Pharmaceutical
Communication
Biosci. Biotech. Res. Comm. 9(4): 694-701 (2016)
Phytoconstituent based mucoadhesive antifungal
vaginal formulation: An effective and innovative
approach
Amit Roy, Ananta Choudhury*, Sanjib Bahadur and Suman Saha
Department of Pharmaceutics, Columbia Institute of Pharmacy, Tekari, Raipur, C.G. pin-493111
ABSTRACT
The present experimental study has been design with an aim to develop a phytoconstitute based mucoadhesive anti-
fungal vaginal gel, for the management of wide range of fungal infections. All formulations were prepared by incor-
porating optimized concentration of curcumin along with uconazole, to overcome the problem related to sensitivity
of uconazole on topical application. Different mucoadhesive polymers like carbopol P934, carbopol 940 and HPMC
K4M either individually or in suitable combination were used to fabricate mucoadhesive gels. Essential in-vitro stud-
ies such as, screening of antifungal activity, rheological property, spreadibility, pH, Content uniformity, capacity of
mucoadhesion, drug release etc. were performed to evaluate the performance of prepared gel in respect of safety and
ef cacy. Results of the study reveal a signi cant increase in antifungal activity of uconazole. Among the different
formulation batches, F5 & F8 showed signi cant mucoadhesive property, spreadibility and In-Vitro release pattern as
compare to others and at the same time no sign of irritation were observe. On the basis of results it can be concluded
that prepared formulations satisfactorily ful ll the desire need as an antifungal vaginal formulation.
694
ARTICLE INFORMATION:
*Corresponding Author: anantachoudhury@gmail.com
Received 26
th
Sep, 2016
Accepted after revision 25
th
Nov, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Since last few decade fungal infections are very common
in all age group patients, but recently its occurrence has
increased signi cantly (Sharma et al., 2010, Choudhury
A et al., 2016). Among different fungal infections caused
by Candida albicans (C. albicans) namely, oral, rectal or
vaginal candidiasis is reported to be most common in
human (Kuleta et al. 2009). Approximately 75% women
population experience vaginal candidiasis during their
whole life and about 40% to 50% of them experience
multiple episodes (Choudhury et al., 2011; Choudhury
et al., 2014). These infections have an unacceptably
high mortality rate may be due to several reasons like,
immunological state of the patient,
restricted number
of commercially available antifungal drugs with many
side effects, Delay in diagnosis of the infection and/ or
the drug resistance of the therapeutic agents (Martins
Amit Roy et al.
et. al., 2009). Therefore, this challenging clinical issue is
required to be addressed on priority basis.
In most of the cases it was found that both systemic
and topical antifungal therapies are required for effec-
tive management of Candida infections.(Hemaiswarya
et al. 2008) Fluconazole is reported to be most effective
molecule for the treatment of virginal candidiasis, (Tsao
et al., 2000) however it shows serious sensitivity issue,
when applied topically at high concentration. Again in
several cases a high risk of development of fungal resist-
ance was also observed (Annette et al., 2014), and on the
other hand if it is introduce in the formulation in very
low concentration perhaps may fail to ful l the desired
needs. (
Oelkrug et al., 2014).
Therefore, there is an urgent need to establish an
alternative way not only to make the existing molecule
like uconazole as effective as they were, rather to make
them therapeutically more effective and safe too. Nat-
ural products are reported as attractive prototypes for
this purpose due to their broad spectrum of biological
activities. The promising results of antimicrobial activ-
ity of curcumin, a natural compound found in the Cur-
cuma longa plant, active against different bacteria, fungi
and parasites, made it a good candidate to enhance the
inhibitory effect of existing antimicrobial agents through
synergism (Sharma et al., 2009). Curcumin reported to
have signi cant inhibitory effect against Candida albi-
cans due to its membrane-lytic activities as well as the
capacity to prevent the adhesion on host epithelial cell
(Shyh et al., 2000). On the other hand, popular antifun-
gal are belongs to azoles category mainly works based
on the mechanism of target heme protein, cytochrome
P450 (Jana et al., 2006 Grossman et al., 2015). There-
fore it is expected that, a twofold effect of curcumin and
uconazole combination may improve the therapeutic
ef cacy against Candida albicans infection.
Therefore, this research work has been designed with
a primary objective to improve ef ciency of uconazole
against Candia albican and develop a safe and effec-
tive antifungal mucoadhesive vaginal gel formulation
for the management of wide range of fungal infections.
An antifungal screening study has been carried out to
establish suitable combination of Curcumin and ucon-
azole. Mucoadhesive gels were prepared incorporating
optimized drug combination using different ratios of
polymers like HPMC, Carbopol P934 and Carbopol 940.
All the prepared formulations were submitted for differ-
ent In-vivo and In –vitro evaluation and nal formula-
tion was selected based on resultant data.
MATERIAL AND METHODS
Materials: The pathogenic antifungal stain of Candida
albicans (MTCC 227) was purchased from MTCC Chan-
digarh, Materials used for the experimental work such
as; uconazole was obtained as gift sample from Cadila
Pharmaceutical ltd. India. Carbopol 940, Carbopol 934
and HPMC were purchased from S.D. ne Pvt. ltd., RPMI
1640 media was and 96 well plates purchased from
sigma, Guar gum & Sodium CMC were purchased from
loba chemie. Ltd., Triethanolamine & Glycerin was pur-
chased from Loba Chemie Ltd. India.
Determination of minimum inhibitory concentration
(MIC) & Fractional inhibitory concentration index (IFCI)
The MIC value of each APIs was evaluated using broth
dilution methods as per standard guideline of NCCLS,
M27. At rst the Candida albicans (MTCC 227) strain
was subculture in Sabouraud dextrose agar media to
ensure purity and viability. After that a standard patho-
genic cell suspension was prepared by suspending few
colonies from a freshly prepared culture, in 5 ml of
saline solution. Final inoculums of 4 x 10
6
cells per mL
were prepared by vortexes the suspension for 30 sec fol-
lowed by adjustment the transmittance as per McFar-
land standard. After that a standard sterile stock solu-
tion of uconazole and curcumin, individually as well
as in suitable combination were prepared. MIC value of
individual drug and drugs combination was measured
on the basis of difference in optical density, through 96
plate method (Mukherjee et al., 2015).
The effect of combination of uconazole and cur-
cumin was investigated based checkerboard experiments
(Gomes et al., 2012; Odds et al., 2003). A 100μl aliquot
of working cell suspension were placed into 96-well
microtitre plate containing RPMI 1640 medium. Again
different concentration of uconazole and curcumin,
alone as well as in combination were placed vertically
and horizontally into the plates. Potentiality of com-
bination was measured after proper incubation for 48
hours. The fractional inhibitory concentration index
(FICI) value was calculated using the following equation
(Gomes et al., Hemaiswarya et al., 2008).
FICI = FIC of curcumin + FIC of Fluconazole
Where,
FIC of curcumin = MIC of curcumin in combination with
FLC/ MIC of curcumin alone,
FIC of uconazole = MIC of uconazole in combination
with CUR / MIC of uconazole alone.
FICI values= 0.5, represent synergistic interactions, 4.0
antagonistic effect and values in between these two rep-
resent no interaction
PREPARATION OF MUCOADHESIVE GEL
Mucoadhesive gels were prepared using different gel
forming polymers namely Carbopal P943 Carbopol 940,
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PHYTOCONSTITUENT BASED MUCOADHESIVE ANTIFUNGAL VAGINAL FORMULATION 695
Amit Roy et al.
Hydroxy-propyl-methyl cellulose either individual or
in combination. Accurately weighted required quanti-
ties of polymers as well as selected antifungal combina-
tion were transferred to beaker containing desire quan-
tity of hydro-alcoholic solvent system. Whole content
were stirred for 5-10 min by means of magnetic stirrer
and allowed to hydrate for 12 hours. After that a few
drops of triethanolamine as neutralizing agent, glyc-
erin as a moistening agent along with propylene glycol
were added to the hydrated mass and mixed slowly with
continuous gentle stirring by means of magnetic star-
rier until the homogenous gel were formed (Basha et al.,
2011; Doaa et al., 2012 and Choudhury et al 2016).
EVALUATION OF PREPARED
MUCOADHESIVE GEL
Visual and Organoleptic Examination
The prepared gel formulations were visually inspected
for their color and appearance. (Choudhury et al., 2016)
It was found that gel formulations were slightly yellow-
ish in color, free from any gritty particles and seems to
be homogeneous
Compatibility Study
In this study physical mixture of individual drugs and
all incorporated polymers in single as well as in combi-
nation were analyzed by means of FTIR study (Mekkawy
et al2013). The major peaks found in physical mixture
of drug with polymer are compared with the peak of
individual APIs.
Spreadability Test
The test was performed as per (Doaa et al., 2012) using
parallel plate method to determine the spreadability.
The prepared formulations were placed in between a set
of 20×20 cm glass slides & around 125 g weights were
placed upon the upper slide to spread the applied gel
uniformly. Then the weight was removed and the excess
of gel adhering to the slide was scrapped off. The set of
slides were xed in such a way that only upper slide
may slip off freely due to the weight tied with it. The
time taken for the upper slide to separate from the lower
slide was noted. The experiment was carried out three
times and the average of three reading was recorded.
Following formula was used for calculation-
S = M.L/T [Where, M = weight tied to upper slide; L =
Length of glass slide; T = Time taken to separate the slide]
Percentage Yield:
In this study weight of empty container as well as of
gel formulation along with container was measured
respectively. Then difference between the weight empty
container and weight of container with gel formulation
were measured, that considered as practical yield where
as the total weight of each ingredient used in each for-
mulation was considered as theoretical weight (Nayak
et al., 2010) The percentage yield was calculated using
the formula as below-
Percentage yield
Percentage yield
Percentage yield
Drug Content Determination
Around 10 gm of prepared gels were transferred into a
100ml volumetric ask containing 50ml of phosphate
buffer pH 4.5., under continuous agitation for 5hr by
means of mechanical rotary shaker. Further the mix-
ture was kept aside for 24hrs in order to get complete
release of drug from gel base. After that the content was
ltered using Millipore lter (0.45μm) and absorbance
was measured After suitable dilution using UV- visible
spectrophotometer (UV – 1700, Shimadzu, Japan) at
max
260 nm and 422 nm respectively using buffer (pH 4.5) as
blank (Choudhury et al., 2010; Choudhury et al. 2016) .
Determination of pH
The pH of gels was determined using a digital Electronic
pH meter. Initially the pH meter was calibrated using
standard buffers of pH 4, 7 and 9. Accurately 5 gm of gel
was weighed and dispersed in 50 ml of double distilled
water. The electrode of pH meter was dipped in disper-
sion and the numerical value displayed in pH meter was
noted (Bachhav et al., 2009; Nayak et al., 2010).
Viscosity and Rheological Studies
The viscosity of gels was determined with the help of
Brook eld viscometer (
Enyyoyt et al., 2014) Formu-
lations were placed in the sample holder and suitable
spindle attached perpendicularly inside the sample. The
spindle was attached to viscometer and allowed to rotate
at a constant speed. The reading displayed on viscometer
was measured.
Determination of mucoadhesion capacity
Pig vaginal mucosa was used as a model for mucoadhe-
sion study. Samples from several newly Sacri ced ani-
mals were obtained from a local slaughterhouse. Vaginal
mucosa was carefully separated from underlying tissues,
washed with normal saline and cut in smaller pieces of
adequate size. After that a single part of mucosal tis-
sue was attached perfectly to the back side of owing
balance such a way that it remain tightly attached till
the completion of study. To complete the study a glass
slide was taken and required amount of formulated gels
were spread over it in such a manner that may cover
696 PHYTOCONSTITUENT BASED MUCOADHESIVE ANTIFUNGAL VAGINAL FORMULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Amit Roy et al.
the whole area of mucosal tissue when come in con-
tact together. The slide and the tissue attached in the
pan were xed for 1min. On the other hand of the pan
a weight of 5gm was applied and determined the time
taken by the tissue to detach from the glass slide were
measured (Enyyoyt et al. 2014; Andrade et al. 2014;
Neves et al. 2016).
In-Vitro Drug Release Study
The apparatus consists of a glass cylinder with both the
ends open, 10 cm in height, 3.8 cm in outer diameter and
3.2 cm in inner diameter was used as a permeation cell.
A cellophane membrane previously soaked in distilled
water for 24 hours was xed to the one end of the cylin-
der. 10 mg of gel was taken in the cell (donor compart-
ment) and the cell was immersed in a beaker containing
100 ml of buffer of pH 4.6 (receptor compartment). The
whole assembly was xed in such a way that the lower
end of the cell containing gel was just touched (1-2 mm
deep) to the diffusion medium, the medium in the com-
partment was agitated using a magnetic stirrer at the
temperature 37±1ºC (Choudhury et al., 2016; Andrade
et al. 2014). Sink condition were maintain throughout
the experiment and after suitable dilution; the sample
was analyzed by using Shimadzu UV visible spectropho-
tometer at 260nm and 422 nm respectively.
Vaginal irritation test
The primary vaginal irritation test was performed
on New Zealand white female rabbit (1.5-2.5kg).
All the animals were kept under standard labora-
tory condition. The total numbers of animals were
divided into four batches, each batch containing
three animals. 1ml of prepared gel was inserted
daily, for 10 days, through a lubricated catheter into
the vagina of rabbits (
Mehta et al. 2012; Rabindranath
et al., 2001). The external genitalia are observed
regularly for any signs of oedema, erythema or
discharge as a reaction to the exposure to the test
materials. The experimental protocol of the study
was approved by the Institutional Animal Ethics
Committee (Regd. No. CIP / IAEC / 2013-14/044).
RESULTS AND DISCUSSION
In-Vitro antifungal effects of pure uconazole and
curcumin alone as well as in combination were tested
against Candida albicans. The MIC value of uconazole
and currcumin alone was found 48ug/ml and 128ug/
ml where as a remarkable fungal growth inhibition was
observer when used in combination of both the APIs. To
explore the nding, further the study was extended to
determine the mechanism involved behind such effect.
The study of fractional inhibitory concentration index
shows that, when the curcumin and uconazole added
in suitable concentration results synergistic action as
mentioned in (table no-02), which helps improve the
potentiality of the combination and ef cacy of ucona-
zole against pathogenic fungi.
The performance and safety issues related to
prepared mucoadhesive vaginal gels were inves-
tigated on nine formulations based on different
in-vitro and in-vivo parameters evaluation. As
per visualization evaluation it was found that all
the prepared mucoadhesive gel formulations were
transparent, smooth, free from any grittiness and
Table 1: Formulation design of Mucoadhesive vaginal gels
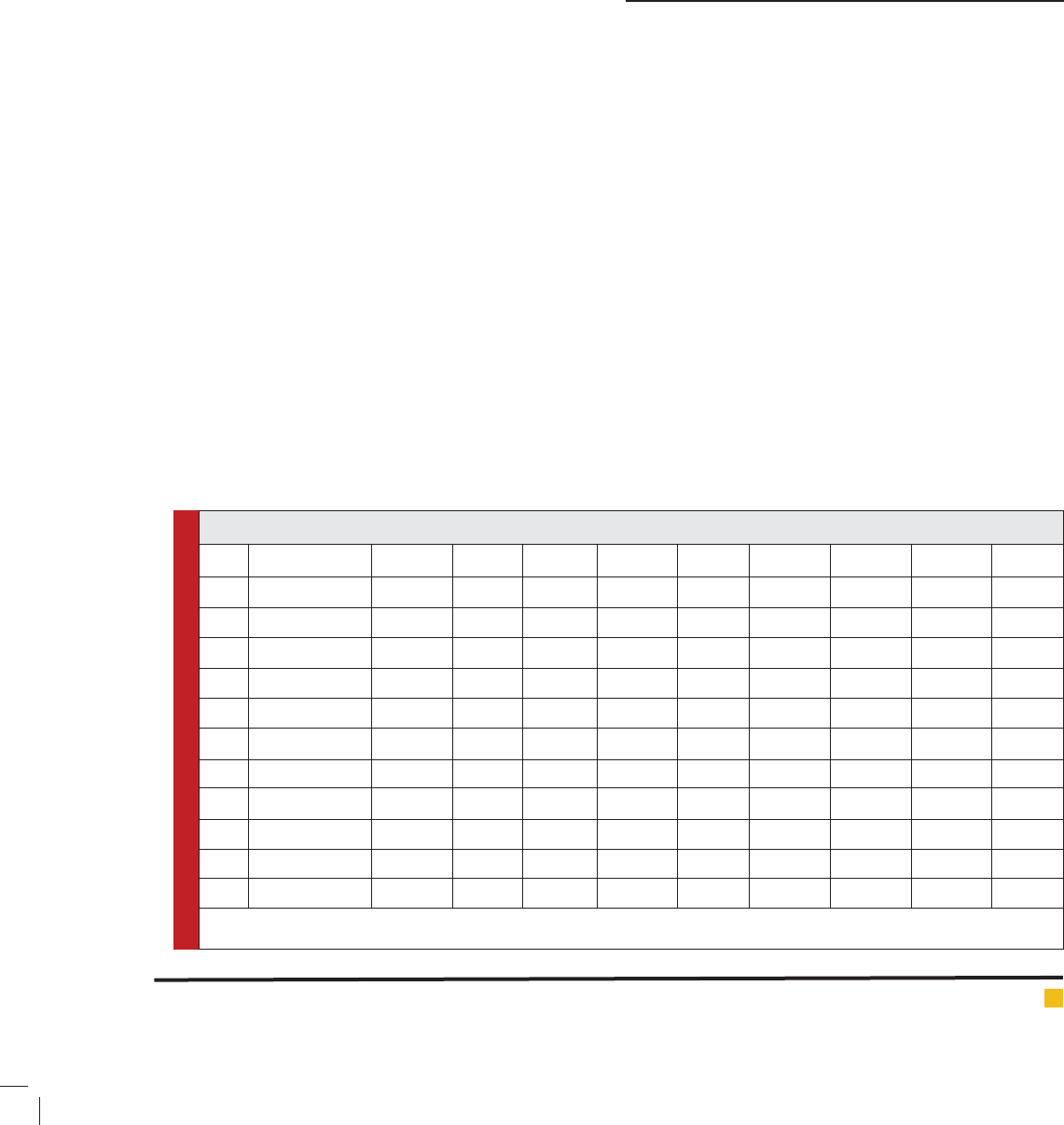
S. N Materials F1 F2 F3 F4 F5 F6 F7 F8 F9
1 Carbopol 934 1 % - 0.5 % 1 % 1.5 % - - -
2 Carbapol 940 - - 1% - - - 0.5% 1% 1.5%
3 HPMC - 1% - 1.5 % 1 % 0.5 % 1.5% 1% 0.5%
4 Water 90ml 90ml 90ml 90ml 90ml 90ml 90ml 90ml 90ml
5 Fluconazole 0.125% 0.125% 0.125% 0.125% 0.125% 0.125% 0.125% 0.125% 0.125%
6 Curcumin 0.62% 0.62% 0.62% 0.62% 0.62% 0.62% 0.62% 0.62% 0.62%
7 Ethanol 5 ml 5 ml 5 ml 5 ml 5 ml 5 ml 5 ml 5 ml 5 ml
8 Propyl paraben 0.08 % 0.08% 0.08 % 0.08 % 0.08 % 0.08 % 0.08% 0.08 % 0.08 %
9 Methyl paraben 0.02 % 0.02 % 0.02 % 0.02 % 0.02 % 0.02 % 0.02 % 0.02 % 0.02 %
10 Glycerin 5 ml 5 ml 5 ml 5 ml 5 ml 5 ml 5 ml 5 ml 5 ml
11 Triethanalamine 0.18ml 0.18ml 0.18ml 0.18ml 0.18ml 0.18ml 0.18ml 0.18ml 0.18ml
*Above table shows the composition of different formulations along with the amount individual component in percentage basis. Where F1,F2, F3, F4, F5,
F6, F7, F8 & F9 are considered as formulation codes, which represent individual combinations.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PHYTOCONSTITUENT BASED MUCOADHESIVE ANTIFUNGAL VAGINAL FORMULATION 697
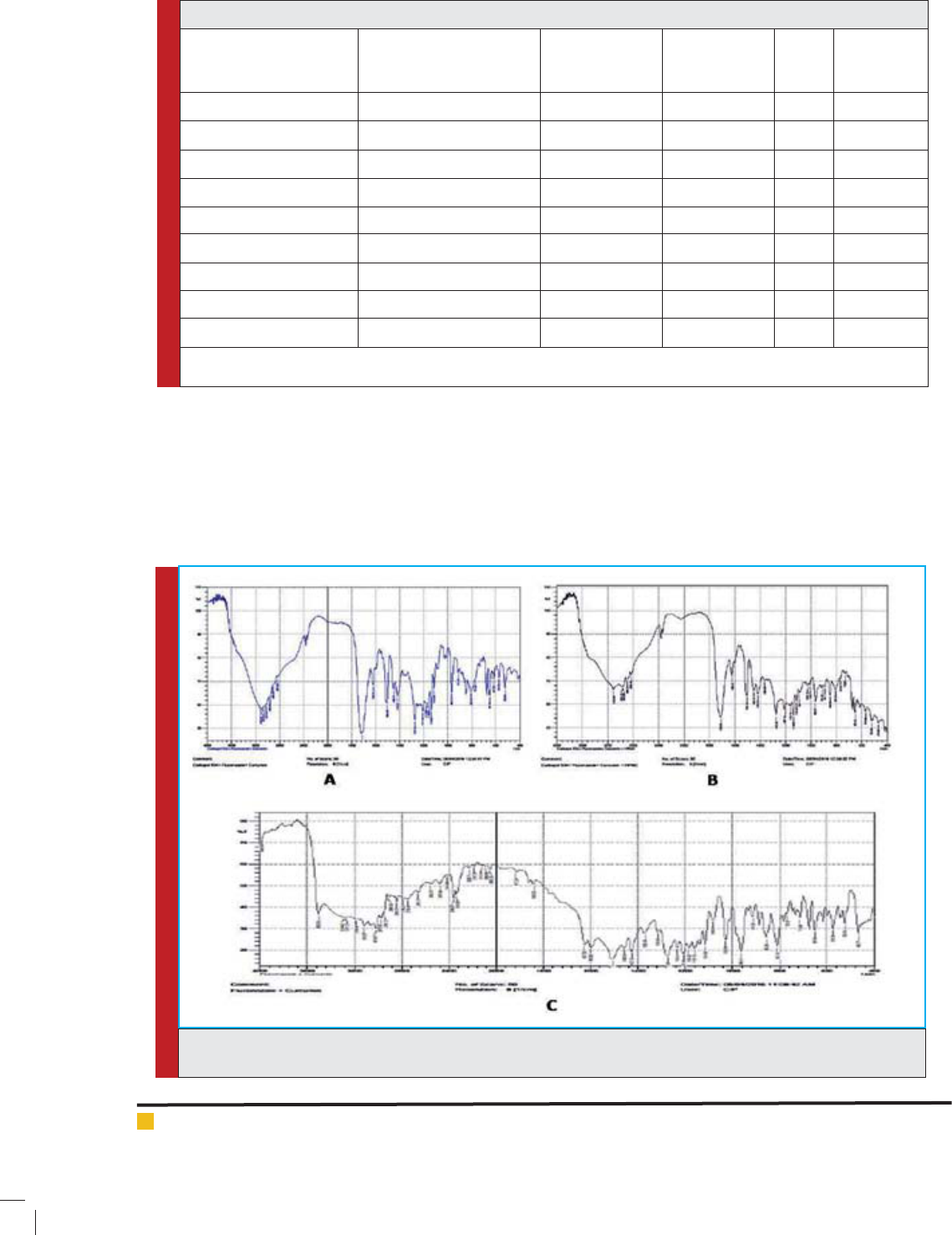
Amit Roy et al.
FIGURE 1. Graphical representation of FTIR study of pure drug and combination of drug and polymer represented
as A, B, C respectively.
Table 2: FICI of Fluconazole and Curcumin combination
Percentage of drug in
combination
Fluconazole + Curcumin
μg/ ml of drug in
combination
Fluconazole + Curcumin
FIC Fluconazole
FIC Curcumin
FICI
Interaction
75%MIC + 25% MIC 36 + 6.25 0.22 0.42 0.642 Antagonism
75%MIC + 12.5% MIC 36 + 3.12 0.20 0.39 0.591 Antagonism
75%MIC + 6.25%MIC 36 + 1.56 0.38 0.73 1.11 Antagonism
50%MIC + 50%MIC 24 + 12.5 0.31 0.60 0.91 Antagonism
50%MIC + 25% MIC 24 + 6.25 0.28 0.53 0.81 Antagonism
50%MIC + 12.5% MIC 24 + 3.12 0.26 0.51 0.77 Antagonism
25%MIC + 75%MIC 12 + 1.56 0.22 0.43 0.65 Antagonism
75%MIC + 50% MIC 12 + 6.25 012 0.24 0.364 Synergistic
25%MIC + 25% MIC 12 + 3.12 0.15 0.30 0.452 Synergistic
*Screening of FICI value based on antifungal activity study, using different ratios of curcumin and uconazole. Concentration
used for the development of ratios was as per the individual MIC value of both the component.
homogeneous in nature. The gel formulations were
slightly yellowish in color with satisfactory yield
value. Compatibility study was performed on phys-
ical mixture of APIs and polymer, which re ects
no major shift or changes in peak value as well
as their location (Fig-1.), hence indicate no inter-
action. All the prepared formulations re ect good
spreadability, which indicate ease of application
of formulations in the vaginal cavity. The pH of
the prepared formulations were ranges within (3.5-
5.3), which complies with pH of vaginal cavities,
hence, consider suitable for vaginal application.
698 PHYTOCONSTITUENT BASED MUCOADHESIVE ANTIFUNGAL VAGINAL FORMULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Amit Roy et al.
Table 3: Result of physicochemical evaluation study of prepared gels.
Formulation
code
Viscosity
(Cp) pH
Spreadibility
(g.cm/sec)
Muco-adhesion
(Dyne/cm2)
Percentage
yield%
Drug
content
F1 1300 ± 1.02 3.6 ± 0.037 0.166 ± 0.0012 12.4 ± 0.0387 92.59 ± 0.88 83 ± 0.645
F2 3180 ± 0.90 4.05 ± 0.014 0.375 ± 0.0027 11.49 ± 0.025 91.82 ± 0.03 86 ± 0.810
F3 1013 ± 1.18 4.27 ± 0.021 0.433 ± 0.0017 14.4 ± 0.2081 93.59 ± 0.093 81 ± 0.391
F4 27400 ± 1.54 4.2 ± 0.029 0.576 ± 0.0018 19.06 ± 0.0095 93.25 ± 0.051 85 ±1.290
F5 28350 ± 0.65 4.3 ± 0.057 0.30 ± 0.170 28.52 ± 0.029 96.00 ± 1.29 83 ±1.290
F6 17800 ± 1.17 5.3 ± 0.180 0.26 ± 0.0250 17.92 ± 0.0216 98.53 ± 0.012 90 ±1.290
F7 6400 ±1.35 4.2 ± 0.29 0.40 ± 0.182 18.24 ± 0.017 89.52 ± 0.015 87 ±1.290
F8 52300 ±1.20 4.3 ± 0.22 0.4 ± 0.182 26.34 ± 0.066 98.51 ± 0.029 85 ±1.290
F9 18300 ±1.38 3.91 ± 0.032 0.2 ± 0.129 15.62 ± 0.029 83.15 ± 0.031 94 ± 0.890
*The above table contains result of essential evaluation parameters. All the data are represented in the format of (Mean ± Standard
deviation).
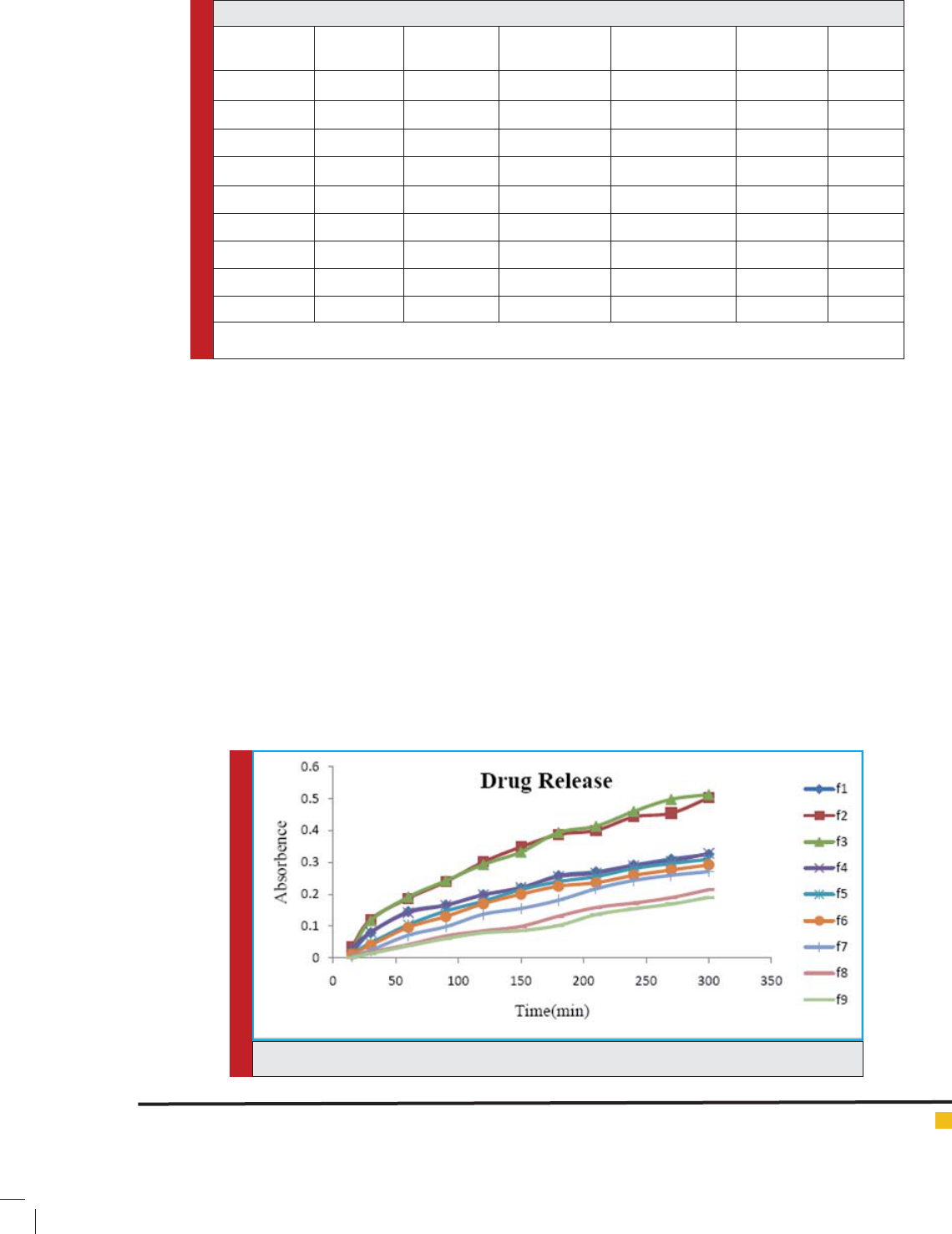
FIGURE 2. Graphical representation of drug release pro le of all the prepared formulations.
Viscosity is considered as an important parameter
for semisolid dosage form intended for vaginal
delivery, since high viscous formulations will bet-
ter adhere to the mucous wall hence, better will
be the retention time. In this contest the viscosity
of prepared formulations was found in the range
of (1013-52300 Cp). The mark difference in the
observed viscosity may be due to the difference in
concentration used, again it has been observed that
formulation fabricated with single polymer shown
less viscosity than the combination of polymer.
Among all, F5 and F8 formulation showed higher
viscosity 28350cp, 52300cp respectively. The result
of mucoadhesion study re ects that formulations
F4, F5 & F8 shows higher mucoadhesion capac-
ity as compare to others; in this connection this is
to mention that good mucoadhesion property shall
improve the residence time of inside vaginal cavity.
It has been also notice that results mucoadhesion
capacity directly related with viscosity and almost
inversely related with spreadib ility parameter of
investigated formulations. Drug content study
indicates that all the prepared formulation contains
around 90-96% of drugs, which consider as a sign
of good formulation. Results of the all the essential
evaluation parameters are shown in (Table no-03)
On the basis of analysis of In-Vitro release data
it was observed that almost all the formulation
were showing 80-90% of drug release within 6-7
hrs.
Clinical signs of irritation include the development
of a rash, in ammation, swelling, scaling, and abnormal
tissue growth in the affected area was not found after
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PHYTOCONSTITUENT BASED MUCOADHESIVE ANTIFUNGAL VAGINAL FORMULATION 699
Amit Roy et al.
RVI test, that indicate the formulations were safe &free
of any kind of irritation, hence, considered not produce
any kind of discomfort to the patients during therapy as
well as may improve patients compliance .
CONCLUSION
The investigation reveals that, incorporation of cur-
cumin leads to increases the antifungal effect of ucon-
azole, which may be due to the mechanism synergism.
Again the evaluation results of prepared mucoadhesive
antifungal gels found to ful ll all the required criteria to
be a suitable vaginal formulation. All the prepared for-
mulations were found satisfy in respect of , formulation
F5 and F8 shown better performance in respect of their
mucoadhesion capacity, property of spreadibility and
drug release study, that may facilitate the vaginal appli-
cation and demand to increase poor patient compliance.
The in vivo animal studies indicate no sign of irritation.
ACKNOWLEDGEMENTS
Author would like to acknowledge IPCA laboratories
ltd. India for providing Fluconazole as drug sample and
Columbia Institute of Pharmacy as research centre for
providing the research facilities.
REFERENCES
Andrade O A, Parente E M, Ares G. Screening of mucoadhe-
sive vaginal gel formulations.
Braz J Pharm Sci 2014; 50(4):
931-942.
Annette W.F., Deanna A.S., McCarthy DI., Wiederhold
N.P.(2014) Impact of New Antifungal Break points on Antifun-
gal Resistance in Candida Species. Journal of Clinical Microbi-
ology. Vol.52 No. 3. Pages 994–997
Bachhav.Y.G., Patravale. V B. (2009) Microemulsion based
vaginal gel of uconazole: formulation, in vitro and in vivo
evaluation. International Journal of Pharmaceutics. Vol. 365.
No.5. Pages 175–179.
Basha B.N., Kalyani P., Divakar G. (2011) Formulation and
evaluation of gel containing uconazole-antifungal agent.
International journal of Drug Delivery Research. Vol.3. No.4.
Pages 109-128
Choudhury A, Das S, Kar M.(2011) A review on novelty and
potentiality of vaginal drug delivery. Int J Pharm Tech Res.
Vol. 3. pages 1033-1044.
Choudhury A., Roy A., Saha S., Bahadur S. (2016) Preparation
and evaluation of phytocontituent based mucoadhesive anti-
fungal vaginal gel, Research Journal of Pharmacognosy and
Phytochemistry. Vol 8. No.3. Pages 116-120
Choudhury. A., Verma. R., Sinha D., Sahu S., Roy. A. (2014)
Development and characterization of topical phyto-formula-
tion for antifungal activity. Vol. 1. No. 2. Pages 18-21
Doaa A.H., Dalia A.E., Sally A.H., Mohamed A.E. (
2012) For-
mulation and evaluation of uconazole topical gel. Interna-
tional journal pharmaceutical sciences.
Vol. 4. No.5, 176-183
Enyyoyt A Z, Karavana Y S, Erac B, Gursel O, Lymoncu M H
G, Baloðlu E. Evaluation of chitosan based vaginal bioadhe-
sive gel formulations for antifungal drugs. Acta Pharmaceutica
2014; 64 (2): 139–156.
Gomes S. G., Curvelo J. A. R., Soares R. M. A., Pereira A.F.(2012)
Curcumin acts synergistically with uconazole to sensitize a
clinical isolate of Candida albicans showing a MDR phenotype.
Medical Mycology. Vol.50. Pages 26–32,
Grossman N.T., Pham C.D., Cleveland A.A., Lockhart S.R.
(2015) Molecular mechanisms of uconazole resistance in
Candida parapsilosis isolates from a U.S. surveillance system.
Antimicrobial Agents and Chemotherapy. Vol.59. No. 2. Pages
1030-1037.
Hemaiswarya S, Kruthiventi A.K., Doble M. (2008) Synergism
between natural products and antibiotics against infectious
diseases. Phytomedicine. Vol. 15. Pages 639–652
Jana C., Julius S. (2006) Resistance mechanisms in ucona-
zole-resistant Candida albicans isolates from vaginal candidi-
asis. International Journal of Antimicrobial Agents. Vol. 27
Pages 403–408.
Kuleta J.K, Kozik R.M, Kozik A.(2009) Fungi pathogenic to
humans: molecular bases of virulence of Candida albicans,
Cryptococcus neoformans and Aspergillus fumigatus. Acta Bio-
chim Polon. vol.55 pages 211 – 224.
Martins C. V. B., DaSilva D. L., Neres A. T. M., Magalha T. F.
F., Watanabe G. A., Modolo L.V., Sabino A.A., Fatima A.D, and
Resende M.A. (2009) Curcumin as a promising antifungal of
clinical interest. Journal of Antimicrobial Chemotherapy. Vol.
63. Pages 337–339
Mehta S, Verstraelen H, Peremens K, Villeirs G, Vermeire S.
Vaginal distribution and retention of a multiparticulate drug
delivery system assessed by gamma scintigraphy and magnetic
resonance imaging. Int. J. Pharm 2012; 426: 44-53.
Mekkawy. A., Fathy M., Sohair E. S. (2013) Formulation and
In vitro evaluation of uconazole topical gels. Br J Pharm Res.
Vol.3 No. 3. Pages 293-313.
Mukherjee P.K., Sheehan D.J., Hitchcock C.A., Ghannoum
M.A.(2005) Combination treatment of invasive fungal infec-
tions. Clinical Microbiology Reviews. Vol.18 No.1. Pages163–
194
Nayak. B.S., Rout P.K., Nayak. U. K., Bhowmik. B. B.(2010)
Development and characterization of bioadhesive gel of micro-
encapsulated metronidazole for vaginal use. Iranian Journal of
Pharmaceutical Research. Vol. 9. No.3. Pages 209-219.
Neves J D, Bahia M F. Gels as vaginal drug delivery systems.
Int. J. Pharm, 2006; 318: 1–14.
Odds F. C. (2003) Synergy, antagonism, and what the chequer
board puts between them, Journal of Antimicrobial Chemo-
therapy Vol. 52. No.1. Pages 203
Oelkrug. C., Lange C.M., Wenzel E., Fricke S., Hartke M.(2014)
Analysis of the tumoricidal and anti-cachectic poten-
700 PHYTOCONSTITUENT BASED MUCOADHESIVE ANTIFUNGAL VAGINAL FORMULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Amit Roy et al.
tial of curcumin. Anticancer Research.Vol.34, pages 4781–
4788.
Rabindranath P, Chakraborty M, Rabindra D, Gupta BK. In-
vitro In-vivo correlation (IVIVC) study of le unomide loaded
microspheres. Int. J Pharm Sci 2009; 1: 165-170.
Roy A, Choudhury A, Nayak T.K. (2014) Importance and Utility
of Vagina as a Route for Drug Delivery System. Asian J. Res.
Pharm. Sci. Vol. 4: no. 2. Pages 86-92.
Sharma M, Manoharlal R, Shukla S, Puri N, Prasad T, Ambud-
kar S V., Prasad R. (2009) Curcumin modulates ef ux mediated
by yeast ABC multidrug transporters and is synergistic with
antifungals, Antimicrobial Agents And Chemotherapy. Vol. 53,
No. 8. Pages 3256–3265
Sharma M, Manoharlal Raman, Negi S.A., Prasad R. (2010)
Synergistic anticandidal activity of pure polyphenol curcumin-
I in combination with azoles and polyenes generates reactive
oxygen species leading to apoptosis. FEMS Yeast Research.
Vol.10. pages 570–578
Shyh M.T., Mei C. Y.(2000) Enhanced inhibitory effect from
interaction of curcumin with amphotericin B or uconazole
against candida species. Journal of Food and Drug Analysis.
Vol. 8. Pages 208-212.
Tsao S.M., Yin M.C., (2000) Enhanced Inhibitory Effect from
Interaction of Curcumin with Amphotericin B or Fluconazole
against Candida Species. Journal of Food and Drug Analysis.
Vol. 8 No. 3. Pages 208-212
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS PHYTOCONSTITUENT BASED MUCOADHESIVE ANTIFUNGAL VAGINAL FORMULATION 701