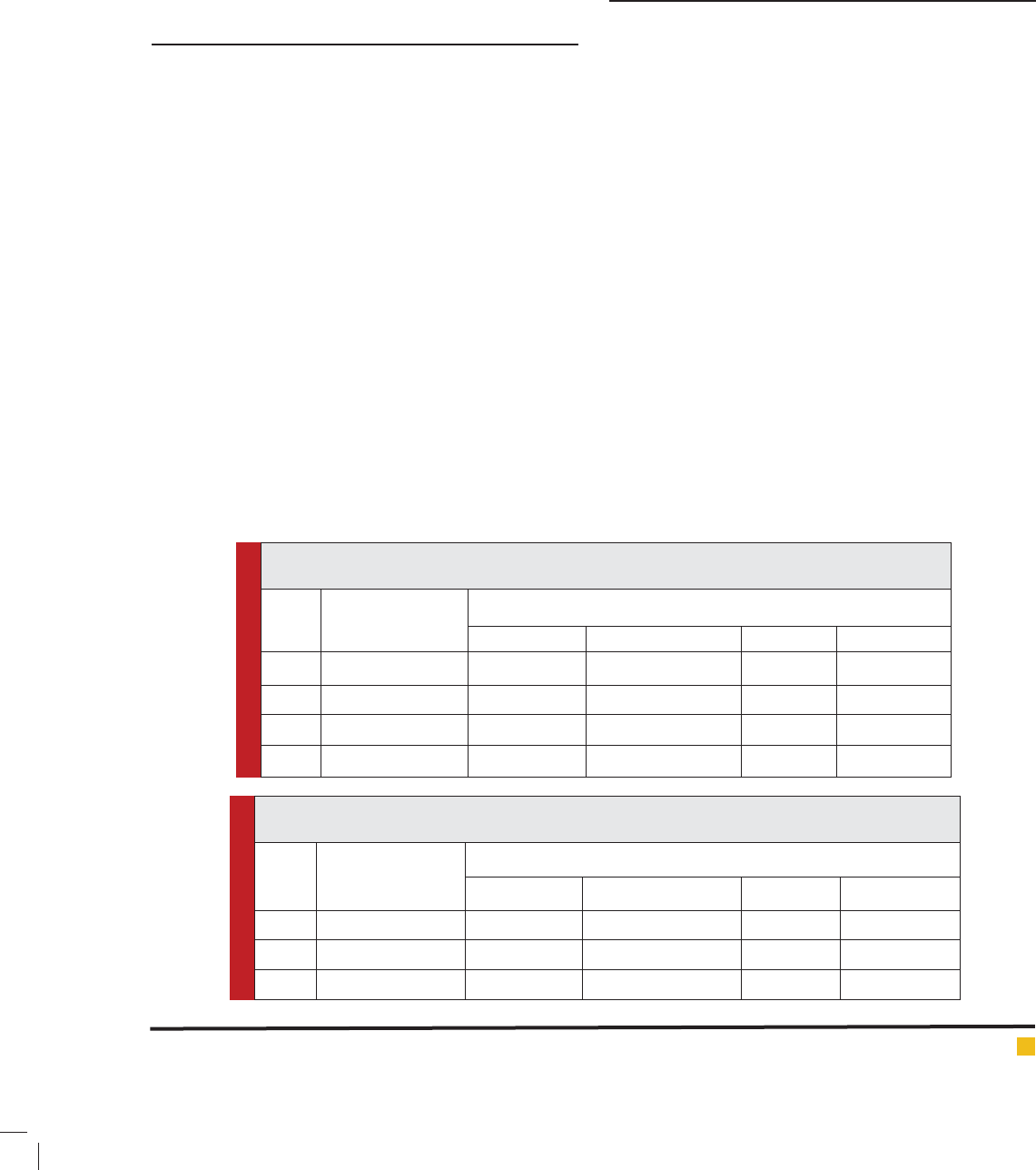
Table 1: Mercuric chloride (1.0 ppm.) induced changes in the Na+/K+-ATPase activity in testis of
H. fossilis (Short duration exposure)
S. No. Exposure Duration
(in hours)
Na+/ K+ ATPase activity in ng pi liberated /mg protein
Control Value Experimental Value Difference Per cent alter
1. 24 57.10 30.12 26.98 -47.25
2. 48 57.10 28.00 29.90 -50.96
3. 72 57.10 26.12 30.98 -54.25
4. 96 57.10 24.35 32.75 -57.35
Table 2: Mercuric chloride (1.0 ppm.) induced changes in the Na+/K+-ATPase activity in testis of H.
fossilis (Long duration exposure)
S. No. Exposure Duration
(in days)
Na+/ K+ ATPase activity in ng pi liberated /mg protein
Control Value Experimental Value Difference Per cent alter
1. 15 57.10 23.00 34.10 -59.71
2. 30 57.10 22.12 34.98 -61.26
3. 45 57.10 18.25 38.85 -60.03
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INDIVIDUAL AND COMBINED EFFECT OF MERCURIC CHLORIDE, MAGNESIUM SULPHATE AND SELENIUM 791
Maheshwari, Prakash and Parihar
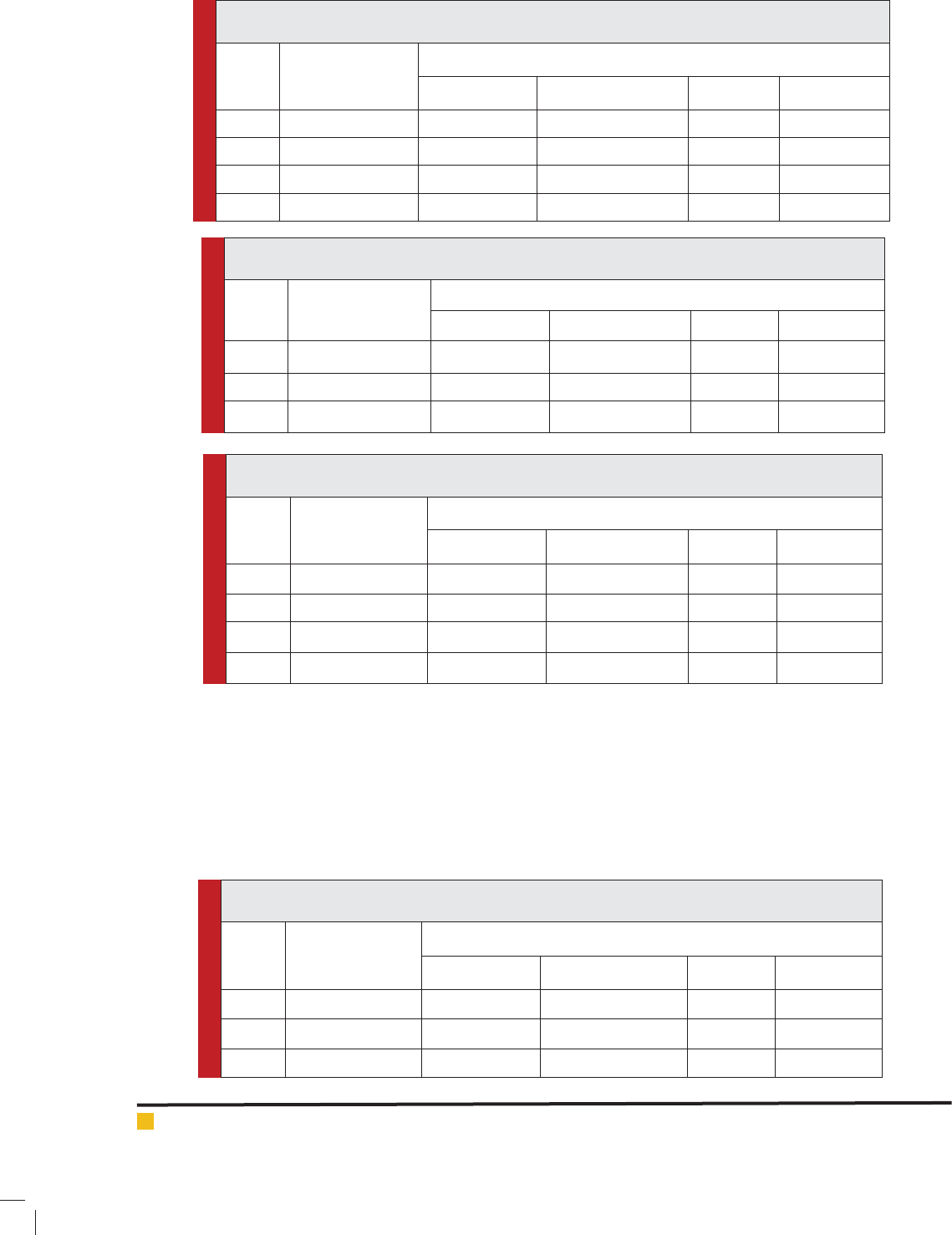
Table 3: Selenium (0.9 ppm.) induced changes in the Na+/K+-ATPase activity in testis of H. fossilis
(Short duration exposure).
S. No. Exposure Duration
(in hours)
Na+/ K+ ATPase activity in ng pi liberated /mg protein
Control Value Experimental Value Difference Per cent alter
1. 24 57.10 45.87 11.23 -19.66
2. 48 57.10 43.56 13.54 -23.71
3. 72 57.10 40.23 16.87 -29.54
4. 96 57.10 37.56 19.54 -34.22
Table 4: Selenium (0.9 ppm.) induced changes in the Na+/K+-ATPase activity in testis of H. fossilis
(Long duration exposure)
S. No. Exposure Duration
(in days)
Na+/ K+ ATPase activity in ng pi liberated /mg protein
Control Value Experimental Value Difference Per cent alter
1. 15 57.10 33.13 23.97 -41.97
2. 30 57.10 32.21 24.89 -43.59
3. 45 57.10 30.34 26.76 -46.86
Table 5: Magnesium sulphate (0.3 ppm.) induced changes in the Na+/ K+ ATPaseactivity in the testis
of H. fossilis (Short duration exposure).
S. No. Exposure Duration
(in hours)
Na+/ K+ ATPase activity in ng pi liberated /mg protein
Control Value Experimental Value Difference Per cent alter
1. 24 57.10 42.15 14.95 -26.18
2. 48 57.10 38.10 19.00 -33.27
3. 72 57.10 37.85 19.25 -33.71
4. 96 57.10 35.17 21.93 -38.40
Table 6: Magnesium sulphate (0.3 ppm.) induced changes in the Na+/ K+ ATPaseactivity in testis of H.
fossilis (Long duration exposure).
S. No. Exposure Duration
(in days)
Na+/ K+ ATPase activity in ng pi liberated /mg protein
Control Value Experimental Value Difference Per cent alter
1. 15 57.10 34.12 22.98 -40.24
2. 30 57.10 30.00 27.10 -47.46
3. 45 57.10 29.12 27.98 -49.00
duction in the testis (Ng and Liu, 1990). Ramalingam
et al. (2001 and 2002) have reported adverse effects of
mercuric chloride on testis and spermatozoa of experi-
mental animals. Nagar and Bhattacharya (2001) also
observed impaired testicular function after an exposure
of Swiss albino rats (30+/2g) to mercuric chloride. In
the present study effect of mercuric chloride (1.0 ppm)
on Na+/K+-ATPase activity of testis of H. fossilis was
investigated and after 96 hrs it was found reduced upto
57.35 per cent. This showed that mercuric chloride pro-
duces toxic effect to testis of H. fossilis and inhibited the
Na+/K+ATPase enzymatic activity. The effect was expo-
sure dependent. Ramalingam and Vimaladevi (2004)
also observed signi cant decrease in the same mem-
792 INDIVIDUAL AND COMBINED EFFECT OF MERCURIC CHLORIDE, MAGNESIUM SULPHATE AND SELENIUM BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Maheshwari, Prakash and Parihar
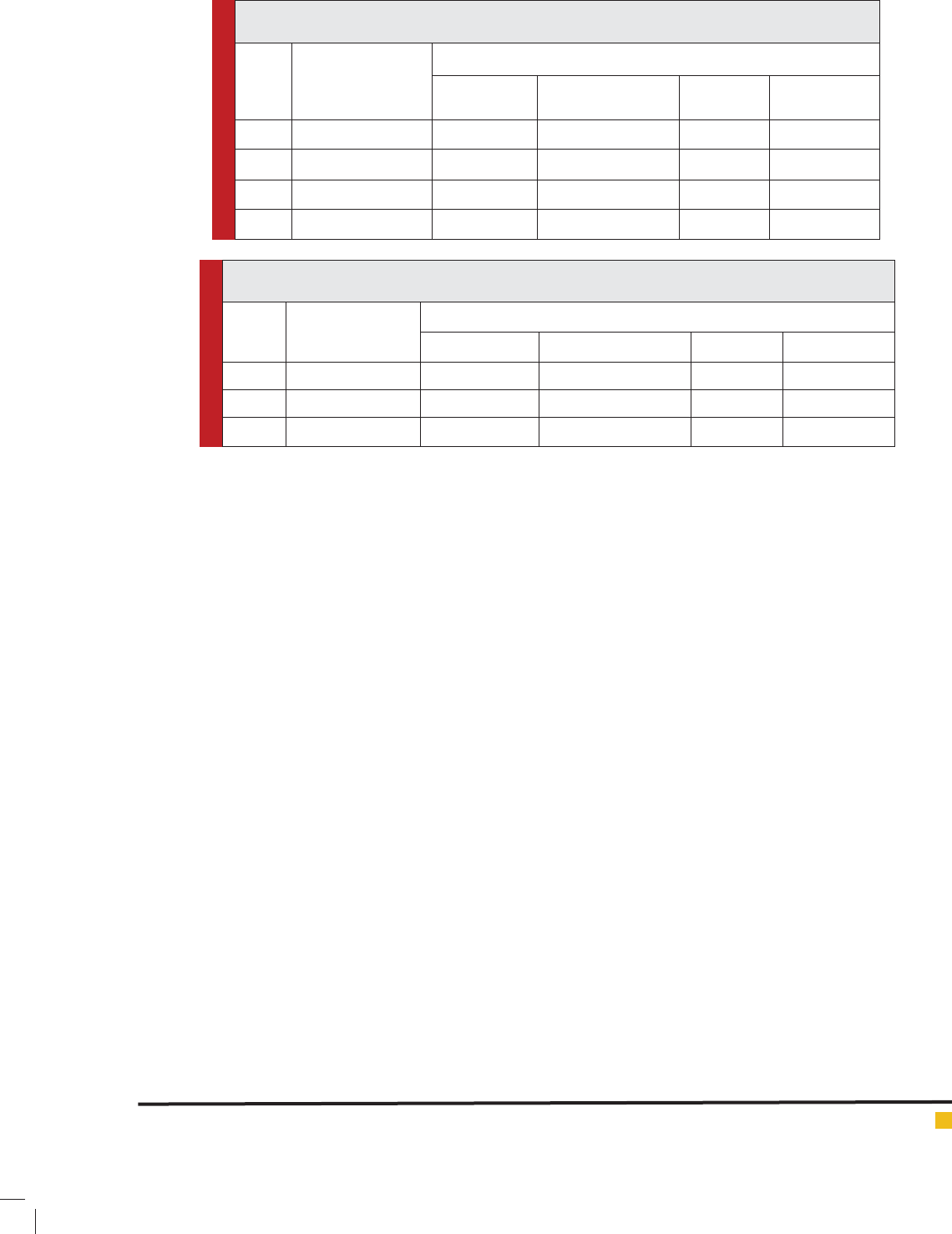
Table 7: Mercuric chloride (1.0 ppm), Selenium (0.9 ppm) and Magnesium sulphate (0.3 ppm.)
induced changes in the Na+/ K+ ATPaseactivityof testis of H. fossilis (Short duration exposure).
S. No. Exposure Duration
(in hours)
Na+/ K+ ATPase activity in ng pi liberated /mg protein
Control Value Experimental Value Difference Per cent alter
1. 24 57.10 35.16 21.94 -38.42
2. 48 57.10 32.17 24.93 -43.66
3. 72 57.10 30.13 26.97 -47.23
4. 96 57.10 10.10 27.00 -47.28
Table 8: Mercuric chloride (1.0 ppm), Selenium (0.9 ppm) and Magnesium sulphate (0.3 ppm.) induced
changes in the Na+/ K+ AT Paseactivity of testis of H. fossilis (Long duration exposure).
S. No. Exposure Duration
(in days)
Na+/ K+ ATPase activity in ng pi liberated /mg protein
Control Value Experimental Value Difference Per cent alter
1. 15 57.10 36.72 20.38 -35.69
2. 30 57.10 38.11 18.99 -33.25
3. 45 57.10 40.33 16.77 -29.36
brane bound enzyme of rat testis when it was treated
with low and high dose of mercuric chloride. Mercury
generally inhibits the function of ion dependent ATPase
leading to disturbances in the ion homeostasis. Distur-
bances in the ion homeostasis results in impaired signal
transduction, altered cellular metabolism, changes in the
cell membrane permeability, integrity and disturbances
of vital functions (Ramalingam and Vimaladevi, 2003).
An inhibition of Na+/K+-ATPase has been shown to be
linked with intracellular accumulation of sodium ,which
reverse the direction of the sodium-calcium exchange
and exacerbates the intracellular calcium ion accumula-
tion (Goddard and Robinson 1976; Akerman and Nicolls,
1982; DiP0l0 and Beauge, 1983) which could further
increase lipid peroxidation,membrane derangement and
excitotoxicity/apoptosis (Farber,1981 and Choi,1993)In
the present study it was inferred that the inhibition of
ATPase in the testis of H. fossilis was due to mercuric
chloride treatment which altered biochemical function-
ing of the testis.
Selenium is an essential trace element, but when its
presence is higher than the normal level in water it causes
adverse health effects, (Beyers and Sodergren, 2002). In
the present study exposure of 0.9 ppm aqueous solu-
tion of selenium to H. fossilis caused depletion in Na+/
K+-ATPase activity in testis. Adverse effects of selenium
to reproductive system of shes were also observed by
Choudhary et al. (1983), Hilton (1986), Lemley (1993),
Kaur and Bansal (2004 and 2005), Demerdash (2004)
and Pyle et al. (2005). In the present study magnesium
sulphate (0.3 ppm) exposure of 96 hrs inhibited Na+/K+-
ATPase activity in testis up to 38.40 percent. According
to Hoffmann et al. (1994) magnesium plays important
role in preventing hypoxia. Hang (1984) also suggested
that magnesium may play direct role in intracellular
potassium homeostasis.
In the present investigation interactions of mercuric
chloride, selenium and magnesium sulphate were also
studied in order to examine the combined effect of these
metals on Na+/K+-ATPase activity of testis. Results
showed reduction of Na+/K+-ATPase up to 47.28 per-
cent, which is less in comparison to the individual expo-
sure of mercuric chloride which was observed up to 60
percent. There was a recovery of Na+/K+-ATPase activ-
ity up to 11 percent. The present data are in agreement
with the statement of Halmy et al. (1987) that uptake of
one metal decreases in the presence of the others, and
thus supports Demerdash (2004) statement that selenium
could be able to antagonize the toxic effect of mercury.
On the basis of present study it is concluded that loss
of Na+/K+-ATPase due to mercuric chloride could be
prevented up to reasonable level by supplementation of
selenium and magnesium sulphate in sh.
REFERENCES
Agha, F.E.; Youness, E.R.; Selim, M.M.H. and Ahmed, H.H.
(2014).Nephroprotective potential of selenium and taurine
against mercuric chloride induced nephropathy in rats.Ren
Fail. 36:704-716
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INDIVIDUAL AND COMBINED EFFECT OF MERCURIC CHLORIDE, MAGNESIUM SULPHATE AND SELENIUM 793
Maheshwari, Prakash and Parihar
Akerman, K.E. and Nicholls, D.G. (1981). Ca2+ transport by
intact synaptosomes.The Voltage –dependent Ca2+ channel
and a re-evaluation of the role of sodium/calcium exchange.
Eur.J.Biochem.117(3):491-497
Aslanturk, A.; Uzunhisarcikli, M.; Kalender, S. And Demir, F.
(2014).Sodium selinite and vitamin E. In preventing mercu-
ric chloride induced renal toxicity in rats. Food Chem Toxicol
70:185-190
Beyers, D.W. and Sodergren, C. (2002). Assessment of expo-
sure of laraval Razorback sucker to selenium in natural waters.
Arch.Environ.contam.Toxicol.42:53-59
Caspers, E.; Serra, R.; Torresani, G.; Andereucci, A. and Gran-
dini, S. (1993).Concentration of Zn, Cu, Fe, and Cd in Liza
ramada and Leuciscus cephalus. Archivio. Veterinario. Ital-
ino.44:166-174
Choi, B.H. (1993).Oxygen, antioxidant and brain dysfunction.
Yonsei.Med.J.34 (1):1-10
Chowdhary, A. R. And Venkata Krishna-Bhatt, H. (1983). Effect
of selenium dioxide on the testis of rat. Ind.J.Physiol.Pharma-
col.27 ((30; 237-240
Demerdash, F.M. (2004). Effect of selenium and mercury on the
enzymatic activities and lipid peroxidation in brain, liver and
blood of rats. J. Environmental Sci. and Health.36:489-499
DiPolo, R. And Deauge, L. (1983). The calcium pump and
sodium-calcium exchange in squid axons.Annu.Rev.Phys-
iol.45:313-324
Durak, D.; Kalender, S.; Uzun, F.G.; Demir, F. And Kalender,
Y. (2010).Mercuric chloride induced oxidative stress and the
protective effect of vitamin C and E in human erythrocyte in
vitro.AJB.9:488-495
Farber, E. (1981). Chemical carcinogenesis.N.Engl.J.Med.305
(23):1379-1389)
Goddard, G.A. and Robinson, J.D. (1976). Uptake and release of
calcium by rat brain synaptosomes.Brains Res.110 (2):331-335
Hilton, J.W.; Hodson, P.V. and Slinger, S. J. (1980). The require-
ment and toxicity of selenium in rainbow trout (Salmo gaird-
eneri). J. Nutr. 110 (12):2527-2535
Hoffman, D. J.; Marro, P. J.; McGowan, J. E.; Mishra, O.P. And
Delivoria-Papadopoulos, M. (1994). Protective effect of mag-
nesium sulphate infusion on nmda receptor binding character-
istics during cerebral cortical hypoxia in the newborn piglet.
Brain Res.644 (10):144-149
Joshi, D.; Mittal, D.K.; Shukla, S.; Srivastava, A.K. and sriv-
astava, S.K. (2014). N-acetyl cystein and selenium protects
mercuric chloride induced oxidative stress and antioxidant
defence system in liver and kidney of rats: A histopathological
approach. Trace Elem Med Biol 28:216-218
Kalender, S.; Uzum, F.G.; Demir, F.; Uzunhisarcikli, M. And
Aslanturk, A. (2013). Mercuric chloride induced testicular tox-
icity in rats and protective role of sodium selinite and vitamin
E.Food Chem Toxicol 55:456-462
Kaur, P. And Bansal, M.P. (2004). Effect of selenium induced
oxidative stress on the oxidation-reduction system and repro-
ductive ability of male mice. Biol. Trace. Elem. Res. (1):83-93
Kaur, P. And Bansal, M.P. (2005). Effect of selenium induced
oxidative stress on the cell kinetics in testis and reproductive
ability of male mice. Nutrition (3): 351-357
Lamley, A.D. (1993). Tetratogenic effect of selenium in natural
population of fresh sh. Ecotoxicol. Environ. Saf. 26(2); 181-
204
Mohandas, N. And Shohet, S.B. (1978). Control of red cell
deformability and shape. Curr. Trop. Hematol. 1:71-125
Moraes-Silva, L.; Siqueira, L.F.: Oliveira, V.A.; Oliveira, C.S.;
Ineu, R.P. and Pedroso,T.F. (2014). preventive effect of CuCl2
on behavioural alterations and mercury accumulation in cen-
tral nervous system induced HgCl2 in new born rats. J.Biochem
Toxicol.28:328-335
Nagar, R.N. And Bhattacharya, L. (2001). Effect of mercury
chloride on testicular activities in mice, Musculus albinus. J.
Environ. Biol. 22 (1):15-18
Ng, T, B. And Liu, W.K. (1990). Toxic effect of heavy metals on
cells isolated from the rat adrenal and testis. Inviro.Cell.Dev.
Bio.26:24-28
Othman, M.S.; Safwat, G.; Aboulkhair, M. And AbdoelMo-
neim, A.E. (2014). The potential effect of berberine in mercuric
induced heptorenal toxicity in albino rats oxygen species in
different region of rat brain.J.Environ Sci Health 32:395-409
Pyle, G.G.; Rajotte, J.W. And Couture, P. (2005). Effect of
industrial metals on wild sh population along a metal con-
tamination gradient. Ecotoxicol. (3):287-312
Ramalingam, V.; Panneerdoss, S. And Giriji, M. (2001). Mer-
curic chloride induced changes in the histology of testis and
serum testosterone in adult albino rats. Poll. Res. 20: 439-
442
Ramalingam, V.; Narmadharaj, R. and Prabhakaran, P. (2002).
Effect of mercuric chloride in the brain of male rats-Impact on
adesino triphosphate. Poll. Res. 21:7-11
Ramalingam, V. and Vimaladevi, V. (2004). Effect of mercury
chloride on membrane-bound enzymes in rat testis. Asian
Journal of andrology (4): 309-311
Rozgaj, R.; Kasuba, V. And Blanus, M. (2005). Mercury chlo-
ride genotoxicity in rats following oral exposure, evaluated
by comet assay and micronucleus text.Arh Hig Rada Toksikol
56:9-15
794 INDIVIDUAL AND COMBINED EFFECT OF MERCURIC CHLORIDE, MAGNESIUM SULPHATE AND SELENIUM BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS