
Biotechnological
Communication
Biosci. Biotech. Res. Comm. 9(4): 702-711 (2016)
In uence of foliar application with amino acids and
citric acid on physiological and phytochemical responses
of
Artemisia abrotanum
produced by in vitro culture
Mohammad M. EL-Zefzafy
1
, Ismail M. A. Shahhat
1,2
, Rania S. Yousef
3
and
Eman R. Elsharkawy
4,5
1,2
National Organization for Drug Control and Research (NODCAR), Medicinal Plants Dept.
(
1
Tissue Culture &
2
Plant Physiology), Egypt.
2
Northern Border University, Biology Dept. Division of Botany (Plant Physiology), Faculty of Science, KSA.
3
Cairo University, Faculty of Agriculture, Biochemistry Dept., Egypt.
4
Department of Environment and Plant Pasture, Desert Research Center, Mathef El-Mataria, Egypt.
5
Northern Border University, Chemistry Department, Faculty of Science, Girl Division, Arar- Kingdom of
Saudi Arabia
ABSTRACT
The physiological and phytochemical responses of Artemisia abrotanum to foliar application of different concentra-
tions of amino acids (tryptophan and phenylalanine) and citric acids acid were studied during two successive seasons
(2014-2015). The results showed that foliar application of either amino acids or citric acid signi cantly promoted the
growth parameters in terms of plant height, a number of branches, fresh, and dry biomass. All treatments led to sig-
ni cant increments in essential oil content and yield. Plant growth and essential oil parameters gradually increased
with amino acids or citric acid concentrations. Gas-liquid chromatographic analysis revealed that the major identi-
ed components of essential oil were Chamazulen, p-Cymen-8-ol and piperitone. Organic and amino acid treatments
resulted in improving quantity and quality of essential oil components.
KEY WORDS: AMINO CIDS AND CITRIC ACID, ARTEMISIA ABROTANUM L., IN VITRO CULTURE
702
ARTICLE INFORMATION:
*Corresponding Author: elsharqawyeman@hotmail.com
Received 29
th
Nov, 2016
Accepted after revision 28
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/

Mohammad M. EL-Zefzafy et al.
INTRODUCTION
The genus Artemisia belongs to one of the largest and
most widely distributed genera of the family Asteraceae.
It is a diverse and economically important genus and
it has more than 500 species. Artemisia is a wind pol-
linated cosmopolitan genus, mainly distributed in tem-
perate areas of mid to high latitudes of the northern
hemisphere, colonizing in arid and semiarid environ-
mental landscape. Most plants within this genus pos-
sess ethnomedical and biological properties related to
antiviral andantimalarial. antifungal, anticoagulant,
hypoglycemic, and antispasmodic activities, some plants
of the genus are used as foodstuff, ornamentals or soil
stabilizers, some are allergenic or toxic, and some are
weeds growing in the elds, (Zafar, et al.,1990 Nin,et
al 1997, Valles & McArthur 2001, Bnouham et al., 2002
and Hayat et al., 2009).
Artemisia species invariably found as small fragrant
shrubs or herbs and most yield essential oils. Some of
these oils found used medicine .The plants themselves
are popular among gardeners as cultivated ornamentals.
essential oil of Artemisia arborescens has been used in
the treatment of in ammation, intestinal trouble, and
diarrhea and antioxidant. A. abrotanum from Cuba had
trans-sabinyl acetate and -terpineol as main oil com-
pounds. The same species collected in Serbia displayed
silphiperfol-5-en-3-one A (14.6%), ascaridole (13.1%),
1,8-cineole (10.5%), -bisabolol oxide A acetate (8.7%)
as main oil components . Similarly, an oil from North-
western Italy had 1,8-cineole (34.7%), bisabolol oxide
(18.4%) and ascaridole (16.0%) as main compounds.
The dominant components in the oil from the Crimea
were 1,8-cineole and camphor. A German A. abrota-
num showed 1,8-cineol as main oil compound . Plant
cultivated in Poland were rich in piperitone (17.5%),
davanone (16.8%), 1,8-cineole (12.5%) and silphiperfol-
5-en-3-ol A (6.3%) (Kordali et al 2005 and Jerkovic et
al., 2003, Orav, et al., 2006, Lopes-Lutz et al., 2008 Sha-
ropov et al 2012, and Ghasemi Pirbalouti et al., 2013).
Amino acids as organic nitrogenous compounds are
the building blocks in the synthesis of proteins (Houn-
some et al., 2008). Amino acids are particularly impor-
tant for stimulation cell growth, they act as buffers
which help to maintain favorable PH value within the
plant cell, since they contain both acid and basic groups;
they remove the ammonia from the cell. (Abdel Aziz et
al., 2010). Amino acid formulations, mixtures of nutri-
ents, hydrolyzed proteins, humic acids, seaweed extracts
and brassinolides are proposed as a commonly used
growth promoters (Thomas et al., 2009). The application
of amino acids can stimulate the performance of plant
(Abdel-Mawgoud et al., 2011). The role of Tryptophan is
well known: it has an indirect role in the growth via its
in uence on auxin synthesis, (Talaat, et al, 2002).The
yield-contributing characters and quality of plants could
be improved by foliar application of glutamine (Amin
et al., 2011).
Citric acid is a six carbon organic acid, having a cen-
tral role in CA cycle in mitochondria that creates cellular
energy by phosphorylative oxidation reactions. It is cre-
ated by the addition of acetyl-CoA to oxaloacetic acid
that is converted to succinate and malate in next steps
(Wills et al., 1981). Despite the proposed bene ts of the
application of amino acids on plant growth, there is not
many studies about the physiological changes induced
by foliar applied amino acids and organic acid together ,
in medicinal plants in which their biochemical constitu-
ents are important. Especially essential oil.
This research was conducted to evaluate the physi-
ological changes induced by foliar applied active formu-
lations of amino acids and citric acid on the Artemisa
plant produced by in vitro culutur. The foliar applica-
tions of three different concentrations of Aminol-Forte
(50,150, 250.) were applied to plant to study their effect
on the growth, presence of phytochemical compounds
and improving of quality and quantity of essential oil.
MATERIAL AND METHODS
A eld experiment was carried out for two successive
seasons (2014 – 2015) in Applied Research Center of
Medicinal Plants (ARCMP), National Organization for
Drug Control and Research (NODCAR), Egypt in order to
examine the physiological and phytochemical responses
of Artemisa plants to foliar applied of amino acids and
citric. To achieve the study purpose, healthy plantlets
(rooting stage in average tall 6 – 8 cm) were selected
from tissue culture laboratory, (ARCMP), National
Organization for Drug Control and Research (NODCAR),
Egypt. Plant materials were washed from phytagel under
running tap water and soaked in a solution of fungicide
(0.2 % Benlet), then transferred to green house in plas-
tic containers (5 cm) full of peat moss/sand (2/1 –V/V)
for ve weeks. Then plants were transferred to plastic
containers (10 cm) full of peat moss /sand (2/1- V/V) for
ve weeks too.
After an acclimatization period nished (ten weeks),
plants were transferred from a greenhouse to open eld
conditions in 1st Marsh of 2014/2015 season. The experi-
mental soil was prepared and divided into ten plots, each
plot area was 7 m2 (3.5 × 2), with six rows at 60 cm apart
and 40 cm between hills. The mechanical and chemi-
cal analysis of the soil were carried out before planting,
according to Jackson (1967), the obtained results were
as shown in Table 1. Ammonium nitrate 33.5% N (200
Kg/fed), Calcium superphosphate 16 %P2O5 (150 Kg/
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID 703
Mohammad M. EL-Zefzafy et al.
fed) and potassium sulfate 48% K2O (50 Kg/fed) were
added in three doses to fertilize the Artemesia plants.
The rst dose was added at 21 days before the rst cut,
the second and third doses were added after 21 days of
the rst and second cuttings, respectively.
The plants were sprayed twice after 60 days from
planting and after rst cut with freshly prepared solu-
tions of amino acids (Tryptophan or phenylalanine) and
citric acid at the concentrations of 50, 150 and 250 mg/l
as well as untreated plants (control; distilled water). The
spraying was done manually using a spraying bottle.
Physiological response parameters:
Two cuts were taken during the growing seasons (the rst
one on June and the second on September).The physi-
ological response parameters were recorded at each cut
as plant height (cm), a number of branches / plant, herb
fresh weight (g/plant) and herb dry weight (g/plant).
Phytochemical response parameters: Determination of
essential oil percentage, %:
The percentage volatile oil of air dried herb for each
treatment was determined by hydro-distillation of veg-
etative parts of plants (100g) of each treatment for 3h
according to British pharmacopeia (1980). The essential
oil was dried over anhydrous Na
2
SO
4
, stored in a dark
glass bottle and kept at 4°C until it was analyzed.
Chemical analysis of the volatile oil:
The analysis of the volatiles was performed using a
Hewlett-Packard 6890 GC linked to a Hewlett- Packard
5973 mass-selective detector. For the analysis a Zebron
ZB-5MS, capillary column (27m × 250μm i. d., 0.25μm
lm thickness) was used. The carrier gas was helium at
1.3 ml/min in constant ow mode. The injector tem-
perature was 250°C, the injection volume 1μl, and the
split ratio 1:20. The initial oven temperature of 60°C was
held for 1minute, then increased at a rate of 5°C/min
up to 220°C, and subsequently at a rate of 15°C/min up
to 280°C, and nally was held isothermal for 1min. The
transfer line to the MSD was set at 280°C and the scan
conditions were: M/Z 40–300, at 1.75 scans/sec. Prior
to analysis 900μl of the volatile fractions were mixed
with 100 μl of biphenyl (2.0 mg/ml in hexane) as an
internal standard. The components of essential oils were
identi ed by comparing their relative retention times
and mass spectra with those of Registry of Mass Spectral
Data and literature citations.
Identi cation of essential oil components:
The components of the were identi ed by comparison
of their mass spectra with those of a computer library or
with the authentic compounds and con rmed by com-
parison of their retention indices with those of authentic
compounds. Kovats, indices (Kova´ ts, 1958) were deter-
mined by co-injection of the sample with a solution
containing a homologous series of hydrocarbons, at a
temperature run identical to that described above.
Statistical analysis:
The experimental treatments were laid out in a rand-
omized complete block design (RCBD) with 3 replicates.
Data on plant height (cm), number of branches per plant,
herb fresh weight (g/plant), herb dry weight (g/plant),
essential oil (%) and yield of essential oil (ml/plant)
were statically analyzed using the COSTAT 6311Win and
means were compared using LSD at a probability level
of 5%.
RESULTS AND DISCUSSION
Artemisia abrotanum plant was sprayed with phenylala-
nine, tryptophan, and citric acid at different concentra-
tions and the results were presented in (Table 2). Results
showed that all treatments signi cantly increased plant
height, the number of branches, fresh and dry weights
of aerial parts per plant. The most pronounced effect
on these growth criteria was obtained at treatment 250
mgl-1 phenylalanine and 250 mgl-1 of citric acid. Foliar
application of phenylalanine resulted in the greatest
effect as compared with other treatments (tryptophan
and citric acid). Data in Table 2 also clearly indicated
Ca
++
1.31
HCO
3
-
0.71 Total N 9.38 Fe 1.34
Mg
++
0.73
Cl
-
1.73
P
2
O
5
4.73 Cu 0.33
Na
+
1.8
SO
4
-
3.38
K
2
O
4.28 Zn 0.89
K
+
1.52 Mn 4.52
CaCO
3
,
%
8.19
0.47
2.59
Soluble anions
(m.equ/l)
Macro elements
(ppm)
Micro elements
(ppm)
pH
E.C,
mmhos/cm
Chemical analysis
3.8
19
Soil texture
Sandy loam
Coarse sand, %
Fine sand %
Silt, %
Clay, %
Mechanical analysis
Soluble cations
(m.equ/l)
15.8
61.4
Table 1: Mechanical and chemical analysis of experimental soil
704 INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mohammad M. EL-Zefzafy et al.
that the lowest records of A. abrotanum growth charac-
teristics were observed in plants cultivated in soil with-
out any foliar application control).
Data in Table 2 show that all applications of amino
acids (phenylalanine or tryptophan) and citric acid sig-
ni cantly enhanced the height of A. abrotanum plants
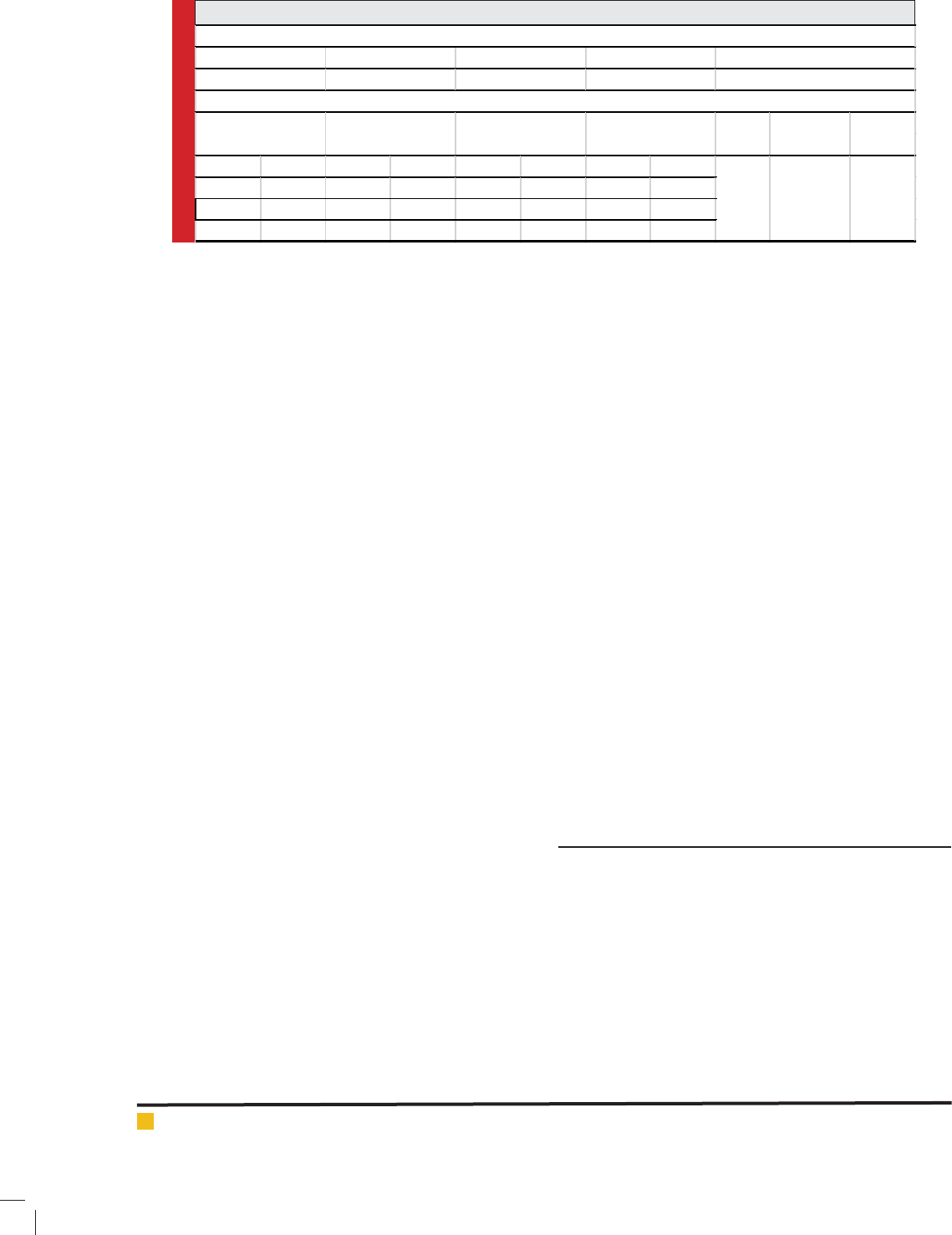
as compared with control in both seasons, (Fig. 1). The
highest plant height (71.50 and 69.56 cm) was recorded
at 250 mgl-1 of phenylalanine and tryptophan, respec-
tively, while the control achieved a minimum plant
height (50.58 cm). It was also observed that there was
no signi cant difference between control and the results
obtained at 50 mgl-1 of all amino acids and citric acid.
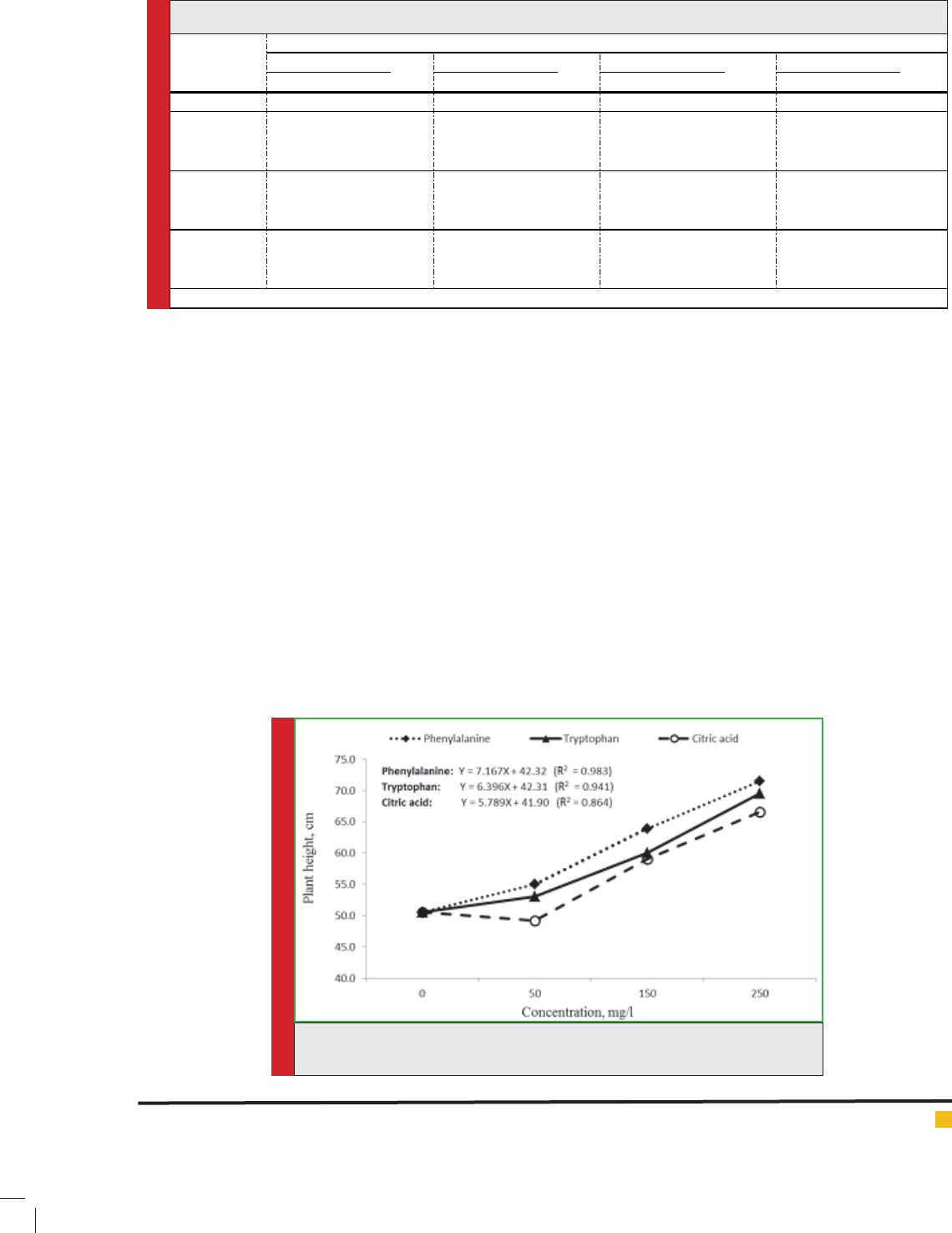
It is obvious from the data in Table 2 that, all amino
acids and citric acid treatments (50, 150 and 250 mgl-1)
gradually increased the number of branches/plant (Fig.
2). Moreover, this increasing was signi cant, except
that at 50 mgl-1 with tryptophan and citric acid, where
there was no signi cant effect of these treatments on the
number of branches. The average minimum of branches
number (31.71±3.55) was recorded with control, while
the high concentration of tryptophan (50 mgl-1) gave
the maximum result of this parameter (52.23±6.37) com-
pared with control. Nevertheless, it was shown that there
was no signi cant difference between tryptophan and
phenylalanine at high concentration (250 mgl-1). Appli-
cation of all treatment concentrations of amino acids
and citric acid observed a higher signi cantly increased
the number of branches in the second season than the
rst season.
Herb fresh weight signi cantly responded to foliar
application of tryptophan, phenylalanine and citric acid
at different concentrations compared with untreated
plants (Table 2). This response was gradual with acid
concentrations (Fig. 3). Phenylalanine was superior to
the other acids (tryptophan and citric acid) at all con-
1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut
22.24 25.57 25.65 27.69 50.58±3.91 13.63 15.57 15.68 18.54 31.71±3.55 32.53 40.35 41.43 46.39 80.35±10.56 12.35 14.57 14.35 18.57 29.92±4.24
50 mg/l 25.45 25.57 28.51 30.42 54.98±5.59 16.35 18.60 19.40 21.80 38.08±4.42 46.27 52.57 51.29 60.47 105.30±9.14 16.24 19.85 19.20 22.64 38.97±4.07
150 mg/l 29.46 31.35 32.49 34.47 63.89±4.35 18.89 20.35 22.59 25.37 43.60±6.17 50.54 57.89 60.75 70.29 119.74±15.99 19.90 22.46 25.30 28.46 48.06±8.06
250 mg/l 31.59 35.23 36.45 39.73 71.5±6.62 22.68 24.47 24.64 30.43 51.11±5.60 67.35 74.35 79.34 82.25 151.70±14.14 22.57 26.35 28.58 31.35 54.43±7.79
50 mg/l 24.46 26.46 26.49 28.63 53.02±2.97 14.13 16.57 16.17 20.54 33.71±4.25 39.57 48.90 44.53 51.88 92.44±5.61 17.35 18.36 19.35 20.36 37.71±2.83
150 mg/l 26.90 30.35 29.41 33.41 60.04±3.94 17.35 20.77 21.39 26.72 43.12±7.06 45.23 60.42 55.23 68.56 114.72±12.83 21.57 23.35 23.35 25.35 46.81±2.67
250 mg/l 30.45 33.54 36.39 38.74 69.56±7.88 21.47 26.25 27.43 29.30 52.23±6.37 61.37 71.47 62.37 71.47 133.34±0.71 23.37 29.45 26.39 29.45 54.33±2.14
50 mg/l 23.65 27.10 26.90 29.20 53.43±
3.78
14.13 18.57 16.40 20.35 34.73±2.86 44.78 50.36 46.75 51.34 96.62±2.09 17.35 19.36 18.55 19.36 37.31±0.85
150 mg/l 26.38 30.16 29.47 32.13 59.07±3.58 17.35 22.50 18.79 24.45 41.55±2.40 46.23 55.42 47.40 61.74 105.40±5.30 22.57 25.35 24.59 26.39 49.45±2.16
250 mg/l 30.28 33.35 33.28 36.28 66.60±4.19 19.47 25.87 21.39 27.84 47.29±2.75 63.37 67.47 68.45 71.51 135.40±6.45 25.37 27.45 27.35 30.44 55.31±3.51
1.76 1.73 1.75 1.52 3.46 1.7 1.79 1.69 1.51 3.39 1.75 1.56 1.73 1.78 6.77 1.75 1.47 1.76 1.79 3.13
Second season
First season
Mean
Plant height, cm
Mean
Control
Treatments
Phenylalanine
Tryptophan
Citric acid
Mean
No. of branches per plant
Herb fresh weight, g/ plant
Herb dry weight, g/ plant
Mean
First season
Second season
First season
Second season
First season
Second season
LSD at 0.05
Table 2: Effect of amino acids and citric acid on physiological responses of A. abrotanum
FIGURE 1. Effect of amino acids and citric acid (type and concentration) on plant
height of A. abrotanum.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID 705
Mohammad M. EL-Zefzafy et al.
1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut 1
st
Cut 2
nd
Cut
1.23 1.47 1.55 1.77 3.01 ± 0.44 0.13 0.18 0.22 0.30 0.41 ± 0.15
50 mg/l 1.60 1.68 1.76 1.85 3.45 ±0.23 0.31 0.31 0.31 0.39 0.65 ±
0.06
150 mg/l 1.89 2.01 1.92 2.06 3.94 ±0.06 0.40 0.40 0.45 0.54 0.89 ±0.14
250 mg/l 2.05 2.15 2.24 2.45 4.45 ± 0.35 0.50 0.50 0.60 0.72 1.16 ±0.12
50 mg/l 1.47 1.68 1.69 1.86 3.35 ± 0.28 0.22 0.26 0.29 0.45 0.61 ±0.18
150 mg/l 1.66 2.00 1.81 2.06 3.77 ± 0.15 0.41 0.40 0.39 0.48 0.84 ±0.04
250 mg/l 1.96 2.10 2.08 2.34 4.24 ± 0.25 0.53 0.53 0.51 0.65 1.11 ±0.06
50 mg/l 1.47 1.68 1.64 1.85 3.32 ± 0.24 0.30 0.26 0.27 0.33 0.58 ±0.03
150 mg/l 1.66 1.91 2.07 1.96 3.71 ±
0.19 0.41 0.41 0.43 0.51 0.88 ±
0.08
250 mg/l 1.71 2.16 2.44 2.07 4.19 ± 0.45 0.52 0.52 0.52 0.69 1.13 ±0.12
0.02 0.05 0.01 0.08 0.20 NS NS 0.11 0.12 0.09
Control
Phenylalanine
Tryptophan
Citric acid
LSD at 0.05
Treatments
Essential oil, %
Yeild of essential oil, ml/plant
First season
Second season
Mean
First season
Second season
Mean
Table 3: Effect of amino acids and citric acid on essential oil % and yield of A. abrotanum
FIGURE 2. Effect of amino acids and citric acid
(type and concentration) on No. of branches of
A. abrotanum.
FIGURE 3. Effect of amino acids and citric acid
(type and concentration) on herb fresh weight of
A. abrotanum.
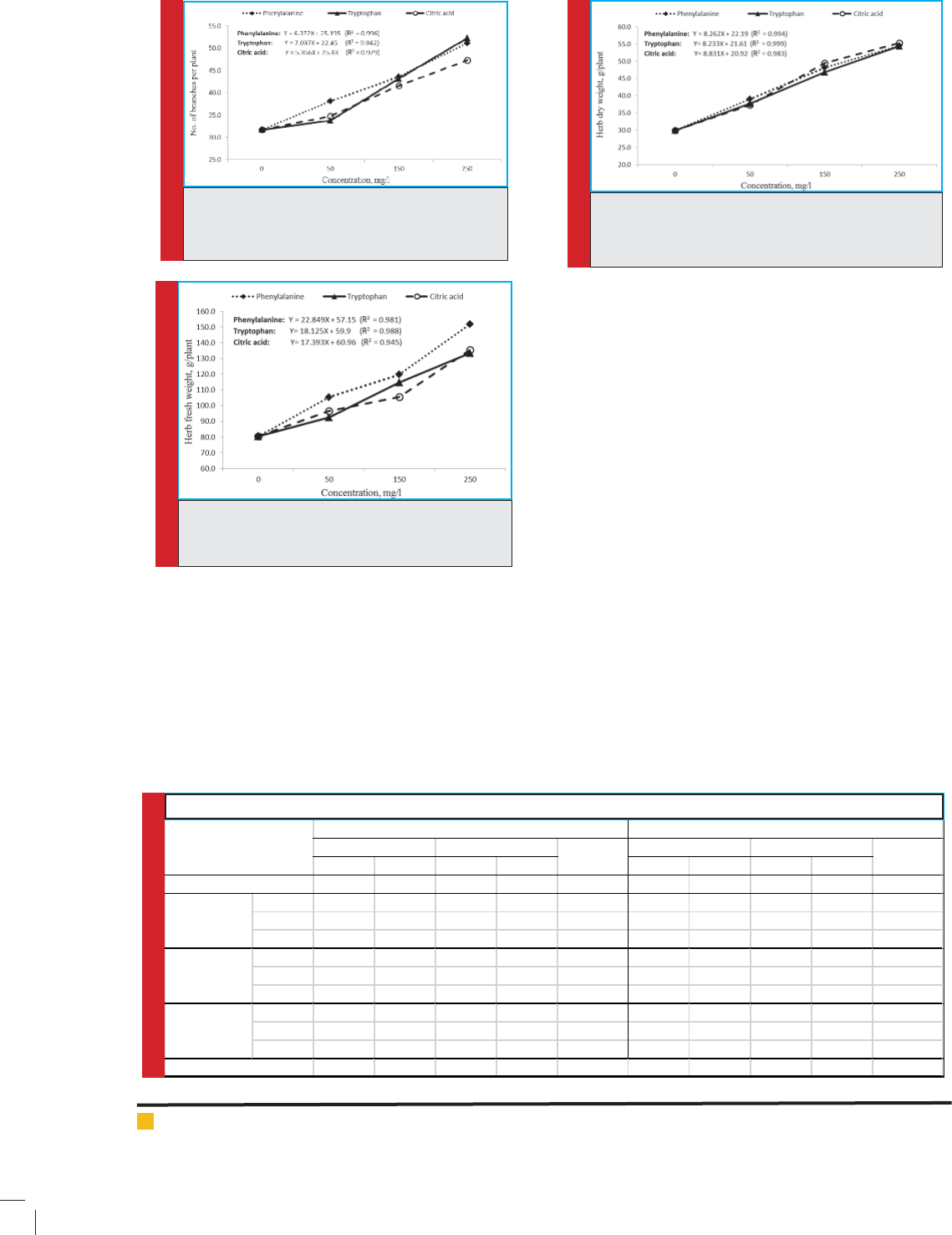
centrations in fresh biomass production. This acid
achieved the highest fresh weight of herb (105.30±9.14
g/plant) with increment about 88.8% at 250 mgl-1 com-
pared with the control which, gave the lowest herb fresh
weight (80.35±10.56 g/plant). According to the statisti-
cal analysis conducted,it was cleared that there was no
signi cant difference between the best treatment, 250
mgl-1, of tryptophan (33.34±0.71 g/plant) and citric acid
(135.40±6.45 g/plant).
FIGURE 4. Effect of amino acids and citric acid (type
and concentration) on herb dry weight of A. abro-
tanum.
The results of herb dry weight presented in Table
2, it was shown clearly that the amino acid and cit-
ric acid had a highly positive signi cant effect on the
average herb fresh yield of A. abrotanum. This effect
was observed in the two growing seasons, as well as the
second season was superior to the rst. Like all previ-
ous growth parameters, dry weight of Artemesia herb
was also gradually increased by increasing the concen-
trations of all amino acids and citric acid (Fig 4). The
increments of herb dry weight of Artemesia were esti-
mated by range from 24.7% to 84.86% at 50 mgl-1 and
250 mgl-1 respectively, with citric acid compared with
the control. Moreover, untreated plants gave the low-
est herb dry weight (29.92±4.24 g/plant). On the other
hand, it was shown that there were no signi cant differ-
encesbetweenall treatments at the same concentration.
The results of essential oil content (%) and its yield
(ml/plant) were presented in (Table 4, Fig. 5 and Fig. 6).
Data in this table shown that the biosynthesis of essen-
tial oil and its average yield were signi cantly promoted
as a result of foliar spraying with amino acids and citric
acid. The increments in essential oil content are ranged
between 10.3% and 47.84%, while in essential oil yield
706 INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mohammad M. EL-Zefzafy et al.
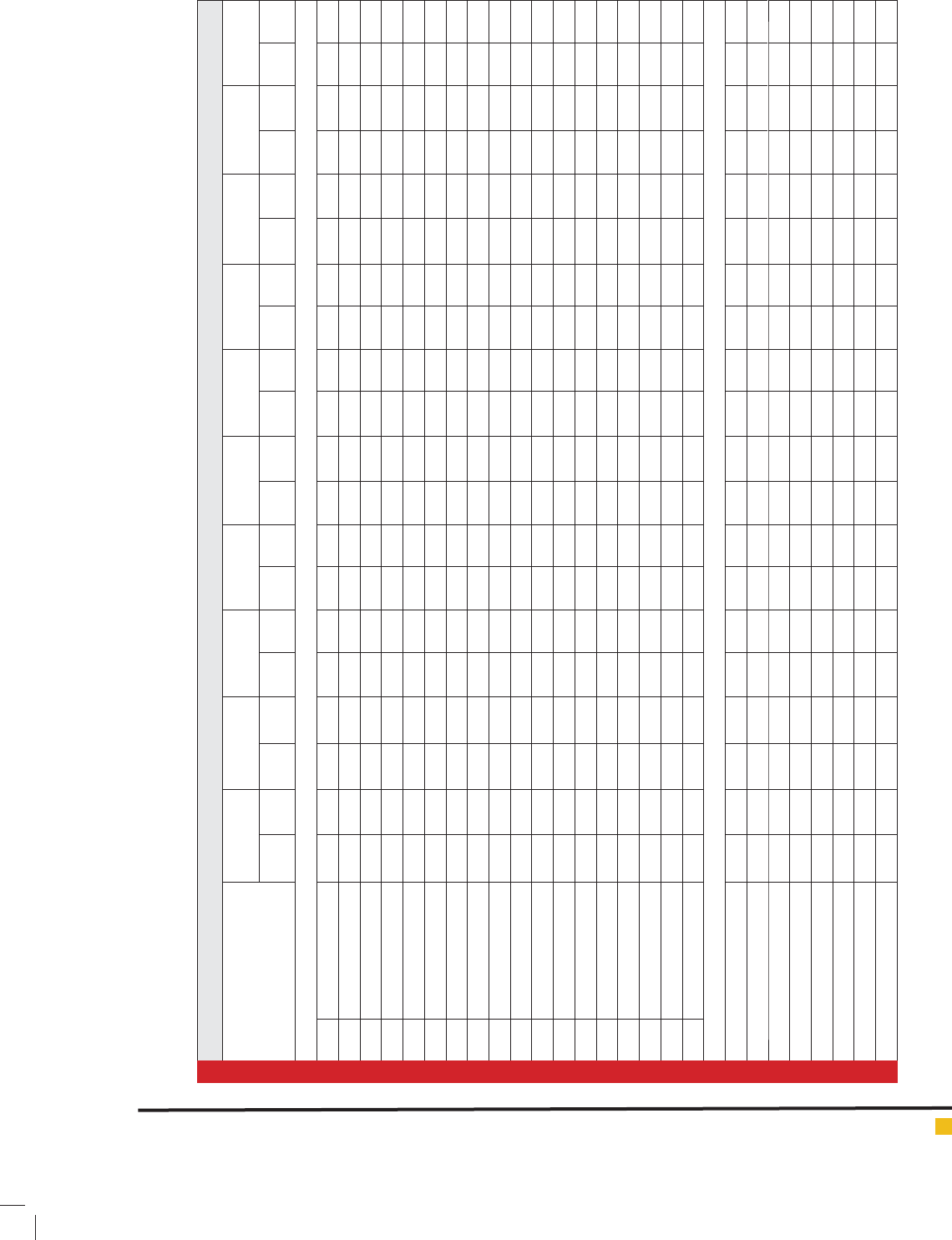
Table 4: Effect of amino acids and citric acid on chemical comparison volatile oil of
A. abrotanum
L plant the rst season.
Compounds Control Phenylalanine
50 m g / L
Phenylalanine
150 mg / L
Phenylalanine
250 mg / L
Tryptophan
50 mg / L
Tryptophan
150 mg / L
Tryptophan
250 mg / L
Citric acid
50 mg / L
Citric acid
150 mg / L
Citric acid 250
mg / L
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1 1,8-cineol 8.85 8.05 8.96 8.87 8.97 9.16 9.23 9.26 9.78 9.88 9.98 9.92 10.22 10.25 8.84 8.96 9.13 9.36 9.46 9.41
2 -terpineol 0.86 3.06 2.06 3.18 2.47 2.68 3.02 3.40 2.97 3.07 2.57 2.80 2.03 3.19 2.54 2.65 2.56 2.97 3.15 3.06
3 Nuciferol butanoate 10.96 11.08 10.90 12.00 10.77 11.68 12.20 12.55 10.56 10.92 10.48 11.11 11.53 11.87 11.03 11.31 11.00 11.99 10.60 12.05
4 Geranyl isobutyrate 1.92 2.15 1.97 2.16 1.92 2.06 2.25 2.59 2.03 2.08 2.63 2.33 2.01 2.37 2.03 2.20 2.03 2.36 2.13 2.02
5 Camphene 0.79 0.96 0.78 1.04 0.48 0.66 0.87 1.08 0.83 1.04 1.27 1.61 0.78 0.98 0.83 1.00 1.15 0.88 0.94 1.09
6 Boroneol 2.40 2.50 2.48 2.92 2.59 2.82 3.02 1.06 2.62 2.80 2.96 3.15 2.82 2.86 2.94 2.99 2.48 3.17 2.64 2.90
7 Terpineol 1.57 1.89 1.66 1.73 1.50 1.58 1.83 1.90 1.36 1.70 1.98 2.25 1.70 1.78 1.64 1.61 1.49 1.06 1.62 1.29
8 Chamazulene 19.03 19.20 19.92 20.00 20.88 21.19 22.37 23.56 22.26 22.93 22.53 22.79 22.87 23.40 19.03 19.58 20.78 21.49 20.39 21.36
9 Sesquisabinenehydrate 3.27 3.48 2.91 3.16 2.97 1.33 3.59 3.87 2.86 3.17 3.48 3.03 3.33 3.67 3.03 3.20 3.41 3.63 3.16 3.17
10 Caryophyllene oxide 3.91 3.97 3.79 3.98 3.30 3.70 4.07 4.36 3.46 3.69 3.92 4.17 3.93 4.16 3.44 3.48 3.17 4.11 3.43 3.37
11 binene 1.95 2.05 2.76 2.87 2.97 3.56 3.73 3.86 3.78 3.88 3.78 4.02 3.22 4.25 3.14 3.26 313 3.36 3.16 3.41
12 Geranyl isobutyrate 1.96 3.06 2.06 3.18 2.47 2.68 3.02 3.40 2.97 3.07 2.57 2.80 2.03 3.19 2.54 2.65 2.56 2.97 3.15 3.06
13 Piperitone 11.05 11.25 11.76 11.87 11.97 12.16 12.23 12.26 11.78 12.88 12.78 12.72 12.82 12.95 11.14 11.26 11.13 11.36 11.16 11.41
14 B- Eudesmol
1.65 1.57 1.89 1.46 1.73 1.50 1.58 1.83 1.90 1.36 1.70 1.98 2.25 1.70 1.78 1.64 1.61 1.49 1.06 1.62
15 Borneol acetate 1.79 1.96 0.78 1.04 0.48 0.66 0.87 1.08 0.83 1.04 1.27 1.61 0.78 0.98 0.83 1.00 1.15 0.88 0.94 1.09
16 Nuciferol propionate 5.30 5.45 4.74 4.93 4.77 4.98 5.28 5.59 4.83 4.97 4.54 4.94 5.10 5.55 4.83 4.18 4.38 5.18 4.39 5.00
17 p-Cymen-8-ol 10.96 11.08 10.90 12.00 10.77 11.68 12.20 12.55 10.56 10.92 10.48 11.11 11.53 11.87 10.96 11.08 10.90 12.00 10.77 11.68
18 Davanone 3.30 3.45 4.74 4.93 4.77 4.98 5.28 5.59 4.83 4.97 4.54 4.94 5.10 5.55 4.83 4.18 4.38 5.18 4.39 5.00
Grouped compounds
Monoterpene hydrocarbons % 0.55 0.75 0.91 0.93 1.07 1.11 1.28 1.58 0.94 0.95 1.17 1.22 1.35 1.61 0.87 0.91 1.10 1.15 1.25 1.52
Oxyenated Monoterpenes % 21.44 21.66 21.93 21.92 21.98 21.99 22.39 22.79 21.95 21.95 21.98 21.99 22.35 22.80 20.90 21.93 20.99 21.97 22.34 22.72
Sesquiterpene hydrocarbons % 5.22 5.82 6.18 6.15 6.21 6.29 6.49 6.75 6.23 6.20 6.21 6.35 6.59 6.77 6.16 6.18 6.25 6.32 6.45 6.71
Oxyenated Sesquiterpene % 17.81 17.93 18.45 18.41 18.47 18.52 18.71 18.94 18.48 18.42 18.47 18.52 18.78 18.95 17.44 18.45 18.49 18.55 18.70 18.91
Di terpenes % 1.11 1.31 1.77 1.79 1.89 1.93 1.98 1.99 1.79 1.80 1.89 1.98 1.98 1.99 1.75 1.77 1.89 1.95 1.95 1.92
Aromatics % 37.55 37.85 37.71 38.67 38.81 38.88 38.92 39.90 37.75 38.70 38.81 38.88 38.95 39.92 36.70 37.71 38.85 38.91 38.90 39.94
Others % 2.77 2.97 3.22 3.33 3.43 3.62 3.72 3.85 3.29 3.37 3.43 3.63 3.75 3.87 3.20 3.22 3.45 3.65 3.70 3.83
Total compounds 85.45 86.99 87.17 91.21 91.86 92.34 93.49 95.80 90.35 91.39 91.96 92.57 93.75 95.92 87.03 90.34 90.83 92.50 93.29 95.55
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID 707

Mohammad M. EL-Zefzafy et al.
was between 41.46% and 182.93% compared with the
control. It is also clear from the obtained data that
essential oil content and its yield gradually increased
with amino acid and citric acid level increases (Fig. 5
and 6). The highest recorded value of the average essen-
tial oil content (4.45±0.35 %) and yield of essential oil
(1.16±0.12 ml/plant) were obtained in the herb of plants
treated with phenylalanine at 250 mg/l. While the con-
trol gave minimum essential oil content (3.01±0.44 %)
and oil yield (0.41±0.15 ml/plant). On the other hand,
there are no signi cant differences between all the three
acids (phenylalanine, tryptophan and citric) at the same
concentration in terms of essential oil contents and
essential oil yield.
In this respect, several investigators have studied the
effect of different amino acids on the total oil percent-
age and oil yield (ml/plant) and found the same results;
Gamal El.Din et al., (1997) on Cymbopogon citratus Hort,
Talaat and Youssef (2002) on basil plant, Karima et al.,
(2005) on Matricaria chamomilla L. Amino acids accu-
mulation in plants plays different roles, an osmolyte,
regulation of iron transport, modulating stomata opin-
ing and detoxi cation of heavy metals. Moreover, amino
acids affect synthesis and activity of some enzymes,
gene expression and redox-homeostasis (Rai, 2002).
Many investigators, Talaat et. al. (2002) and Orner
et.al. (2013), mentioned that foliar application of amino
acids signi cantly increased essential oil percentage and
yield. Also, Taraf et.al.(1999) on lemongrass, Eid et.al.
(2010) on Jasminum grandi orm and Safaa et.al. (2011)
on geranium reported that foliar application of citric
acid caused a pronounced increment in the percentage
and yield of essential oil. It has been documented that
the exudation of citrate and malate from roots of cal-
cicole plants (plants growing in alkaline soils) enables
them to extract P and Fe from such soils (Lopez-Bucio
and Nieto-Jacobo, 2000). Use of citric acid alone or in
combinations with malic acid increased the essential oil
production of sweet basil (Jaafari and Hadavi, 2012).
Foliar sprays of citric acid alone or in combination
with Fe sources have been used to recover many plants
from the iron chlorosis (Eidyan et al., 2014). Later stud-
ies revealed that the effect of citric acid is not just due
to pH change and there are a variety of physiological
responses to applied citric acid.
The results of the GC/MS analysis of the essential oil
of A. abrotanum. during the rst and second season as
are shown in Table (4,5). There are 18 identi ed com-
pounds. Chamazulen was identi ed as the major com-
pound in the different treatments ranged from 19.3 %
to .23.5 %, the second main component, p-Cymen-8-ol
ranged (12.39% and 12.55. %) in the essential oil fol-
lowed with piperiton which was identi ed as the third
main constituent in the essential oil and its relative
percentage accounted for (12.16% and 12.23. %), while
some compound found in traces amount as Camphen
(0.79% and 1.61%).
Foliar application of amino acid indicate that pheny-
lalanine at 250 mg/1 revealed that, maximum relative
percentage of Chamazulen was (22.37% and 23.5 % ) for
1st and 2nd cuts, respectively during 1st season). In the
2nd season, the foliar application of phenylalanine at
250 mg/1 resulted in the highest relative percentage of
Chamazulen 2nd cut, (Table, 4, 5) in both seasons. The
data indicated that Cymen-8-ol reached to its maximum
values 11.3% and 12.5. % for 1st and 2nd cuts, respec-
tively) as a result of application of phenylalanine at 250
mg/ L during the 1st season. On the other hand, trypto-
phan at 250 mg /l gave the highest relative percentage
1,8-cineol which ranged between (10.22 to 10.95%) in
the 1st and 2nd cuts during two seasons, as compared
with control. while citric acid did show any signi cant
change in some essential oil concentration than amino
acid, like Eudesmol, pipeitone, Terpenol, camphene,
Gerenyl isobutrate, terpenol and 1.8-cineol in First sea-
son, while second season citric acid showed signi cant
decrease in the concentration of some oil component
especially at low concentration 50 mg of citric acid.
The essential oil composition varies according to cuts
or different treatments and was characterized by a high
percentage of oxygenated compounds ranged from 21.9
to 22.8 %. The components of the essential oil in herb
FIGURE 5. Effect of amino acids and citric
acid concentrations on essential oil content.
FIGURE 6. Effect of amino acids and citric
acid concentrations on essential oil yield.
708 INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mohammad M. EL-Zefzafy et al.
Table 5: Effect of amino acids and citric acid on chemical comparison volatile oil of
A. abrotanum
L plant the second season
Compounds Control Phenylalanine
50 mg / L
Phenylalanine
150 mg / L
Phenylalanine
250 mg / L
Tryptophan
50 mg / L
Tryptophan
150 mg / L
Tryptophan
250 mg / L
Citric acid
50 mg / L
Citric acid
150 mg / L
Citric acid 250
mg / L
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1st
Cut
2nd
Cut
1 1,8-cineol 9.78 9.88 9.87 9.98 9.99 10.16 10.23 10.26 10.78 10.89 10.98 10.92 10.92 10.95 9.89 10.26 10.53 10.47 10.66 10.71
2 -terpineol 1.55 2.06 3.46 3.38 3.77 3.68 3.72 3.80 3.97 3.99 3.57 3.80 3.03 3.19 2.54 2.65 2.56 2.97 3.15 3.06
3 Nuciferol butanoate 10.76 11.08 10.90 12.00 10.77 11.68 12.20 12.55 10.56 10.92 10.48 11.11 11.53 11.87 11.03 11.31 11.00 11.99 10.60 12.05
4 Geranyl isobutyrate 2.92 2.15 1.97 2.16 1.92 2.06 2.25 2.59 2.03 2.08 2.63 2.33 2.01 2.37 2.03 2.20 2.03 2.36 2.13 2.02
5 Camphene 1.79 0.96 0.78 1.04 0.48 0.66 0.87 1.08 0.83 1.04 1.27 1.61 0.78 0.98 0.83 1.00 1.15 0.88 0.94 1.09
6 Boroneol 3.45 2.50 2.48 2.92 2.59 2.82 3.02 1.06 2.62 2.80 2.96 3.15 2.82 2.86 2.94 2.99 2.48 3.17 2.64 2.90
7 Terpineol 2.57 1.89 1.66 1.73 1.50 1.58 1.83 1.90 1.36 1.70 1.98 2.25 1.70 1.78 1.64 1.61 1.49 1.06 1.62 1.29
8 Chamazulene 19.23 19.20 19.92 20.00 20.88 21.19 22.37 23.56 22.26 22.93 22.53 22.79 22.87 23.40 19.03 19.58 20.78 21.49 20.39 21.36
9 (Z) Sesquisabinenehydrate 3.27 3.48 2.91 3.16 2.97 1.33 3.59 3.87 2.86 3.17 3.48 3.03 3.33 3.67 3.03 3.20 3.41 3.63 3.16 3.17
10 Caryophyllene oxide 3.91 3.97 3.79 3.98 3.30 3.70 4.07 4.36 3.46 3.69 3.92 4.17 3.93 4.16 3.44 3.48 3.17 4.11 3.43 3.37
11 binene 2.95 2.05 2.76 2.87 2.97 3.56 3.73 3.86 3.78 3.88 3.78 4.02 3.22 4.25 3.14 3.26 313 3.36 3.16 3.41
12 Geranyl isobutyrate 2.96 3.06 2.06 3.18 2.47 2.68 3.02 3.40 2.97 3.07 2.57 2.80 2.03 3.19 2.54 2.65 2.56 2.97 3.15 3.06
13 piperitone 11.35 11.25 11.76 11.87 11.97 12.16 12.23 12.26 11.78 12.88 12.78 12.72 12.82 12.95 11.14 11.26 11.13 11.36 11.16 11.41
14 B- Eudesmol 2.65 1.57 1.89 1.46 1.73 1.50 1.58 1.83 1.90 1.36 1.70 1.98 2.25 1.70 1.78 1.64 1.61 1.49 1.06 1.62
15 Borneol acetate 2.79 1.96 0.78 1.04 0.48 0.66 0.87 1.08 0.83 1.04 1.27 1.61 0.78 0.98 0.83 1.00 1.15 0.88 0.94 1.09
16 Nuciferol propionate 6.30 5.45 4.74 4.93 4.77 4.98 5.28 5.59 4.83 4.97 4.54 4.94 5.10 5.55 4.83 4.18 4.38 5.18 4.39 5.00
17 p-Cymen-8-ol 11.96 11.08 10.90 12.00 10.77 11.68 12.20 12.55 10.56 10.92 10.48 11.11 11.53 11.87 10.96 11.08 10.90 12.00 10.77 11.68
18 Davanone 4.30 3.45 4.74 4.93 4.77 4.98 5.28 5.59 4.83 4.97 4.54 4.94 5.10 5.55 4.83 4.18 4.38 5.18 4.39 5.00
Grouped compounds
Monoterpene hydrocarbons % 1.05 1.21 1.33 1.45 1.51 1.35 1.58 1.75 0.91 1.22 1.95 1.35 1.52 1.99 1.10 1.17 1.15 1.25 1.61 1.96
Oxyenated Monoterpenes % 21.45 21.61 21.99 21.95 21.99 22.35 22.79 22.89 21.93 21.99 22.90 22.35 22.72 22.89 20.99 21.98 21.97 22.34 22.80 22.93
Sesquiterpene hydrocarbons % 5.33 5.82 6.38 6.25 6.69 6.59 6.75 6.79 6.18 6.35 6.86 6.59 6.71 6.86 6.25 6.21 6.32 6.45 6.77 6.98
Oxyenated Sesquiterpene % 17.84 16.99 18.49 18.44 18.57 18.78 18.94 18.99 18.45 18.52 18.74 18.78 18.91 18.85 18.49 18.47 18.55 18.70 18.95 18.96
Di terpenes % 1.16 1.31 1.78 1.82 1.95 1.98 1.99 2.91 1.77 1.98 1.95 1.98 1.92 2.79 1.89 1.89 1.95 1.95 1.99 2.58
Aromatics % 37.66 37.81 37.77 38.73 38.88 38.95 39.90 39.93 37.71 38.88 36.78 38.95 39.94 38.98 38.85 38.81 38.91 38.90 39.92 38.96
Others % 2.81 3.92 3.26 3.39 3.69 3.75 3.85 3.88 3.22 3.63 3.79 3.75 3.83 3.88 3.45 3.43 3.65 3.70 3.87 3.85
Total compounds 87.30 88.67 91.10 92.03 92.34 93.75 95.80 96.27 90.34 92.57 92.97 93.75 95.55 96.24 90.83 91.96 92.50 93.29 95.92 96.22
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID 709

Mohammad M. EL-Zefzafy et al.
for different treatments during 2 cuts during both sea-
sons were shown in Tables (4 and 5). The increment in
oil% might be due to the increase in vegetative growth
and nutrient uptake. Similar results were reported by
Gharib (2006). The chemical composition of A. abro-
tanum oils is very variable, the chemovarieties and the
environmental conditions caused to this fact. The major
components from different origins were found to be lin-
alool, 1,8-cineol, piperitone, davanone and silphiperfol-
5-en-3-ol, several workers reported that essential oil
components of are varied according to growth condi-
tions., foliar application of salicylic of (Ocimum basili-
cum L.) and (Majorana hortensis) increased the produc-
tion of quantity and quality of oil ( Gharib et al 2006).
These results are similar to those obtained by (Orav,
et al., 2006).
CONCLUSIONS
We conclude that there is a pure effect by sprayed of all
treatments enhanced growth parameters, plant height ,the
number of branches, the fresh and dry weight of plant,
oil percentage and yield per plant for two cuttings rela-
tive to untreated controls. from the above explanation the
important role of amino acids in enchaning the growth
and production of different plant constituents which is
in accordance with our results, also our results are in
accordance with many investigators who reported that
the application of amino acids increases the concentra-
tion of essential oil and growth rate. Foliar application
of phenylalanine, gave the best effect on growth param-
eters at 250 mg,and increase the quantity and quality of
essential oil, by incrasing the yield percentage of oil and
increasing the concentration of major compounds in the
oil, while the application of citric acid shows variable
effect according to concentration and seasons.
DISCLOSURE STATEMENT
No potential con ict of interest was reported by the
authors.
REFERENCES
Attoa, G.E., H.E. Wahba and A.A. Farahat, (2002). Effect of
some amino acids and sulphur fertilization on growth and
chemical composition of Iberis amara L. plants. Egyption J.
Hort., 29:17-37.
Audesirk G. and Audesirk T. (1986). Biology Life and Earth.
MACmillan Publising Company, New York, London. pp: 494-
507.
Bnouham, M., Mekh , H., Legssyer, A., Ziyyat, A. (2002).
Forummedicinal plants used in the treatment of diabetes in
Morocco. Int. J. Diabetes & Ethnopharmacology.
Chialva F, Liddle PAP, Doglia G. (1983)Chemotaxonomy of
wormwood (Artemisia absinthium L.) Composition of the
essential oil of several chemotypes. Z Lebensm UntersForsch.,
176: 363-366.Google Scholar.
Eid RA. (2010) Physiological Properties studies on essential oil
of Jasminum grandi orum L. as affected by some vitamins.
Ozean Journal of Applied Sciences, 3,1: 87- 96.
Eidyan, B., Hadavi, E., and Moalemi, N. (2014). Pre-harvest
foliar application of iron sulfate and citric acid combined
with urea fertigation affects growth and vase life of tuberose
(Polianthes tuberosa L.)‘por-par’. Hortic. Environ. Biotechnol.
55, 9–13. doi: 10.1007/s13580-014-0061-2.
El-Bahar, K.M., A.S. Ghanem and E.A. Omer (1990). Responses
of Datura metel L. cell cultures to some amino acids. Egypt J.
Appl. Sci., 5: 192-199.
Fatmaa A. E. G. (2006).Effect of Salicylic Acid on the Growth,
Metabolic Activities and Oil Content of Basil and Marjoram,
Int. J. Agri. Biol., Vol. 8, No. 4, 2006.
Gamal El-Din (1997), Physiological studies on the effect of
some amino acids and microelements on growth and essential
oil content in lemongrass (Cymbopogon citrates Hort). Agric.
Sci. Mansoura Vniv., 22, 4229-4241.
Ghasemi Pirbalouti A, Firoznezhad M, Craker L, Akbarzadeh
M. 2013. Essential oil compositions, antibacterial and antioxi-
dant activities of various populations of Artemisia chamae-
melifolia at two phenological stages. Rev Bras Farmacogn; 23:
861-9.
Hayat MQ, Ashraf M, Khan MA, Jabeen S, 2009, Ethnobotany
of the genus Artemisia L. (Asteraceae) in Pakistan. Ethnobot
Res, 7: 147-162.
Hendawy, S.F. (2000): Physiological and chemical studies on
Echinaceae purpurea L., plant. Ph.D. Thesies, Fac., Agric.,
Moshtohor, Zaga. Univ. Egypt.
Jaafari, N., and Hadavi, E. (2012). Growth and essential oil
yield of basil (Ocimum basilicum L.) as affected by foliar spray
of citric acid and salicylic acid. Z. Arznei Gewurzp . 17, 80–83.
Jackson, M. L.(1967). Soil chemical analysis.printice - Hall of
India, P.144 - 197.
Jerkovic I, Mastelic M, Milos M, Juteau F, Masotti V, Viano
J (2003) Chemical variability of Artemisia vulgaris L. essen-
tial oils originated from the Mediterranean area of France and
Croatia. Flavour Fragrance J.18: 436-440. 10.1002/ffj.1246.
Karima MG and MSA Abd EL-Wahed.(2005)Effect of Some
Amino Acids on Growth and Essential Oil Content of Chamo-
mile Plant. International Journal of Agriculture & Biology 7,
3: 376 – 380.
Kordali S, Kotan R, Mavi A, Cakir A (2005) Determination
of the chemical composition and antioxidant activity of the
essential oil of Artemisia dracunculus L. and of the antifun-
gal and antibacterial activities of turkish A. dracunculus, A.
absinthium and Santonicum essential oil. J Agric Food Chem.
53: 9452-9458..View ArticleGoogle Scholar.
Ljung Karin (2013). Auxin metabolism and homeostasis during
plant development. Development 140, 943-950.
710 INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Mohammad M. EL-Zefzafy et al.
Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP.
(2008). Screening of chemical composition, antimicrobial and
antioxidant activities of Artemisia essential oils. Phytochem-
istry; 69:1732-8.
McArthur E.D & D.J. Fairbanks. (2000). Proceedings of Provo,
Utah. US Department of Agriculture, Rocky Mountain Research
Station, Ogden.
Omer, E.A.; H.A.H. Said-Al Ahl; A.G. El Gendy; Kh. A. Shaban
and M.S. Hussein (2013). Effect of amino acids application on
production, volatile oil and chemical composition of chamo-
mile cultivated in saline soil at Sinai. J. Appl. Sci. Res., 9(4):
3006-3021. Metabolism, 10: 33-50.
Opez-Bucio, J., and Nieto-Jacobo, M. I. (2000). Organic acid
metabolism in plants: from adaptive physiology to transgenic
varieties for cultivation in extreme soils. Plant Sci. 160, 1–13.
doi: 10.1016/S0168-9452(00)00347-2.
Orav A, Raal A, Arak E, Müürisepp M, Kailas T. (2006). Com-
position of the essential oil of Artemisia absinthium L. of dif-
ferent geographical origin. Proc. Estonian Acad. Sci. Chem. 55
(3): 155-165. Google Scholar.
Phillips, L.D.J., (1971). Introduction to the Biochemistry and
Physiology of Plant Growth Hormones. Mc. Graw-Hill Book Co.
Rai, V.K. (2002). Role of amino acids in plant responses to
stresses. Biologia Plantarum, 45(4): 481-487.
Saffaa RE et. al. (2011). Effect of ribo avin, ascorbic acid
and dry yeast on vegetative growth, essential oil pattern and
antioxidant activity of geranium (Pelargonium graveolens L.).
American-Eurasian J. Agric. & Environ. Sci.10,5: 781-786.
Sharopov FS, Sulaimonova VA, Setzer WN. Composition of the
essential oil of Artemisia absinthium from Tajikistan. Rec Nat
Prod 2012;6:127-34.
Sun, Y.L., Hong, S.K., (2011): Effects of citric acid as an impor-
tant com ponent of the responses to saline and alkaline stress
in the halophyte Leymus chinensis (Trin.). Plant Growth Reg.
64, 129-139.
Talaat, IM. and AA. Youssef (2002) The role of the amino acids
lysine and ornithine in growth and chemical constituents of
Basil plants.Egyptian J. Appl. Sci. 17: 83–95.
Taraf, S.A., (1999). The response of vegetative growth and
essential oil of lemongrass (Cymbopogon citrates Hort) to foliar
application of ascorbic acid, nicotinamid and some micronu-
trients. Arab Univ. of Agric. Sci.; 7: 247-259.
Tucker, A.O., Maciarello, M.J. and Sturtz, G. (1993). The essen-
tial oils of Artemisia Powis Castle and its putative parents,
A. absinthium and A. arborescens. Journal of Essential Oil
Research, 5: 239-242.
Valles, J. & E.D. McArthur. 2001. Artemisia systematic and
phylogeny: Cytogenetic and molecular insights. Pp 67-74 in
Shrubland Ecosystem Genetics and Biodiversity.
Wahba H. E., et. al.( 2015) Growth and chemical composition
of Urtica pilulifera L. plant as in uenced by foliar applica-
tion of some amino acids. 1. Mater. Environ. Sci. 6 (2) 499-
506.
Waller, G.R. and E. Nawacki, (1978). Alkaloid Biology and
Metabolism in Plants. Phanum, Press, New York, pp; 152.
Youssef, AA. and Talaat I M,. (2003). Physiological response
of rosemary plants to some vitamins. Egypt.Pharm.J.;1:
81-93.
Zafar, M.M., M.E. Hamdard and A. Hameed. (1990). Screening
of Artemisia bsenthium for antimalarial effects on Plasmodium
berghei in mice: a preliminary report. J. Ethnopharmacol.,
30:223-226.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS INFLUENCE OF FOLIAR APPLICATION WITH AMINO ACIDS AND CITRIC ACID 711