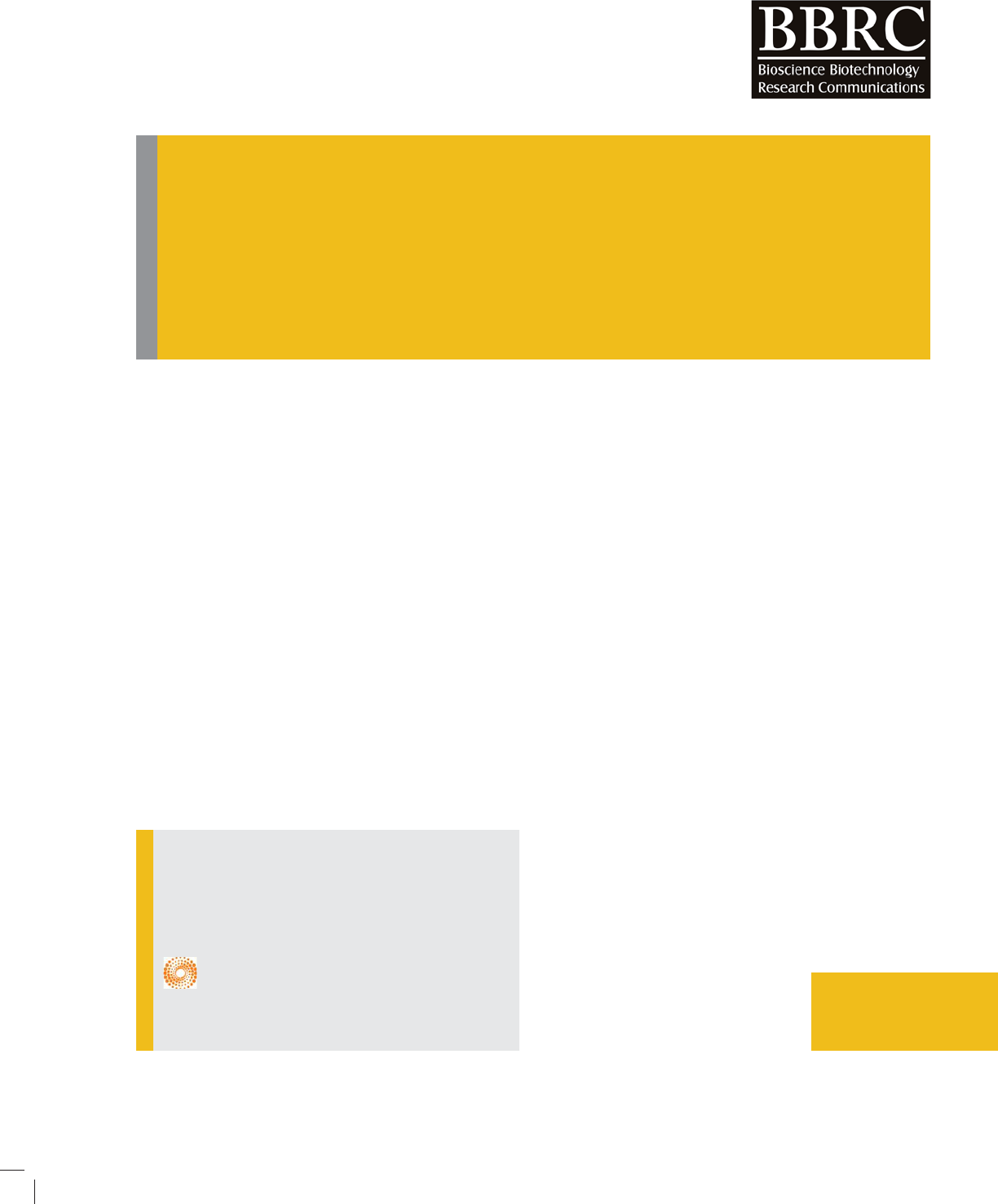
Medical
Communication
Biosci. Biotech. Res. Comm. 9(4): 653-665 (2016)
Af ictions of enteric diseases in human population
with reference to diarrhoea – A Review
Pooja Rawat,
1#
Riddhi Doshi,
2#
Pawan Kumar Singh,
1
* and Vipin Kumar
1
1
Value Addition Research and Development-Human Health, National Innovation Foundation-India, Satellite
Complex, Jodhpur Tekra, Premchand Nagar Road, Satellite, Ahmedabad -380015, Gujarat, India
2
Society for Research and Initiatives for Sustainable Technologies and Institutions (SRISTI), Near Gujarat
University, Navrangpura, Ahmedabad – 38009, Gujarat, India
ABSTRACT
Diarrhoea is a worldwide profound problem for the people of all age groups including new born, young children, adults
as well as old age people. About 88% of diarrhoea associated mortality is mainly attributed to poor sanitization and lack
of awareness. Despite the global decline in diarrhoea associated mortality by 50% between 2000 and 2013, the disease
still carries a high burden of morbidity. Commonly used treatment regimen includes the measures for preventing dehy-
dration and use of antibiotics.Exclusive breastfeeding, vitamin A and Zn supplementation have been recommended as
preventive strategies. Recently, two live oral rotavirus vaccines have been licensed in more than 100 countries, including
India, which are very effective in lowering the incidence of diarrhoea and frequency of death. Treatment options for
diarrhoea are ORT such as glucose-based ORS, intravenous infusions, normal saline, Zn supplementation, antibiotics,
and anti-motility drugs such as loperamide hydrochloride.Ef cacy of the traditional phyto-medicines is evident from
long history of use of several plants to treat diarrhoea.Approximately 80% of world population relies on traditional
medicines using plant extracts or their active constituents.Pre-clinical evaluations of anti-diarrhoeal activity of several
medicinal plants have been extensively carried out which supports their traditional uses. However, lack of clinical data
is a major limiting factor towards development of phyto-drugs against diarrhoea. Phytochemicals identi cation from
these plants and their clinical studies are an excellent area to explore towards development of safe phyto-pharmaceuti-
cals for management of diarrhoea and associated enteric disorders. Probiotics are also one of the safe alternative options
which need to be given attention in future researches.Thorough review of different research databases was carried out
and compiled to depict overview of the disease, its pathophysiology, intervention strategies, drawbacks of current treat-
ment methods and safe alternate treatment options available against diarrhoea.
KEY WORDS: ENTERIC DISORDERS, DIARRHOEA, TRADITIONAL MEDICINE, PATHOGENS
653
ARTICLE INFORMATION:
*Corresponding Author: pawan@ni ndia.org
Received 20
th
Sep, 2016
Accepted after revision 12
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
654 AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Pooja Rawat et al.
FIGURE 1. Percentage of deaths among children under age 5 attributable to
diarrhoea, 2013 (Source: UNICEF analysis based on WHO-CHERG estimates for
child causes of death 2000–2013).
INTRODUCTION
Despite substantial progress in the understanding of
pathogenesis and management, diarrhoeal diseases are
the second leading cause of death (after pneumonia) in
infants and young children, and are responsible for around
18% of all deaths, more than 5000 every day. The World
Health Organization (WHO) and UNICEF have estimated
that worldwide about two billion cases of diarrhoea occur
annually, mostly in developing countries (Fig 1). Diar-
rhoea has created a massive economic burden on health
services, and accounts for more than 578,000 deaths per
year in paediatric patients younger than 5 years in low
and middle income countries (Leung et al., 2016).
During last 15 years, various preventive and cura-
tive solutions were developed and systematically imple-
mented worldwide to combat diarrhoeal diseases. From
2000 to 2013, signi cant reduction of more than 50%
(from 1.2 million to 0.6 million approximately) in total
annual number of deaths due to diarrhoea was observed.
Oral rehydration and zinc supplementation are the key
therapies which have been recommended by WHO and
UNICEF for treating diarrhoea. Despite the cost effec-
tiveness, affordability and easy implementation, oral
rehydration therapy (ORT) covers only about 40 per cent
of the children under 5 years of age. From 2009 - 2013,
in sub-Saharan Africa and South Asian countries with
most death of children attributed to diarrhoea, the per-
centage of children receiving ORT encompasses 36% and
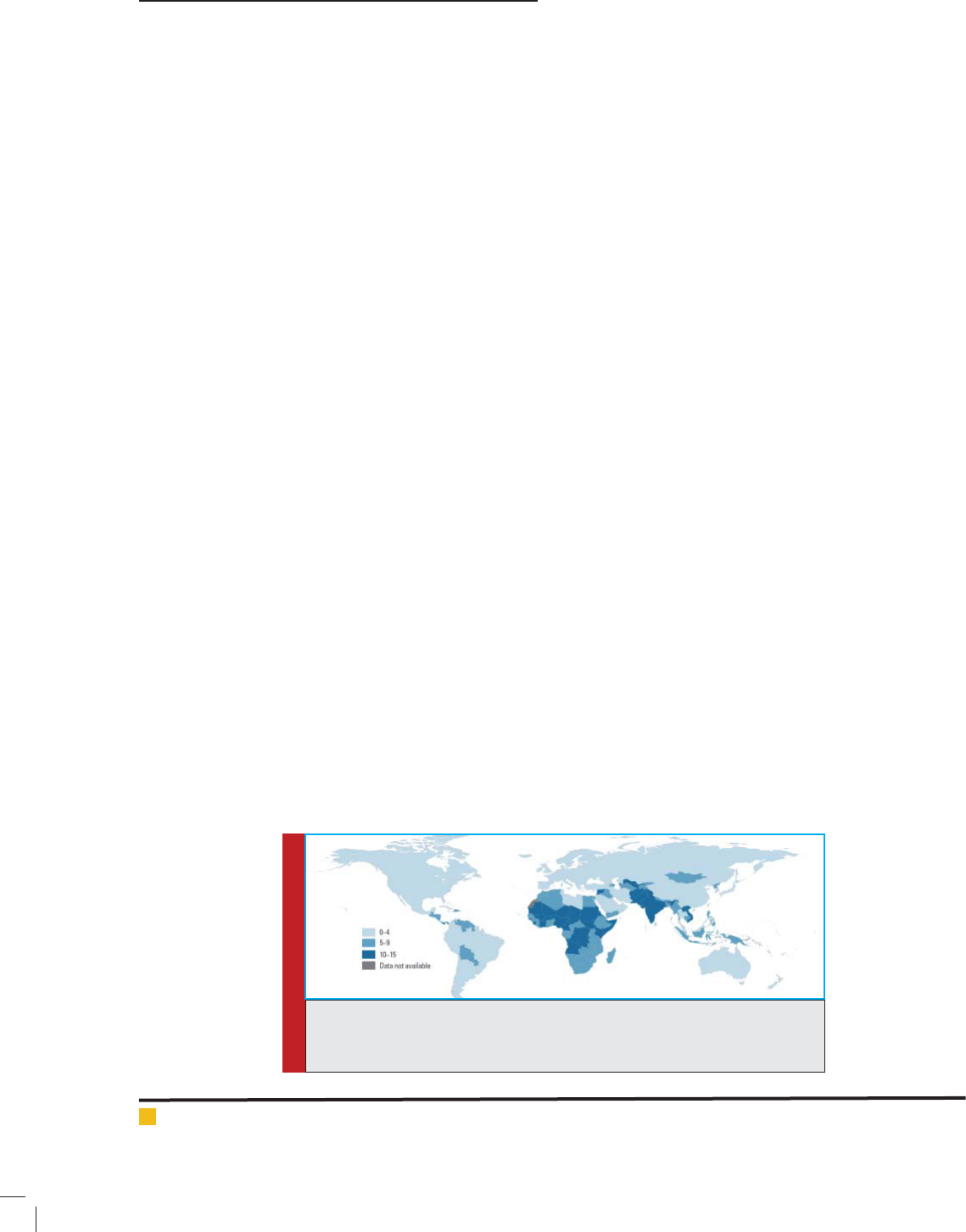
38% respectively. Surveys and studies conducted during
2009-2013 shows that the countries with highest num-
ber of child deaths due to diarrhoea are having lowest
number of coverage by ORT (Fig 2).
It is evident from the literature that about 78% of
child deaths due to diarrhoea occur in the African and
South-East Asian countries. Among 15 highest burden
countries, India is grouped in top three countries with
the maximum child deaths due to pneumonia and diar-
rhoea. Compared to developing countries, lesser number
of deaths are recorded in the less developed countries.
In African, South-East Asian and least developed coun-
tries, generally children from high income family groups
are more likely to receive ORT than the children from
low income families. Children in low and middle-income
countries have high risk of getting frequent diarrhoea
epidemics (Julian, 2016).
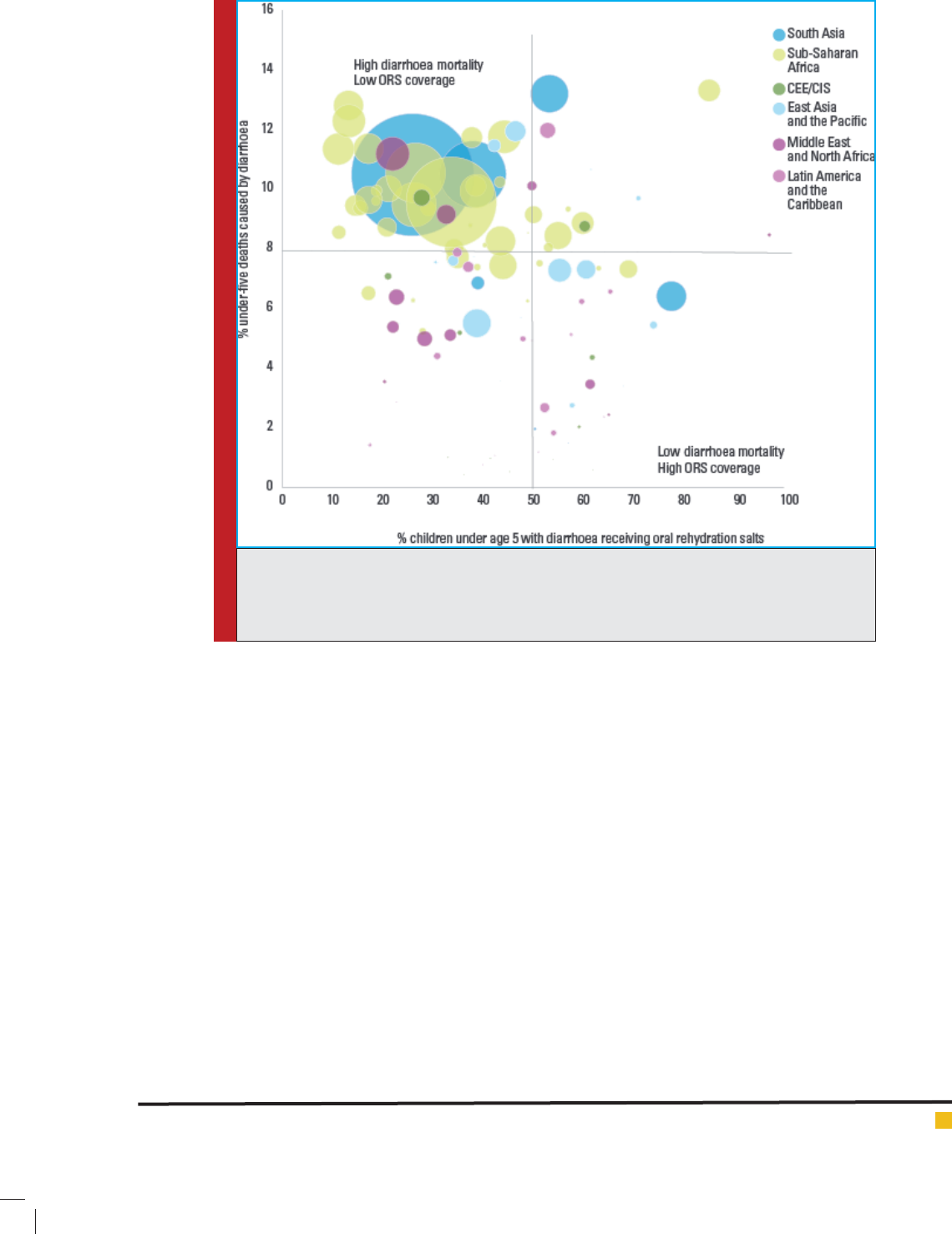
In 2013, the Global Action Plan for the Prevention
and Control of Pneumonia and Diarrhoea (GAPPD) was
developed and released by the WHO and UNICEF, with
an objective to eradicate the preventable pneumonia and
diarrhoeal mortality in the children by 2025. WHO and
UNICEF have adopted this cohesive approach because
of interdependency of many of the solutions desired
to combat diarrhoea and pneumonia. As per Pneumo-
nia and Diarrhoea progress report (2014), published by
International Vaccine Research Center (IVAC), India and
Nigeria are the two countries with highest burden of
child deaths. The slow implementation and poor acces-
sibility of children under ve to vaccination programs
are key reasons for continued child deaths. About 20%
of the Asian countries have introduced rotavirus vac-
cination programs, compared to 47% of the countries in
the Africa. Various factors suggested for delayed intro-
duction of rotavirus vaccine in these regions are poor
acceptance by end users, logistic challenges and sup-
ply issues. Diarrhoeal treatment rates are far below the
GAPPD target (90%) in all the 15 highest-burden coun-
tries in which coverage of Zn supplements are extremely
lower than the ORS. Inclusive GAPPD intervention
scores in the 15 countries having highest burden of child
mortality due to diarrhoea and pneumonia, ranges from
23 to 63 per cent which is far below the set target (Fig 3).
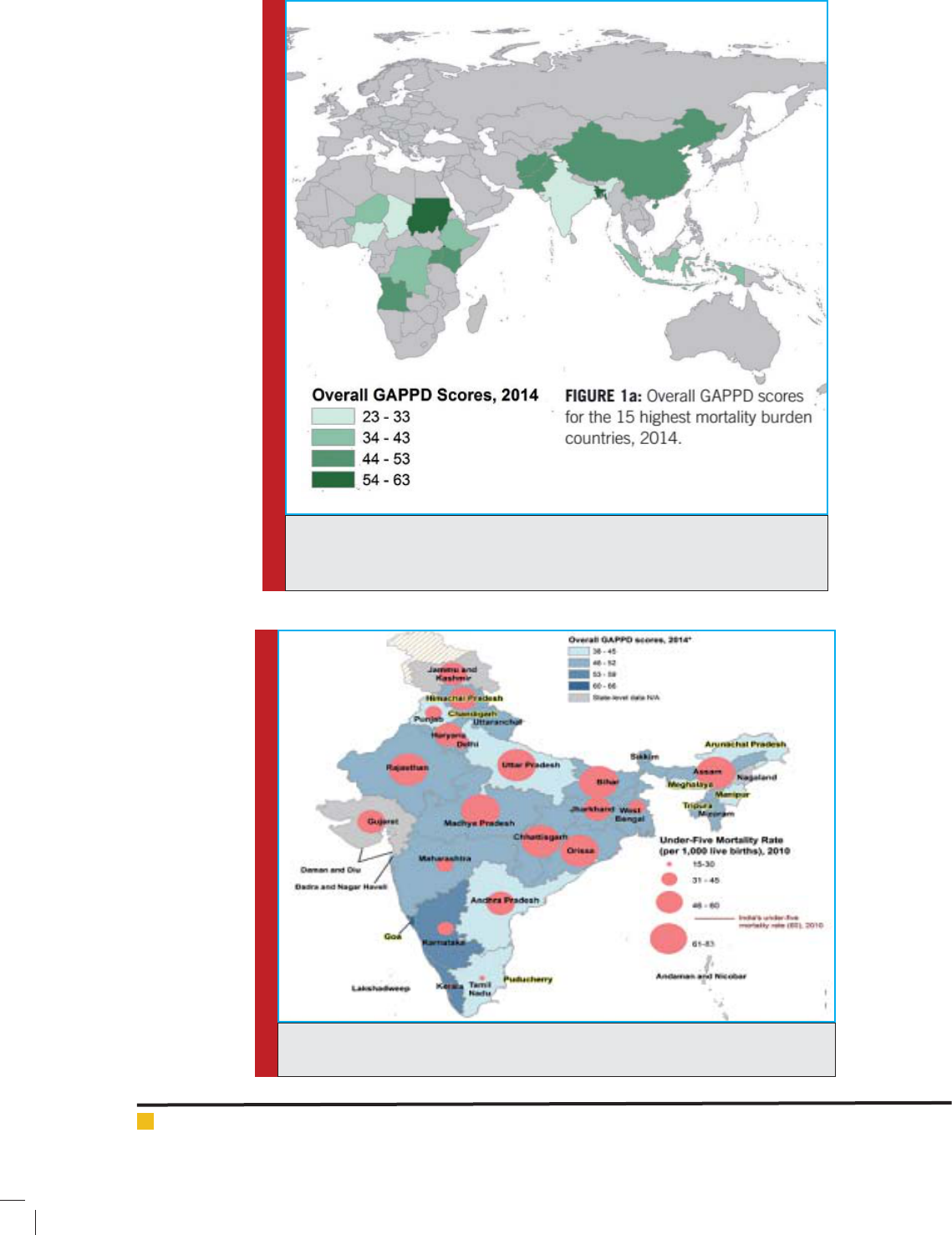
Zinc supplementation coverage in India has increased
substantially from 2005-06 to 2012-13. Goa was among
the top performing states in terms of zinc and ORS cov-
erage with GAPPD score of about 66%. According to
IVAC report, 2014, overall GAPPD score in India across
all the states ranged from 38 to 66% (Fig 4).
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION 655
Pooja Rawat et al.
FIGURE 2. Diagram showing correlation between percentage of children with diarrhoea receiving
Oral rehydration therapy and percentage mortality caused by diarrhoea for children under age
ve [Source: UNICEF global databases, 2014, based on DHS, MICS, and other national household
surveys, 2009-2013].
CLINICAL MANIFESTATIONS
Gastroenteritis is a generic medical term for various
pathological conditions of the gastrointestinal tract. The
primary indication of gastroenteritis is diarrhoea; which
may be generally accompanied by mild abdominal pain,
nausea and vomiting. Diarrhoea gets more complicated
due to occurrence of other gastrointestinal tract syn-
dromes such as emesis, abdominal pain and distension
(China, 2005). Diarrhoea, as de ned by WHO, is having
three or more stools per day, or having more stools than
is normal (Ahs et al., 2010). There are several causes for
diarrhoea which includes overeating or eating unhealthy
food, inadequate personal hygiene, food putrefaction in
intestine, nervous irritability, microbial fermentation
due to inadequate digestion of carbohydrate, intestinal
infection, overuse of antibiotics, drug reaction, food
intolerance and excess ingestion of purgatives. (Tarri-
cone et al., 2016, McQuade et al., 2016).
Rotavirus is the leading cause of severe diarrhoea in
children across the globe (Bahl et al., 2005, Kumar et al.,
2016). A wide variety of protozoans inhabits human intes-
tinal tract, but majority of them are non-pathogenic or
cause very mild diseased condition. Virulent strains includ-
ing Entamoeba histolytica, Giardia, Cryptosporidium par-
vum, Cyclospora species and microsporidia (Hashmey et al.,
1997) contributes to very less percentage (0-12%) of acute
traveler’s diarrhea. However, prevalence of such infection
is as high upto 30% in people with HIV or immune-com-
promised individuals (Ericsson et al., 2001). The microbial
species causing intestinal diseases include Shigella, Bacil-
lus, Vibrio, Salmonella, Listeria, Escherichia, Clostridium
(Hosokawa et al., 2016, DeMeo, 2016).
PATHOPHYSIOLOGY OF DIARRHOEA
Absorption and secretion of ions and solutes is the fun-
damental process taking place throughout the length
of intestine, starting from duodenum to distal part of
colon. Secretion process takes place through a cyclic
AMP-dependent chloride channel, also known as cystic
brosis trans-membrane conductance regulator (CFTR),
656 AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Pooja Rawat et al.
FIGURE 3. Overall GAPPD scores for the 15 countries with highest mortality, 2014
[Source: International Vaccine Research Center (IVAC) 2014- Pneumonia Diarrhoea
Progress Report].
FIGURE 4. GAPDD score relative to child Mortality in India, 2014 [Source: Interna-
tional Vaccine Research Center (IVAC) 2014- Pneumonia Diarrhoea Progress Report)].
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION 657
Pooja Rawat et al.
present in the apical or luminal membrane of crypt epi-
thelial cells. The channel regulates secretion of Cl
-
into
the lumen which leads to movement of Na
+
through
creation of electric potential. The osmotic gradient cre-
ated by net movement of NaCl results in the secretion
of water by crypt epithelial cells (Frizzell and Hanrahan,
2012, Camilleri et al., 2016).
Pathophysiology of enteric diseases involves several
mechanisms by which infectious agents interact with
intestinal mucosal cells. Infection with enterotoxin pro-
ducing bacteria can lead to diarrhoea through toxigenic
effects or in ammation. Cholera, an infection of small
intestine is caused by some strains of bacterium, Vibrio
cholera. The disease is characterized by severe diarrhoea
leading to dehydration and electrolyte imbalance. The
diarrhoea symptoms are due to the secretion of toxin,
known as cholera toxin (CT) by V. cholera. CT is an
84-kDa protein consisting of a dimeric A subunit and
ve identical B subunits. CT irreversibly activates adenyl
cyclase resulting in increased mucosal concentration of
cAMP. Increased cAMP results in the increased secretion
of Cl
-
into the lumen, leading to loss of water through
creation of osmotic pressure (Fig 5). The toxin also medi-
ates its effect through inhibition of NaCl absorption by
decreasing the activity of NHE and Cl
-
bicarbonate anti-
porter which leads to electrolyte imbalance. However, CT
mediated toxigenic mechanism does not have any effect
on glucose-stimulated Na
+
absorption, which is inhibited
in case of infection with Shigella spp. or Salmonella spp.
(Anand et al., 2016, Barrett, 2016).
Diarrhoeal diseases in case of enterotoxigenic strains of
E. coli is caused by two enterotoxins, a heat-labile toxin
(LT) and a heat-stable toxin (ST). Action of LT is simi-
lar to CT, which mediates its action through activation
of adenylate cyclase. However, ST activates guanylate
cyclase, resulting in increased mucosal cyclic GMP. cGMP
has similar effects on ion transport as cAMP leading to
water secretion and impaired absorption. Enterotoxin
released by rotavirus has been identi ed to be nonstruc-
tural protein (NSP4), which mediates its effect through
impairment of lactase enzymatic activity in brush border
of human enterocyte-like Caco-2 cells (Beau et al., 2007).
An intact intestinal mucosa was detected in the histologi-
cal analysis of proximal intestinal biopsy samples from
infected individuals. Mild in ammatory in ltration into
the lamina propria was also observed in infected indi-
viduals (Troeger et al., 2009).
TYPES OF DIARRHOEA
Osmotic diarrhoea
Osmotic diarrhoea occurs when too much water is drawn
into the lumen and happens after ingestion of large
amount of poorly absorbable osmotically active solutes
such as lactulose, sorbitol etc. The condition may also
result when a person with a particular absorption defect
ingests such nutrients. Examples include lactose intoler-
ance in lactase de cient individuals, mal-digestion in
case of pancreatic insuf ciency and hydrolysis of unab-
sorbed carbohydrates into short chain fatty acids, which
exceed beyond the absorptive capacity of the colon.
Secretory diarrhoea
Secretory diarrhoea results due to overstimulation of
intestinal tract’s secretory capacity or due to inhibition
of absorption. Bacterial toxins, luminal secretagogues
(such as bile acids or laxatives), reduced absorptive
surface area caused by disease or resection, circulat-
ing secretagogues (such as various hormones, drugs,
and poisons), and medical problems that compromise
regulation of intestinal function (Schiller, 1999) are the
key factors for secretory diarrhoea. The most common
example is cholera toxin that stimulates the secretion
of anions, especially Cl ions and subsequently results in
movement of Na along with water to maintain a charge
balance (Thiagarajah et al., 2015).
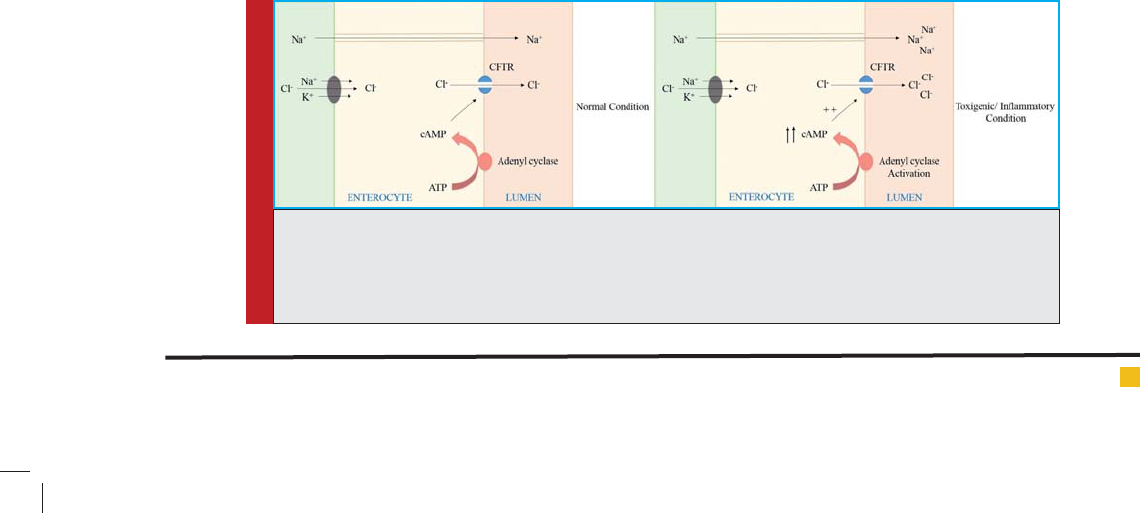
FIGURE 5. Secretion of Chloride ion in small intestine. Toxicity or in ammation caused by infection
results in activation of adenyl cyclase and increased cAMP production. Increased cAMP activates CFTR
to cause increased secretion of Cl- ion. Increased Cl- concentration in the lumen results in increased
passive transport of Na+. Loss of water follows as a result of osmotic effect of Na+ and Cl- ions.
Pooja Rawat et al.
658 AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
In ammatory and infectious diarrhoea
Gatroenteritis or infectious diarrhoea, is an in ammation
of the gastrointestinal tract that comprises the stomach
and small intestine. Epithelium destruction causes exu-
dation of serum and blood into the lumen and also asso-
ciated with the destruction of absorption function. In
such cases, absorption of water occurs very inef ciently
resulting in diarrhoea. Pathogens frequently associated
with infectious diarrhea include bacteria (Salmonella,
E. coli, Campylobacter), viruses (rotaviruses, coronavi-
ruses, parvoviruses, norovirus) and protozoa (Coccidia
sp., Cryptosporium, Giardia).
Diarrhoea associated with deranged motility
Disorders in motility may lead to poor absorption result-
ing in diarrhoea. Both increase and decrease in gut motil-
ity can lead to diarrhoea. Examples of the former are
dysthyroidism (Daher et al., 2009). Decreases in effective
motility in the small intestine due to large diverticula,
smooth muscle damage (scleroderma, dematomyositis,
amyloidosis, muscular dystrophy, or radiation injury), or
autonomic neuropathy (diabetic, idiopathic) can result
in bacterial overgrowth which may lead to diarrhoea.
Genetic factors implicated in susceptibility to enteric
disease
A number of studies have implicated association of
genes with the susceptibility of an individual to infec-
tions with enteric disease causing pathogens. Genes
associated with the susceptibility to enteric diseases
have been summarized in Table 1.
Preventive interventions
Several preventive measures have been recommended
by WHO on the basis of a systematic review. Exclusive
breastfeeding to infants has been recommended as one
of the preventive strategy for diarrhoeal disease (Shah
et al., 2012). Breast milk contains several antimicrobial
factors and exclusive breastfeeding excludes the con-
sumption of contaminated food and water. It has been
reported that breastfed children below age 6 months are
6 times less likely to die due to diarrhoea than other
infants (Victoria, 2000). Fewer than 4 in 10 children
worldwide are exclusively breastfed during their rst six
months of life (Unicef, 2015).
Data from 2005- 2013 for 15 highest burden coun-
tries shows that the exclusive breastfeeding percent-
age of infants ranges from <3% to 64%. In India, this
percentage is 46% and less than GAPPD target of 50%.
WHO has also recommended vitamin A supplementation
for all HIV-infected and exposed infants and children
aged 6 months to 5 years, in doses given every 6 months
(100 000 IU for those aged 6–12 months and 200 000
IU for those aged > 12 months). Though no effect of
vitamin A supplementation was observed on occurrence
of diarrhoea in infants and children less than 6 months
(Shah et al., 2012, Organization, 2010). Zinc supple-
mentation (10 mg elemental Zn for 14 days for children
aged 2-6 months and 20 mg/day for older children) is
an important preventive measure to lower the incidence
rate, mortality and morbidity associated with diarrhoea
(Shah et al., 2012).
Changes in public health policy also results in signi -
cant reduction in overall prevalence of diarrhoea (Emina
Table 1: Genes implicated in susceptibility to enteric diseases
Pathogens Genes implicated in susceptibility
Bacteria
EAEC IL-8 (Jiang et al., 2003)
Clostridium dif cile IL-8 (Jiang et al., 2006)
Salmonella spp. HLA-DRB1 (Dunstan et al., 2014), TNFA, IL-12B, IL-12RB1,
IFNGR1, HLA-DQB18*0201-3 allele (Dunstan et al., 2001)
V. cholera O blood group (Glass et al., 1985)
Virus
Norwalk Virus FUT2 (Lindesmith et al., 2003)
Protozoans
Cryptosporidium parvum/ hominis DQB1*301 allele, DQB1*301/DRB1*1101, HLA class 1B*15
(Kirkpatrick et al., 2008)
Entamoeba histolytica DQB1*0601/DRB1*1501(Duggal et al., 2004)
Pooja Rawat et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION 659
and Kandala, 2012). A median reduction of 55% (range
20–82%) in child mortality was observed with improved
access to sanitation facilities. Effective immunization
with the vaccines can have major impact on diarrhoea
mortality in developing countries. Since 2009, the WHO
has recommended inclusion of rotavirus vaccine in all
the national immunization programmes. The diarrhoeal
disease caused by the rotavirus kills about 500,000 chil-
dren annually; of which more than 85 per cent deaths
are from developing nations. (Verma et al., 2012).
Recently, two live oral rotavirus vaccines (one derived
from attenuated human strain of rotavirus and second
contains ve bovine-human re-assortant strains) have
been licensed in more than 100 countries, including
India, which are very effective in lowering the incidence
of diarrhoea and frequency of death (Morris et al., 2012,
Glass et al., 2006). Eighty one countries have introduced
Rotarix or RotaTeq rotavirus vaccines into their national
immunization program (Burnett et al., 2016, Kuate Defo
and Lee, 2016).
There is, however, a signi cant debate on the intro-
duction of such vaccines in India, mainly because of
their high costs. Killed whole cell vaccines are being
used for vaccinization for cholera. Both oral and injected
vaccines are safe to use and effective for up to two years
after single dose and three to four years after annual
booster dose (Sinclair et al., 2011, Graves et al., 2010).
Killed whole cell oral cholera vaccine (Dukoral®) is rec-
ommended to combat the infections due to Entero toxi-
genic Escherichia coli (ETEC) bacteria, common cause of
travelers’ diarrhoea in the adults and children in devel-
oping countries. It contains a recombinant B subunit of
cholera toxin and is similar to heat labile toxin of ETEC.
However, an assessment of twenty four randomized con-
trolled trials (RCTs) was carried out which indicated lack
of suf cient evidences for use of Dukoral® for protecting
travelers’ against ETEC diarrhoea (Ahmed et al., 2013).
Several whole cell oral cholera vaccines have been tested
and found to be effective against diarrhoea (Desai et al.,
2015, Baik et al., 2015, Desai et al., 2016a, Desai et al.,
2016b).
Treatment methods
WHO has recommended following strategies for the
treatment of diarrhoea:
Oral and Intravenous Rehydration therapy
The introduction of ORT has played a crucial part in
reducing the mortality rate due to diarrhoea. For more
than 35 years, WHO and UNICEF have recommended a
single formulation of glucose-based ORS to prevent or
treat dehydration, irrespective of the cause or age group.
Administration of appropriate solutions by mouth, is
now routine therapy for managing diarrhoea. Modi ed
ORS such as polymer-based ORS is found to be better
than the standard one, due to its cost effectiveness in
managing acute gastroenteritis and also reduces hospi-
talization requirements in both developed and develop-
ing countries (Suh et al., 2010). Apart from oral rehy-
dration therapy, a number of solutions for intravenous
infusions are also available including Ringer’s Lactate
Solution (also called Hartmann’s Solution for Injection)
and Ringer’s lactate solution with 5% dextrose. Normal
saline (isotonic or physiological saline) is also an accept-
able solution, however it does not contain a base to cor-
rect acidosis and does not replace potassium losses.
Zinc Supplementation
Use of Zinc has been recommended by WHO for the
treatment of children with diarrhoea. Zinc supplementa-
tion is recommended for a period of 10–14 days, with
increased uids and continued feeding, for all HIV-
infected and -exposed children with diarrhoea (10 mg
per day for infants under 6 months of age, 20 mg per
day for infants and children over 6 months) .
Antibiotic treatment
Antibiotics aim at treating dehydration, shortening the
length of illness and reducing the infection period (Allen
et al., 2003). Formerly used antibiotics such as ampi-
cillin, doxycycline, and trimethoprim-sulfamethoxazole
for the treatment of traveler’s diarrhoea have become
less effective because of increasing microbial resist-
ance. Loperamide is the agent of choice for antimotility
in the adults but not in children below 2 years of age.
Cipro oxacin and azithromycin are indicated drugs for
moderate to severe disease to reduce the duration of ill-
ness. In recent times, rifaximin, a semi-synthetic, poorly
absorbed, broad-spectrum antibiotic with minimal
effects on gut ora, has been added for the treatment
of noninvasive forms of traveler’s diarrhoea (Ouyang-
Latimer et al., 2011).
Although antibiotics are bene cial in certain types of
acute diarrhoea, these are customarily not used except
in speci c conditions. This is because drug resistance to
human pathogenic bacteria has been frequently reported
in recent years. In addition, antibiotics are sometimes
associated with adverse effects on host, including hyper-
sensitivity, depletion of bene cial gut and mucosal
micro-organism, immuno-suppression and allergic reac-
tions. Besides, antibiotics may disturb the natural bal-
ance of human intestinal tract as well as colonization
resistance of the gut ora. This may lead to overgrowth
of certain enteropathogens such as C. dif cile, leading
to antibiotic-associated diarrhoea (Johnston et al., 2011,
Hempel et al., 2012).
Antimicrobials that are ineffective for treatment of
Shigellosis include metronidazole, streptomycin, tetra-
Pooja Rawat et al.
660 AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
cyclines, chloramphenicol, sulfonamides, amoxicillin,
nitrofurans (e.g. nitrofurantoin, furazolidone), amino-
glycosides (e.g. gentamicin, kanamycin), rst and second
generation cephalosporins (e.g. cephalexin, cefaman-
dole). Antidiarrhoeal treatments also include adsorbents
such as; kaolin, attapulgite, smectite, activated charcoal,
cholestyramine, antimotility drugs such as; loperamide
hydrochloride, diphenoxylate with atropine, tincture
of opium, camphorated tincture of opium, paregoric,
codeine and Bismuth subsalicylate. These antidiarrhoeal
drugs are however not recommended for treatment due
to lack of practical bene t. Resistant strains of a number
of microbes including V. cholerae, Shigella dysenteriae
type I, Salmonella enterica subsp. Typhi, Enteropatho-
genic E. coli, have been reported over the past few dec-
ades (Cooke and Wain, 2004, Mwansa et al., 2007).
In developing countries, the major problem with anti-
microbials is the way these antibiotic therapies are used.
Most of such medicines are taken without prescription
and without monitoring the usage by the patients, not
completing the prescribed drug regimen resulting in
multidrug resistance. Another major factor contributing
to increased resistance is the substandard drugs availa-
ble in the market, containing doses lower than required,
further predisposing the population towards increased
drug resistant.
Traditional plant based medicines
Owing to the enormous clinical problems associated
with diarrhoea, there is a need to develop alternative
anti-microbial drugs for its treatment. Since ancient
times, traditional medicines have been in use to cure
several diseases (Rawat et al., 2016, Farag et al., 2016,
Patel et al., 2014). In developing countries, healthcare
management by the people living in rural areas depends
upon use of traditional plant based medicines (Gaikwad
et al., 2015, Pandit et al., 2015, Kumar, 2016).
As per WHO, approximately eighty percent of world
population relies on traditional medicines using plant
extracts or their active constituents (Umamaheswari et
al., 2008). The biological activity of extracts, combina-
tion of extracts, fractions and compounds of several
plants has been investigated against diarrhoea. These
plants are reported to have anti-spasmodic effects, gut
motility suppression activity, intestinal transit delay,
water adsorption stimulation or reduction in electrolyte
secretion. Numerous phyto-chemicals such as tannins,
alkaloids, avonoids and terpenes identi ed in these
medicinal plants have been stated to be responsible for
anti-diarrhoeal activity. Anti-microbial properties of
medicinal plants are also being reported from different
parts of the world (Namita and Mukesh, 2012).
Several RCTs have been conducted to evaluate the
anti-diarrhoeal potential of herbal medicines. In one
of the study, compared with the patients in placebo
group, patients in the group treated with Chinese herbal
formulations showed signi cant improvement in the
symptoms (Bensoussan et al., 1998). Pre-clinical safety
and ef cacy studies of SP-300, a standardized botani-
cal extract formulated from the latex of Croton lechleri
was conducted. Ef cacy studies in cholera mouse model
demonstrated signi cant inhibitory effect on uid secre-
tion into the intestinal lumen. The effect was found to
be mediated through inhibition of cAMP mediated Cl
-
ion secretion. Clinical studies of SP-303 was also con-
ducted in travelers’ diarrhoea. Signi cant reduction in
diarrhoea and improvement in the subjective symptoms
such as; relief from cramping and urgency was observed
compared to placebo controlled group. Crofelemer, a
puri ed proanthocyanidin oligomer from bark latex of
the plant has been investigated against secretory diar-
rhoea. It was found that the oligomer inhibits the Cl
-
channel with maximum inhibition of 60% and an IC
50
7μM (Tradtrantip et al., 2010). Indigenous anti-diar-
rhoeal plants such as; Acacia burkei, Brachylaena trans-
vaalensis, Cissampelos hirta, Sarcostemma viminale,
Psidium guajava, Catharanthus roseus, Melia azedarac,
Sclerocarya birrea and Strychnos madagascariensis etc.
are reported from KwaZulu-Natal Province, South Africa
(de Wet et al., 2010, Of ah et al., 2011).
Holarrhena antidysenterica, Curcuma amada, Ficus
glomerata and Butea monosperma are reportedly used
for treating diarrhoeal condition by tribals from Mad-
hya Pradesh, India (Singh and Sharma, 2011). Pharma-
cological activity against diarrhoea was investigated for
Acacia nilotica, Acanthospermun hispidum, Gmelina
arborea, Parkia biglobosa and Vitex doniana, the plants
used for diarrhoea treatment in Kaduna State, Nigeria
(Agunu et al., 2005). Randomized controlled trials for
the herbs; Curcuma longa, Cynara scolymus, Hypericum
perforatum, Iberis amara, Maranta arundinacea, Men-
the piperita, Paeonia lacti ora and Plantago psyllium
revealed that herbs are effective in management of IBS
associated symptoms. However, no relief was observed
in case of herbal preparations made up of Aloe vera,
Curcuma xanthorriza and Fumaria of cinalis. Apart
from single herbs, several polyherbal preparations such
as; Carmint, Padma Lax, STW 5, Tong-xie-ning and
Tong-Xie-Yao-Fang (traditional Chinese herbal prepa-
ration) and DA-IBS have also been found effective in
management of symptoms. STW-5 is most ef cacious
among these preparation, with different mechanisms of
action such as anti-in ammatory activity, prosecretory
activity, and effect on gastrointestinal motility (Rahimi
and Abdollahi, 2012).
Antidiarrhoeal activity of Psidium guajava Linn.
(Myrtaceae) is well studied (Salgado et al., 2006,
Gakunga et al., 2013). The plant is reported to show anti-
Pooja Rawat et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION 661
diarrhoeal activity through several mechanisms such as;
anti-microbial activity (Lutterodt et al., 1999), reduction
in gastrointestinal motility (Ezekwesili et al., 2010), inhi-
bition of adherence and invasion by pathogen (Birdi et
al., 2010). Broad spectrum antibacterial properties was
observed in case of Terminalia chebula and Syzygium
cumini.. The study revealed inhibition of multidrug
resistant strain of V. cholerae non-O1, non-O139 (strains
PC4 and PC65), Klebsiella pneumoniae strain PC36, A.
hydrophila strain PC16, Escherichia coli strain PC80
(ETEC), E. coli strain VT3 (Enterohaemorrhagic E. coli,
EHEC), Pseudomonas aeruginosa ATCC 15442 and Bacil-
lus subtilis ATCC 6623, with MBC ranging from 0.25
to 4 mg/ml (Acharyya et al., 2009). Medicinal Plants,
Caesalpinia bonducella, Gardenia gummifera and Acacia
arabica from Melghat Forest in India, showed antibac-
terial potential against Escherichia coli, Staphylococcus
aureus, Klebsiella pneumoniae, Proteus vulgaris, Sal-
monella typhi, Shigella exneri, Salmonella paratyphi,
Salmonella typhimurium, Pseudomonas aeruginosa, and
Enterobacter aerogenes (Tambekar et al., 2009).
Apart from these, several plant preparations men-
tioned in Ayurveda such as; Pashanbhed churna,
Arjuna churna, Bilba churna, Amla churna, Gokharu
churna, Panchasakar churna, Trikatu churna, Avipatti-
kar churna, Chandanadi churna and Pushyanug churna
used for treatment of various infectious diseases have
been shown to possess antibacterial activity (Tambekar
et al., 2010). The natural compounds have proven to be
a rich source of biologically active materials. This has
resulted in the development of several new lead chemi-
cals for pharmaceuticals. The plant extracts and their
phyto-constituents can therefore be utilized as an alter-
native to antibiotics and other medicines for treatment
of enteric infections and diseases caused by microbes.
The plant based medicines though considered to be safe
for human consumption also presents with the problem
of drug resistance, known as herbal antimicrobial drug
resistance (HADR), with cases of resistance observed in
case of a number of clinical and non-clinical isolates
(Kavanaugh and Ribbeck, 2012, Pattiyathanee et al.,
2009, Vadhana, 2015).
Probiotics
Probiotics, are live organisms which when consumed in
adequate amount confer a health effect on host (Hotel,
2001). There is mounting evidence that several de ned
strains of non-pathogens such as; Lactobacilli and Bi -
dobacterium are safe for human consumption and ben-
e cial for prevention and treatment of acute infectious
diarrhoea (Allen et al., 2010, Johnston and Thorlund,
2009) as well as antibiotic associated and travelers’ diar-
rhea byrestoring the natural balance in the intestinal
tract. Culturelle, one of the probiotics, reduced the inci-
dence of diarrhoea by 41% in one of the study. The daily
dose for Culturelle is one pill containing 10 billion CFU.
There are no reports suggesting its signi cant side-
effects except for the rare cases of people having com-
promised immune systems. In one of the metaanalysis
study of sixty-three randomized and quasi-randomized
controlled trials, effect of probiotic agent was studied
and it was concluded that the probiotics provide bene -
cial effects in shortening of the duration and reduction
in the stool frequency in case of acute infectious diar-
rhoea (Allen et al., 2010). Similar results were observed
in another meta-analysis study evaluating the use of
probiotics for prevention and treatment of antibiotic
associated diarrhoea (AAD). A total of 82 RCTs of pro-
biotics (Lactobacillus, Bi dobacterium, Saccharomyces,
Streptococcus, Enterococcus, and/or Bacillus) were ana-
lyzed. Signi cant association of probiotic administra-
tion with reduction in AAD was observed(Hempel et al.,
2012).
SUMMARY
Child mortality due to diarrhoea in developing coun-
tries can primarily be attributed to infections caused
by pathogenic microorganisms. Signi cant reduction
in mortality has been achieved over the past 15 years
by adopting simple prevention initiatives such as; better
sanitization practices, encouraging longer breast feed-
ing of infants and vaccination. Complete eradication
of the diarrhoea related mortality is possible by timely
management and prevention of severe dehydration
associated with the disease. Oral rehydration therapy is
simple and cost effective to achieve the targets. Herbal
medicines and phyto-ingredients based therapeutics is
well proven through scienti c studies and long history
of their traditional use. These potential therapeutics are
economical to produce and easily accessible to the peo-
ple who generally don’t have easy access to antibiotics
and other treatment methods. Phytochemicals therefore
represent a potentially effective management strategy
for diarrhoea in high risk populations.
ACKNOWLEDGEMENTS
Authors are grateful to Prof Anil Gupta, Executive Vice
Chair, National Innovation Foundation India for his
honorary guidance and encouragement for carrying out
research activities.
REFERENCES
Acharyya S, Patra A & Bag PK (2009). Evaluation of the antimi-
crobial activity of some medicinal plants against enteric bac-
Pooja Rawat et al.
662 AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
teria with particular reference to multi-drug resistant Vibrio
cholerae. Tropical journal of pharmaceutical Research, 8.
Agunu A, Yusuf S, Andrew GO, Zezi AU & Abdurahman EM
(2005). Evaluation of ve medicinal plants used in diarrhoea
treatment in Nigeria. Journal of Ethnopharmacology, 101:27-30.
Ahmed T, Bhuiyan TR, Zaman K, Sinclair D & Qadri F (2013).
Vaccines for preventing enterotoxigenic Escherichia coli
(ETEC) diarrhoea. Cochrane Database Syst Rev, 7.
Ahs JW, Tao W, Löfgren J & Forsberg BC (2010). Diarrheal dis-
eases in low-and middle-income countries: incidence, preven-
tion and management. The Open Infectious Diseases Journal,
4:113-24.
Allen SJ, Martinez EG, Gregorio GV & Dans LF (2010). Probiot-
ics for treating acute infectious diarrhoea. Cochrane Database
Syst Rev, 11.
Allen SJ, Okoko B, Martinez E, Gregorio G & Dans LF (2003).
Probiotics for treating infectious diarrhoea. Cochrane Database
of Systematic Reviews, 4.
Anand S, Mandal S, Patil P & Tomar S (2016). Pathogen-
induced secretory diarrhea and its prevention. European Jour-
nal of Clinical Microbiology & Infectious Diseases, 35:1721-39.
Ankolekar C & Labbé RG (2009). Survival during cooking and
growth from spores of diarrheal and emetic types of Bacillus
cereus in rice. Journal of Food Protection®, 72:2386-89.
Bahl R, Ray P, Subodh S, Shambharkar P, Saxena M, Parashar
U, Gentsch J, Glass R & Bhan M (2005). Incidence of severe
rotavirus diarrhea in New Delhi, India, and G and P types of
the infecting rotavirus strains. Journal of Infectious Diseases,
192:S114-S19.
Baik YO, Choi SK, Olveda RM, Espos RA, Ligsay AD, Montel-
lano MB, Yeam JS, Yang JS, Park JY & Kim DR (2015). A ran-
domized, non-inferiority trial comparing two bivalent killed,
whole cell, oral cholera vaccines (Euvichol vs Shanchol) in the
Philippines. Vaccine, 33:6360-65.
Barrett KE 2016. Rethinking cholera pathogenesis-No longer
all in the same “camp”. Taylor & Francis.
Beau I, Cotte‐Laf tte J, Géniteau‐Legendre M, Estes MK & Servin
AL (2007). An NSP4‐dependant mechanism by which rotavirus
impairs lactase enzymatic activity in brush border of human
enterocyte‐like Caco‐2 cells. Cellular microbiology, 9:2254-66.
Bensoussan A, Talley NJ, Hing M, Menzies R, Guo A & Ngu
M (1998). Treatment of irritable bowel syndrome with Chi-
nese herbal medicine: a randomized controlled trial. Jama,
280:1585-89.
Birdi T, Daswani P, Brijesh S, Tetali P, Natu A & Antia N (2010).
Newer insights into the mechanism of action of Psidium gua-
java L. leaves in infectious diarrhoea. BMC complementary and
alternative medicine, 10:33.
Burnett E, Yen C, Tate JE & Parashar UD (2016). Rotavirus vac-
cines: current global impact and future perspectives. Future
Virology, 11:699-708.
Camilleri M, Sellin JH & Barrett KE (2016). Pathophysiology,
Evaluation, and Management of Chronic Watery Diarrhea.
Gastroenterology.
China C (2005). Review Research on The Literature of Diarrhea
Disease in China.
Cooke FJ & Wain J (2004). The emergence of antibiotic resist-
ance in typhoid fever. Travel medicine and infectious disease,
2:67-74.
Curtis V & Cairncross S (2003). Effect of washing hands with
soap on diarrhoea risk in the community: a systematic review.
The Lancet infectious diseases, 3:275-81.
Daher R, Yazbeck T, Jaoude JB & Abboud B (2009). Conse-
quences of dysthyroidism on the digestive tract and viscera.
World J Gastroenterol, 15:2834-38.
Davis GR, Morawski SG, Santa Ana CA & Fordtran J (1983).
Evaluation of chloride/bicarbonate. Exchange in the human
colon in vivo. Journal of Clinical Investigation, 71:201.
De Wet H, Nkwanyana MN & Van Vuuren SF (2010). Medicinal
plants used for the treatment of diarrhoea in northern Maputa-
land, KwaZulu-Natal Province, South Africa. Journal of Eth-
nopharmacology, 130:284-89.
Deborah Chen H & Frankel G (2005). Enteropathogenic Escher-
ichia coli: unravelling pathogenesis. FEMS Microbiology
Reviews, 29:83-98.
Demeo MT (2016). Gastrointestinal Food Allergy and Intol-
erance. Practical Gastroenterology and Hepatology Board
Review Toolkit:227.
Desai SN, Akalu Z, Teferi M, Manna B, Teshome S, Park JY,
Yang JS, Kim DR, Kanungo S & Digilio L (2016a). Comparison
of immune responses to a killed bivalent whole cell oral chol-
era vaccine between endemic and less endemic settings. Tropi-
cal Medicine & International Health, 21:194-201.
Desai SN, Akalu Z, Teshome S, Teferi M, Yamuah L, Kim DR,
Yang JS, Hussein J, Park JY & Jang MS (2015). A randomized,
placebo-controlled trial evaluating safety and immunogenicity
of the killed, bivalent, whole-cell oral cholera vaccine in Ethio-
pia. The American journal of tropical medicine and hygiene,
93:527-33.
Desai SN, Pezzoli L, Alberti KP, Martin S, Costa A, Perea W &
Legros D (2016b). Achievements and challenges for the use of
killed oral cholera vaccines in the global stockpile era. Human
Vaccines & Immunotherapeutics:00-00.
Dolin R, Levy AG, Wyatt RG, Thornhill TS & Gardner JD (1975).
Viral gastroenteritis induced by the Hawaii agent: jejunal his-
topathology and serologic response. The American journal of
medicine, 59:761-68.
Duggal P, Haque R, Roy S, Mondal D, Sack RB, Farr BM, Beaty
TH & Petri WA (2004). In uence of Human Leukocyte Anti-
gen Class II Alleles on Susceptibility to Entamoeba histolytica
Infection in Bangladeshi Children. Journal of Infectious Dis-
eases, 189:520-26.
Dunstan SJ, Hue NT, Han B, Li Z, Tram TTB, Sim KS, Parry CM,
Chinh NT, Vinh H & Lan NPH (2014). Variation at HLA-DRB1
is associated with resistance to enteric fever. Nature genetics,
46:1333-36.
Dunstan SJ, Stephens HA, Blackwell JM, Duc CM, Lanh MN,
Dudbridge F, Phuong C, Luxemburger C, Wain J & Ho VA
Pooja Rawat et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION 663
(2001). Genes of the class II and class III major histocompat-
ibility complex are associated with typhoid fever in Vietnam.
Journal of Infectious Diseases, 183:261-68.
Emina JB & Kandala N-B (2012). Accounting for recent trends
in the prevalence of diarrhoea in the Democratic Republic of
Congo (DRC): results from consecutive cross-sectional surveys.
BMJ open, 2:e001930.
Ericsson CD, Steffen R & Okhuysen PC (2001). Traveler’s diar-
rhea due to intestinal protozoa. Clinical Infectious Diseases,
33:110-14.
Ezekwesili J, Nkemdilim U & Okeke C (2010). Mechanism of
antidiarrhoeal effect of ethanolic extract of Psidium guajava
leaves. Biokemistri, 22.
Farag M, Mohammed MS, Ahmed WJ & Foud I (2016). The
Role of Medicinal Plants in the Treatment of Type-2 Diabetes.
Flint JA, Van Duynhoven YT, Angulo FJ, Delong SM, Braun P,
Kirk M, Scallan E, Fitzgerald M, Adak GK & Sockett P (2005).
Estimating the burden of acute gastroenteritis, foodborne dis-
ease, and pathogens commonly transmitted by food: an inter-
national review. Clinical infectious diseases, 41:698-704.
Frizzell RA & Hanrahan JW (2012). Physiology of epithelial
chloride and uid secretion. Cold Spring Harbor perspectives
in medicine, 2:a009563.
Gaikwad SLR, Ramana DN, Solanki RP, Sahay NS, Patel J,
Ravikumar R & Amol S (2015). Ef cacy of an indigenous
veterinary medication to control endoparasite infestation in
clinically diagnosed large ruminants affected with diarrhoea
amongst eld conditions, Gujarat, India. European Journal of
Experimental Biology, 5:81-84.
Gakunga JN, Mirianga B, Muwonge H, Sembajwe LF & Kat-
eregga J (2013). Antidiarrheal activity of ethanolic fruit extract
of Psidium guajava (Guava) in castor oil induced diarrhea in
albino rats. National Journal of Physiology, Pharmacy and
Pharmacology, 3:191-97.
Glass RI, Holmgren J, Haley CE, Khan M, Svennerholm A, Stoll
BJ, Hossain KB, Black RE, Yunus M & Barua D (1985). Predis-
position for cholera of individuals with o blood group possible
evolutionary signi cance. American journal of epidemiology,
121:791-96.
Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Wid-
dowson M-A, Jiang B & Gentsch JR (2006). Rotavirus vac-
cines: current prospects and future challenges. The Lancet,
368:323-32.
Graves PM, Deeks JJ, Demicheli V & Jefferson T (2010). Vac-
cines for preventing cholera: killed whole cell or other subunit
vaccines (injected). Cochrane Database Syst Rev, 8.
Guarino A, Vecchio AL & Canani RB (2012). Chronic diarrhoea
in children. Best practice & research Clinical gastroenterology,
26:649-61.
Gulig PA, Bourdage KL & Starks AM (2005). Molecular patho-
genesis of Vibrio vulni cus. J. Microbiol, 43:118-31.
Hahn S, Kim S & Garner P (2002). Reduced osmolarity oral
rehydration solution for treating dehydration caused by acute
diarrhoea in children. Cochrane Database Syst Rev, 1.
Harrington SM, Dudley EG & Nataro JP (2006). Pathogenesis of
enteroaggregative Escherichia coli infection. FEMS microbiol-
ogy letters, 254:12-18.
Hashmey R, Genta RM & White AC (1997). Parasites and diar-
rhea. I: Protozoans and diarrhea. Journal of travel medicine,
4:17-31.
Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shan-
man R, Johnsen B & Shekelle PG (2012). Probiotics for the
prevention and treatment of antibiotic-associated diarrhea: a
systematic review and meta-analysis. Jama, 307:1959-69.
Hill Z, Kirkwood B & Edmond K (2004). Family and commu-
nity practices that promote child survival, growth and devel-
opment. Geneva: WHO.
Hosokawa A, Nishikawa J, Ando T & Sugiyama T (2016).
Chemotherapy-induced Small Bowel Injury. Internal Medicine,
55:1023-23.
Hotel ACP (2001). Health and nutritional properties of probiot-
ics in food including powder milk with live lactic acid bacteria.
Prevention, 5.
Huang DB, Mohanty A, Dupont HL, Okhuysen PC & Chiang T
(2006). A review of an emerging enteric pathogen: enteroag-
gregative Escherichia coli. Journal of medical microbiology,
55:1303-11.
Jafari F, Shokrzadeh L, Hamidian M, Salmanzadeh-Ahrabi
S & Zali MR (2008). Acute diarrhea due to enteropathogenic
bacteria in patients at hospitals in Tehran. Jpn J Infect Dis,
61:
269-73.
Jiang Z-D, Dupont HL, Garey K, Price M, Graham G, Okhuysen
P, Dao-Tran T & Larocco M (2006). A common polymorphism
in the interleukin 8 gene promoter is associated with Clostrid-
ium dif cile diarrhea. The American journal of gastroenterol-
ogy, 101:1112-16.
Jiang Z-D, Okhuysen PC, Guo D-C, He R, King TM, Dupont
HL & Milewicz DM (2003). Genetic Susceptibility to Entero-
aggregative Escherichia coli Diarrhea: Polymorphism in the
Interleukin-8 Promotor Region. Journal of Infectious Diseases,
188:506-11.
Johnston BC, Goldenberg JZ, Vandvik PO, Sun X & Guyatt
GH (2011). Probiotics for the prevention of pediatric antibiotic-
associated diarrhea. Cochrane Database Syst Rev, 11.
Johnston BC, Supina AL, Ospina M & Vohra S (2007). Probiot-
ics for the prevention of pediatric antibiotic-associated diar-
rhea. Cochrane Database Syst Rev, 2.
Johnston BC & Thorlund K (2009). Probiotics for the preven-
tion of Clostridium dif cile associated diarrhea in adults and
children. The Cochrane Library.
Joshi PC, Kaushal S, Aribam BS, Khattri P, D’aoust O, Singh
MM, Marx M & Guha-Sapir D (2011). Recurrent oods and
prevalence of diarrhea among under ve children: observa-
tions from Bahraich district, Uttar Pradesh, India. Global
health action, 4.
Julian TR (2016). Environmental transmission of diarrheal
pathogens in low and middle income countries. Environmental
Science: Processes & Impacts, 18:944-55.
Pooja Rawat et al.
664 AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Kaur P, Chakraborti A & Asea A (2010). Enteroaggregative
Escherichia coli: an emerging enteric food borne pathogen.
Interdisciplinary perspectives on infectious diseases, 2010.
Kavanaugh NL & Ribbeck K (2012). Selected antimicrobial
essential oils eradicate Pseudomonas spp. and Staphylococ-
cus aureus bio lms. Applied and environmental microbiology,
78:4057-61.
Kirkpatrick BD, Haque R, Duggal P, Mondal D, Larsson C,
Peterson K, Akter J, Lockhart L, Khan S & Petri WA (2008).
Association between Cryptosporidium Infection and Human
Leukocyte Antigen Class I and Class II Alleles. Journal of
Infectious Diseases, 197:474-78.
Kuate Defo Z & Lee B (2016). New approaches in oral rotavirus
vaccines. Critical reviews in microbiology, 42:495-505.
Kumar A, Basu S, Vashishtha V & Choudhury P (2016). Bur-
den of rotavirus diarrhea in under- ve Indian children. Indian
pediatrics, 53:607-17.
Kumar V (2016). Importance of Alangium salviifolium and
Its Pharmacological Update. European Journal of Medicinal
Plants, 12:1.
Kuntz TB & Kuntz ST (1999). Enterohemorrhagic E. coli infec-
tion. Primary Care Update for OB/GYNS, 6:192-96.
Leung DT, Chisti MJ & Pavia AT (2016). Prevention and Con-
trol of Childhood Pneumonia and Diarrhea. Pediatric clinics of
North America, 63:67-79.
Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lind-
blad L, Stewart P, Lependu J & Baric R (2003). Human sus-
ceptibility and resistance to Norwalk virus infection. Nature
medicine, 9:548-53.
Lozoya LX, Chavez SMA & Rivera AE 2006. Improved extracts
of psidium guajava l., methods for its obtaining and use for the
treatment of gastrointestinal disorders. Google Patents.
Lutterodt G, Ismail A, Basheer R & Baharudin HM (1999). Anti-
microbial effects of Psidium guajava extracts as one mecha-
nism of its antidiarrhoeal action. Malaysian J Med Sci, 6:17-
20.
Mcquade RM, Stojanovska V, Abalo R, Bornstein JC & Nurgali
K (2016). Chemotherapy-Induced Constipation and Diarrhea:
Pathophysiology, Current and Emerging Treatments. Frontiers
in Pharmacology, 7.
Morris SK, Awasthi S, Khera A, Bassani DG, Kang G, Parashar
UD, Kumar R, Shet A, Glass RI & Jha P (2012). Rotavirus mor-
tality in India: estimates based on a nationally representa-
tive survey of diarrhoeal deaths. Bulletin of the World Health
Organization, 90:720-27.
Mwansa J, Mwaba J, Lukwesa C, Bhuiyan N, Ansaruzzaman M,
Ramamurthy T, Alam M & Nair GB (2007). Multiply antibiotic-
resistant Vibrio cholerae O1 biotype El Tor strains emerge dur-
ing cholera outbreaks in Zambia. Epidemiology and infection,
135:847-53.
Namita P & Mukesh R (2012). Medicinal plants used as antimi-
crobial agents: a review. Int Res J Pharm, 3:31-40.
Nataro JP & Kaper JB (1998). Diarrheagenic escherichia coli.
Clinical microbiology reviews, 11:142-201.
Nightingale K, Schukken Y, Nightingale C, Fortes E, Ho A, Her
Z, Grohn Y, Mcdonough P & Wiedmann M (2004). Ecology
and transmission of Listeria monocytogenes infecting rumi-
nants and in the farm environment. Applied and Environmen-
tal Microbiology, 70:4458-67.
Niyogi SK (2005). Shigellosis. Journal of microbiology (Seoul,
Korea), 43:133-43.
Ochoa TJ, Barletta F, Contreras C & Mercado E (2008). New
insights into the epidemiology of enteropathogenic Escheri-
chia coli infection. Transactions of the Royal Society of Tropi-
cal Medicine and Hygiene, 102:852-56.
Of ah NV, Makama S, Elisha IL, Makoshi MS, Gotep JG, Dawu-
rung CJ, Oladipo OO, Lohlum AS & Shamaki D (2011). Eth-
nobotanical survey of medicinal plants used in the treatment
of animal diarrhoea in Plateau State, Nigeria. BMC Veterinary
Research, 7:1.
Ojewole JA, Awe EO & Chiwororo WD (2008). Antidiarrhoeal
activity of Psidium guajava Linn.(Myrtaceae) leaf aque-
ous extract in rodents. Journal of Smooth Muscle Research,
44:195-207.
Organization WH (1997). Health and environment in sustain-
able development: Five years after the Earth Summit: Execu-
tive Summary.
Organization WH (2004). Antibiotics in the management of
shigellosis. Weekly Epidemiological Record, 79:349-56.
Organization WH (2010). WHO recommendations on the man-
agement of diarrhoea and pneumonia in HIV-infected infants
and children: integrated management of childhood illness
(IMCI).
Ouyang-Latimer J, Jafri S, Vantassel A, Jiang Z-D, Gurleen K,
Rodriguez S, Nandy RK, Ramamurthy T, Chatterjee S & Mcken-
zie R (2011). In vitro antimicrobial susceptibility of bacterial
enteropathogens isolated from international travelers to Mex-
ico, Guatemala, and India from 2006 to 2008. Antimicrobial
agents and chemotherapy, 55:874-78.
Palombo EA (2006). Phytochemicals from traditional medici-
nal plants used in the treatment of diarrhoea: modes of action
and effects on intestinal function. Phytotherapy Research,
20:717-24.
Pandit R, Singh PK & Kumar V (2015). Natural Remedies
against Multi-Drug Resistant Mycobacterium tuberculosis.
Journal of Tuberculosis Research, 3:171.
Pattiyathanee P, Vilaichone R-K & Chaichanawongsaroj N
(2009). Effect of curcumin on Helicobacter pylori bio lm for-
mation. African Journal of Biotechnology, 8.
Rahimi R & Abdollahi M (2012). Herbal medicines for the man-
agement of irritable bowel syndrome: a comprehensive review.
World J Gastroenterol, 18:589-600.
Rawat P, Singh PK & Kumar V (2016). Anti-hypertensive
Medicinal Plants and their Mode of Action. Journal of Herbal
Medicine.
Reid G, Jass J, Sebulsky MT & Mccormick JK (2003). Potential
uses of probiotics in clinical practice. CLINICAL microbiology
Reviews, 16:658-72.

Pooja Rawat et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS AFFLICTIONS OF ENTERIC DISEASES IN HUMAN POPULATION 665
Sairam K, Hemalatha S, Kumar A, Srinivasan T, Ganesh J,
Shankar M & Venkataraman S (2003). Evaluation of anti-diar-
rhoeal activity in seed extracts of Mangifera indica. Journal of
ethnopharmacology, 84:11-15.
Salgado HRN, Roncari A, Michelin D & Moreira RRD (2006).
Evaluation of antidiarrhoeal effects of Psidium guajava
L.(Myrtaceae) aqueous leaf extract in mice. Revista de Ciências
Farmacêuticas Básica e Aplicada:89-92.
Sansonetti PJ, Arondel J, Cavaillon J-M & Huerre M (1995).
Role of interleukin-1 in the pathogenesis of experimental shig-
ellosis. Journal of Clinical Investigation, 96:884.
Sarin R & Bafna P (2012). Herbal antidiarrhoeals: a review. Int
J Res Pharm Biomed Sci, 3:637-49.
Schiller LR (1999). Secretory diarrhea. Current gastroenterol-
ogy reports, 1:389-97.
Schraft H & Grif ths MW (2006). Bacillus cereus gastroenteri-
tis. Foodborne Infections and Intoxications, 15:563-77.
Schreiber DS, Blacklow NR & Trier JS (1973). The mucosal
lesion of the proximal small intestine in acute infectious non-
bacterial gastroenteritis. New England Journal of Medicine,
288:1318-23.
Schreiber DS, Blacklow NR & Trier JS (1974). The small intesti-
nal lesion induced by Hawaii agent acute infectious nonbacte-
rial gastroenteritis. Journal of Infectious Diseases, 129:705-08.
Shah D, Choudhury P, Gupta P, Mathew JL, Gera T, Gogia S,
Mohan P, Panda R & Menon S (2012). Promoting appropri-
ate management of diarrhea: a systematic review of literature
for advocacy and action: UNICEF-PHFI series on newborn and
child health, India. Indian pediatrics, 49:627-49.
Sinclair D, Abba K, Zaman K, Qadri F & Graves PM (2011).
Oral vaccines for preventing cholera. Cochrane Database Syst
Rev, 3.
Patel K, Kumar V, Singh PK & Kumar V (2014). Phytochemistry
and Pharmacology of Abies pindrow. Pharmagene, 2:36-39.
Singh R & Sharma A (2011). Medicinal plants used for diar-
rhoea by tribals from Majhgawan Block of District Satna, Mad-
hya Pradesh, India.
Suh J-S, Hahn W-H & Cho B-S (2010). Recent advances of
oral rehydration therapy (ORT). Electrolytes & Blood Pressure,
8:82-86.
Surawicz CM (2003). Probiotics, antibiotic-associated diar-
rhoea and Clostridium dif cile diarrhoea in humans. Best Prac-
tice & Research Clinical Gastroenterology, 17:775-83.
Tambekar D, Dahikar S & Dahikar S (2010). Antibacterial
potential of some herbal preparation: An alternative medicine
in treatment of enteric bacterial infection. Int J Pha Pharm
Sci, 2:176.
Tambekar DH, Khante B, Chandak B, Titare A, Boralkar S &
Aghadte S (2009). Screening of antibacterial potentials of some
medicinal plants from Melghat forest in India. African Journal
of Traditional, Complementary and Alternative Medicines, 6.
Tarricone R, Koush DA, Nyanzi-Wakholi B & Medina-Lara A
(2016). A systematic literature review of the economic impli-
cations of chemotherapy-induced diarrhea and its impact
on quality of life. Critical reviews in oncology/hematology,
99:37-48.
Thapar N & Sanderson IR (2004). Diarrhoea in children: an
interface between developing and developed countries. The
Lancet, 363:641-53.
Thiagarajah JR, Donowitz M & Verkman AS (2015). Secre-
tory diarrhoea: mechanisms and emerging therapies. Nature
Reviews Gastroenterology & Hepatology, 12:446-57.
Tradtrantip L, Namkung W & Verkman AS (2010). Crofelemer,
an antisecretory antidiarrheal proanthocyanidin oligomer
extracted from Croton lechleri, targets two distinct intestinal
chloride channels. Molecular pharmacology, 77:69-78.
Troeger H, Loddenkemper C, Schneider T, Schreier E, Epple
H-J, Zeitz M, Fromm M & Schulzke JD (2009). Structural and
functional changes of the duodenum in human norovirus
infection. Gut, 58:1070-77.
Umamaheswari A, Shreevidya R & Nuni A (2008). In vitro anti-
bacterial activity of Bougainvillea spectabilis leaves extracts.
Adv Biol Res, 2:1-5.
Unicef (2015). Committing to Child Survival: A Promise
Renewed, Progress Report 2014. 2014. New York, USA: UNICEF.
Vadhana P (2015). Emergence of Herbal Antimicrobial Drug
Resistance in Clinical Bacterial Isolates. Pharmaceutica Ana-
lytica Acta, 2015.
Verma R, Khanna P & Chawla S (2012). Rotavirus vaccine
can save millions of children’s lives in developing countries.
Human vaccines & immunotherapeutics, 8:272-74.
Victoria C (2000). Effect of breastfeeding on infant and child
mortality due to infectious diseases in less developed coun-
tries: a pooled analysis. Lancet (British edition), 355:451-55.
Walker CLF, Perin J, Aryee MJ, Boschi-Pinto C & Black RE
(2012). Diarrhea incidence in low-and middle-income coun-
tries in 1990 and 2010: a systematic review. BMC public health,
12:220.
Xin X, Jia B, Huang W, Liu C & Li C (2011). In vitro susceptibil-
ity of some antimicrobials against bacteria enteropathogens.
African Journal of Microbiology Research, 5:4702-07.