
Medical
Communication
Biosci. Biotech. Res. Comm. 9(4): 633-642 (2016)
In vitro
evaluation of antifungal effects of nanoliposomal
uconazole against uconazole susceptible and resistant
Candida
species isolated from patients
Asadi Mehrdad
1
, Hashemi Seyed Jamal
2
*, Hamishehkar Hamed
3
, Kordbacheh Parivash
4
,
Ghasemi Zeinab
5
, Mojtaba Didehdar
6
, Nazari Maryam
7
and Sadeghi Sanam
8
1
Department of Medical Parasitology and Mycology, Faculty of Public Health, Tehran University of Medical
Sciences, Tehran-Iran
2
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical
Sciences, Tehran, Iran
3
Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical
Sciences, Tehran, Iran
5
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical
Sciences, Tehran, Iran
6
Department of Medical Parasitology and Mycology, Arak University of Medical Sciences, Arak, Iran
7
Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Department of Food Science and Technology, Faculty of Agriculture, University of Tabriz, Tabriz, Iran
8
Department of Nanobiotechnology, Faculty of Biological Sciences, University of Tarbiat Modares, Tehran, Iran
ABSTRACT
The aim of this study was to produce uconazole loaded liposomal nanoparticles, to analyze their physicochemical
properties and to compare their antifungal effects with the free uconazole drug in vitro against the uconazole
susceptibleand resistant Candida species isolated from patients.Six common candida species including C.albicans,
C.parapsilosis, C.tropicalis, C.glabrata, C.krusei and C.guilliermondii were tested. The Liposomal nanoparticles
were prepared using thin layer hydration method and soybean lecithin, cholesterol, and uconazole at a ratio of
10: 1: 1. The nanoparticles were analyzedin terms of size, poly dispersity index, zeta potential, morphology,
entrapment ef ciency of drug and the amount of drug released. To investigate the antifungal effects of liposomal
633
ARTICLE INFORMATION:
*Corresponding Author: sjhashemi@tums.ac.ir
Received 13
th
Nov, 2016
Accepted after revision 19
th
Dec, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
634 IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Asadi Mehrdad et al.
nanoparticles and compare them with the free form of uconazole, we used Broth Microdilution as described in CLSI
M27-A3.The results were analyzed using Student’s T-test and indicated the greater antifungal effects of the liposomal
nanoparticles containing uconazole than the normal form of the drug. It was shown that MIC of Fluconazole was put in
the range of sensitive species after exposure with the Fluconazole Nanoliposomal in most Fluconazole resistant Candida
species except for the krusei species. Therefore, it is likely that we can use the new system for drug delivery to prevent
drug release from the cell. In addition, this is the rst research using uconazole lipid nanoparticles against C.krusei.
KEY WORDS: NANOLIPOSOME, FLUCONAZOLE, ANTIFUNGAL ACTIVITY, CANDIDA
INTRODUCTION
Nowadays, the opportunistic pathogenic fungi are
among the life threatening infections in patients with
impaired immune systems. Yeasts, especially the can-
dida species are the most common fungi that are isolated
from human infections. Despite many advances in the
eld of health care and methods of treatment, the inci-
dence of invasive systemic candidiasis is signi cantly
increasing. Although Candida albicans was and is the
most common agent responsible for infections in differ-
ent clinical forms of candidiasis, the other species belong
to the Candida types such as Candida tropicalis, Candida
glabrata, Candida krusei, Candida parapsilosis, Candida
guilliermondii and so on, are more or less isolated from
patients. The importance of the non-albicans species has
increased due to the relative resistance in some of these
species like Candida tropicalis and Candida glabrata to
the antifungal drugs in the recent years, (Price etal 1994,
Pfaller, 1995, Wingard, 1995 Neppelenbroek et al 2006).
The increase of the different and various reports from
all over the world about the drug resistance increase
among the fungi and Candida species, and on the other
hand, the new antifungal drugs production indicates the
need for testing the susceptibility to these drugs and
makes the researchers eager to determine susceptibility
pattern for the various antifungal drugs, (White et al.,
2002 Mohammadi et al 2016).
The side effects of anti-fungal drugs is one of the
main reasons of extensive research on new anti-fungal
compounds and their therapeutic effects, (Falkiewicz-
Dulik and Macura, 2008). Meanwhile nano particles are
of the particular importance, among the metal nano par-
ticles, silver nano particles has attracted many research-
ers due to their interesting physicochemical traits (S.
Schultz et al., 2000). Recently nano particles synthesis
by microbes has been considered as a suitable alterna-
tive for the mass production of nano particles, (Verma et
al 2009 and Omidi et al., 2014). Solid lipid nanoparticles
(SLNs) were introduced for the rst time in 1991 as an
alternative to common colloidal carriers such as emul-
sions and polymeric nanoparticles. SLNs are colloidal
carriers with sizes ranging from 50 to 1000 nm, which
are formed from biolipids (Thassu et al., 2007, Yadav
et al., 2013).
Recently, these particles are used as antifungal drug
carriers such as Itraconazole, (Mohanty et al., 2015),
ketoconazole (Souto and Muller, 2005), Griseofulvin
(Aggarwal and Goindi, 2013) and Miconazole ( Mendes
et al., 2013). The small size of these lipid nanoparticles
increases their access to the tissues and the more in u-
ence of drugs( Jenning et al., 2000). These drug delivery
systems bring the controlled release of drugs, increas-
ing chemical stability of the trapped drugs. In addition,
these systems are among the safe and secure carriers
that can be easily produced on a large scale (Muhlen et
al., 1998 and Mehnert and Mader, 2001).
The treatment using azole antifungal agents, espe-
cially, uconazole is introduced as an effective solution
for treating infections caused by Candida, but the drug
resistances have faced this treatment method with dif-
culties ( Revankar et al., 1996, Silva et al., 2012).
Thus, for treating these types of cases, that are not
curable applying the conventional methods, the use of
new treatment strategies seems to be essential. In this
research, nanoliposome-containing uconazole is pre-
pared, and its antifungal effects are studied in vitro
on the uconazole susceptible and resistant strains of
C.albicans, C.parapsilosis, C.glabrata, C.tropicalis and
the resistant C.krusei species comparing to the conven-
tional form of uconazole.
MATERIALS AND METHODS
87 positive cultures of yeast organisms isolated from
cutaneous, mucosal and systemic fungal lesions of
patients referring Razi hospital in Tehran, Sina Hospital
in Tabriz city and another private lab in Tabriz were col-
lected. Clinical specimens included skin, nail, mucous
discharges and the BAL samples.
Isolation of yeast: clinical samples were cultured on
Sabouraud dextrose agar medium with chloramphenicol,
and after the growth of yeast colonies were transferred
to sterile Eppendorf tubes containing distilled water
and 20% glycerol so were kept in the freezer with -20 °
C.CHROMagar Candida culture medium: Yeasts isolated
from patients were cultured applying linear method on
the CHROMagar Candida medium in HIMEDIA Company
and incubated for 48 hours at 35 ° C and then the plates
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE 635
Asadi Mehrdad et al.
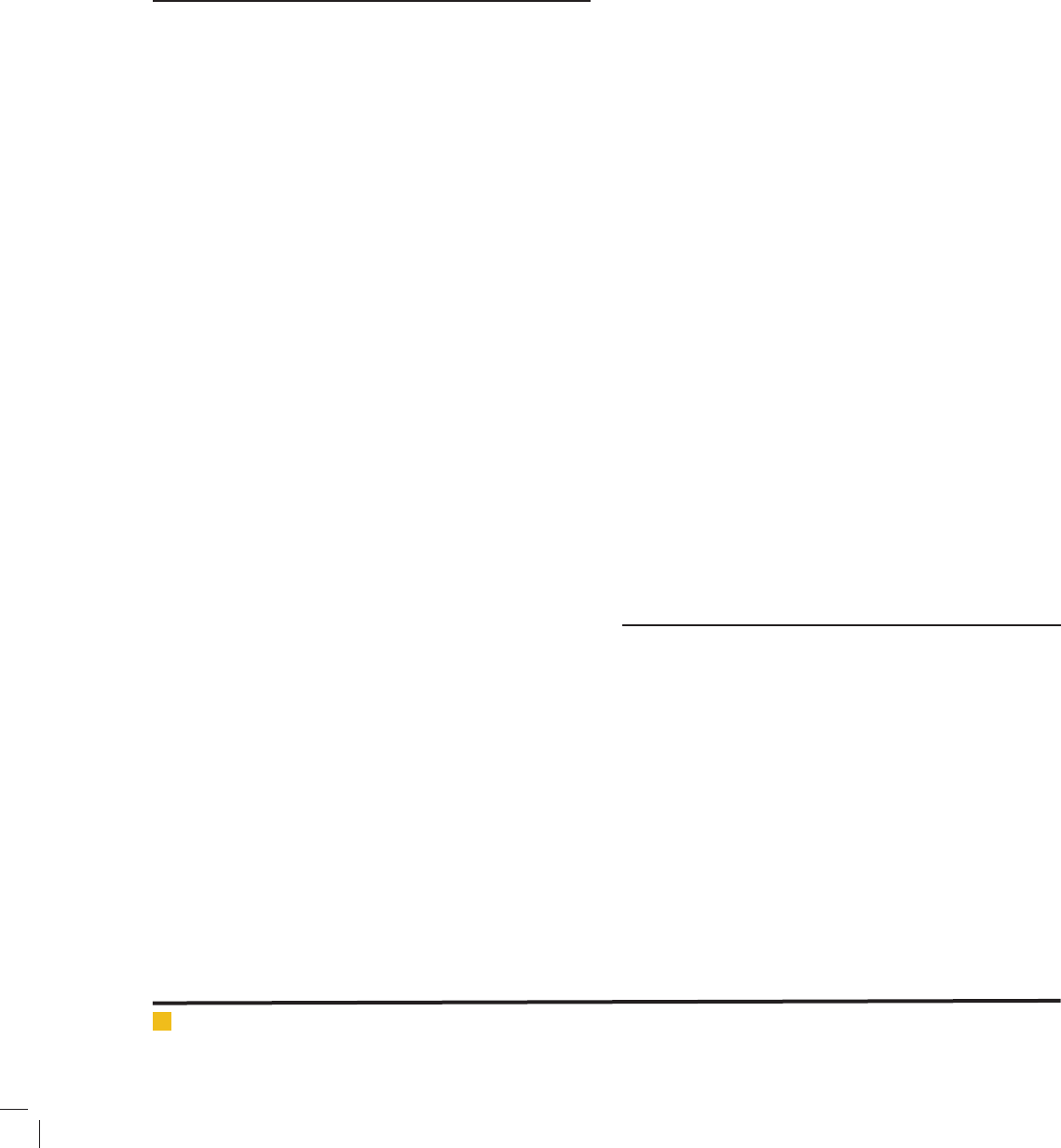
FIGURE 1. Gel electrophoresis of ITS-PCR prod-
ucts of six candida species, L is 50 bp molecular
marker, well 1) C. guilliermondii, well 2) C. par-
apsilosis, well 3) C. glabrata, well 4) C. krusei, 5)
C. albicans, well 6) C. tropicalis.
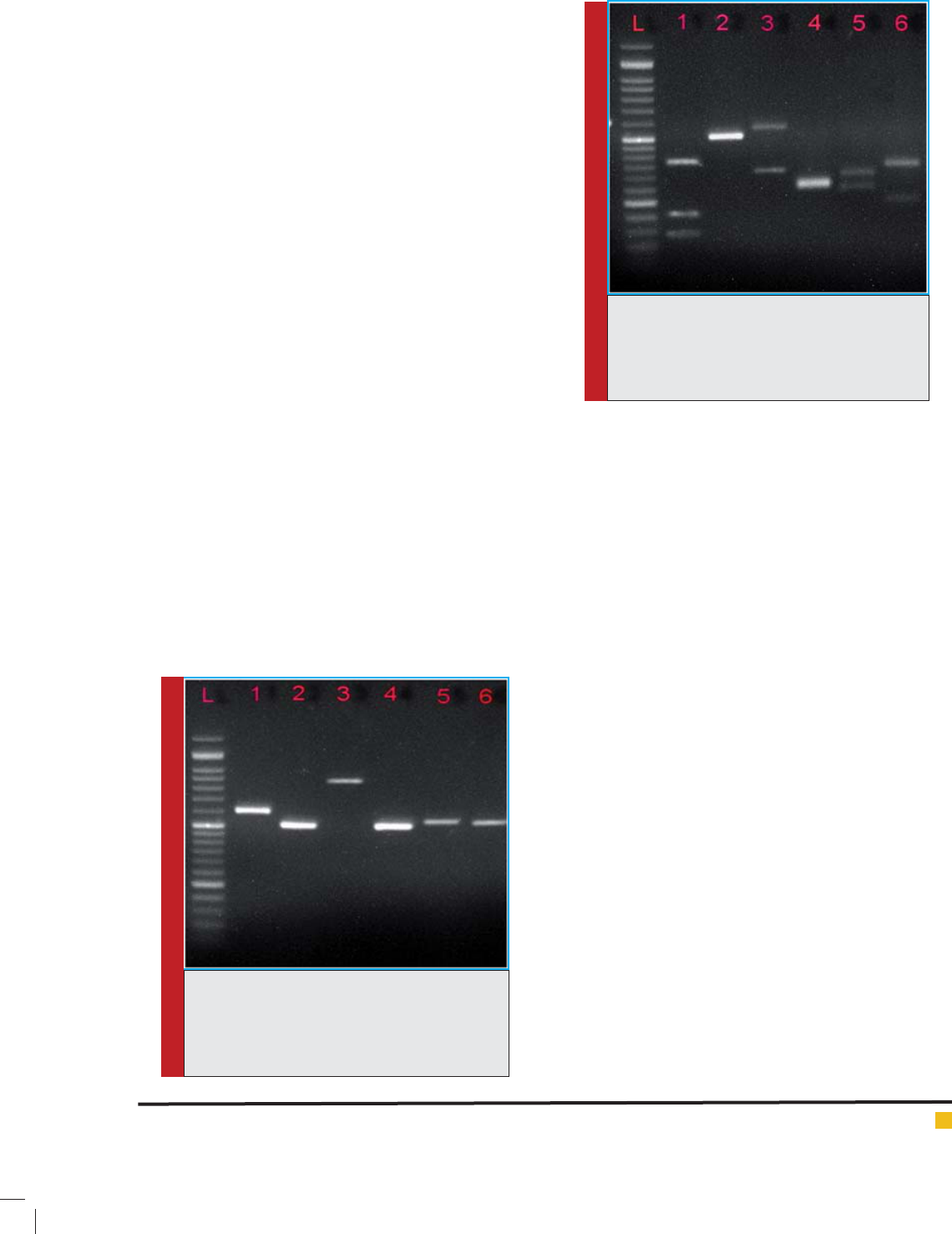
FIGURE 2.PCR-RFLP electrophoresis pattern
with the HpaII enzyme on Agarose gel, L is 50
bp molecular marker, well 1) C. guilliermondii,
well 2) C. parapsilosis, well 3) C.glabrata, well 4)
C. krusei, well 5) C. albicans, well 6) C. tropicalis.
were examined macroscopically. For the green samples
testing of Candida albicans/dubliniensis, chlamydocan-
idia and mycelium forms production using corn meal
agar medium containing Tween 80 for 48 hours at 30 °
C was carried out.To differentiate albicans species from
dubliniensis the test of chlamydoconidia production by
dubliniensis species in Niger seed agar medium and the
inability of C.albicans was used in the production of
chlamydoconidia in the mentioned medium.
The identi cation of Non-Albicans candidaisolates:
all of the yeasts that their colony color was not exclu-
sive to identify them in CHROMagar medium and iso-
lates that their color was blue and purple in CHROMagar
Candida agar medium were identi ed using PCR-RFLP.
This method is based on ampli cation of ribosomal DNA
fragments in ITS1-5.8S-ITS2 PCR method using univer-
sal primers ITS1, ITS4 and then digestion the PCR prod-
ucts ampli ed by restriction enzyme HpaII. ( gure 1, 2)
PREPARATION OF LIPOSOME
Fluconazole and cholesterol were purchased from Sigma
company, and soybean lecithin was purchased from Lip-
oid Company. Liposomal formulation uconazole was
prepared by the method of thin layer lm hydration (H.
Ola et al., 2010).
For the preparation of liposomes, lecithin, choles-
terol and uconazole were used with a ratio of 10: 1:
1. A thin layer was formed by solving these two sub-
stances in the organic solvent of chloroform-methanol
(1: 1) containing uconazole at a rate of 5.12mg / ml,
then evaporation of the solvent in the rotary evaporator
under the temperature of 45 ° C. Then, it was hydrated
by 9% sucrose slowly at 65 ° C. The homogenization
action of samples was performed by a homogenizer at
20000 rpm and ata temperature above the liposomes
phase transition (70) for 10 minutes. The liposomal sam-
ple sonication operation was carried out in an ice bath.
The amount of the drug that was not loaded, by the
amicon lter was removed by centrifugation at 4000 × g
for 4 minute and was passed for sterilization twice using
0/22 micron lter needle. This new formulation trans-
ferred to the laboratory and used freshly to evaluate the
antifungal activity.
BLANK LIPOSOMAL PREPARATION
To prepare this formulation, it was treatedin accordance
with the formulation of uconazole liposomal formula-
tions and the difference was that it was prepared with-
out uconazole.Determining the size of nanoliposomes
containing uconazole:Particle size, average diameter,
nanoliposome zeta potential distribution was measured
by zetasizer device based on laser light scattering. Par-
ticle size distribution in the device, was evaluated based
on PDI (Poly Dispersity Index).
NANOPARTICLE STRUCTURE
For this purpose, the scanning electron microscope
device was used. That a quantity of sample is placed on
a glass surface with dimensions of 1 x 1 cm. Then it was
placed inside an incubator at 37 ° C until the sample is
completely dry. After that the particles were coated with
gold and the images were taken with the magni cation
of 20,000 and 40,000.
636 IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Asadi Mehrdad et al.
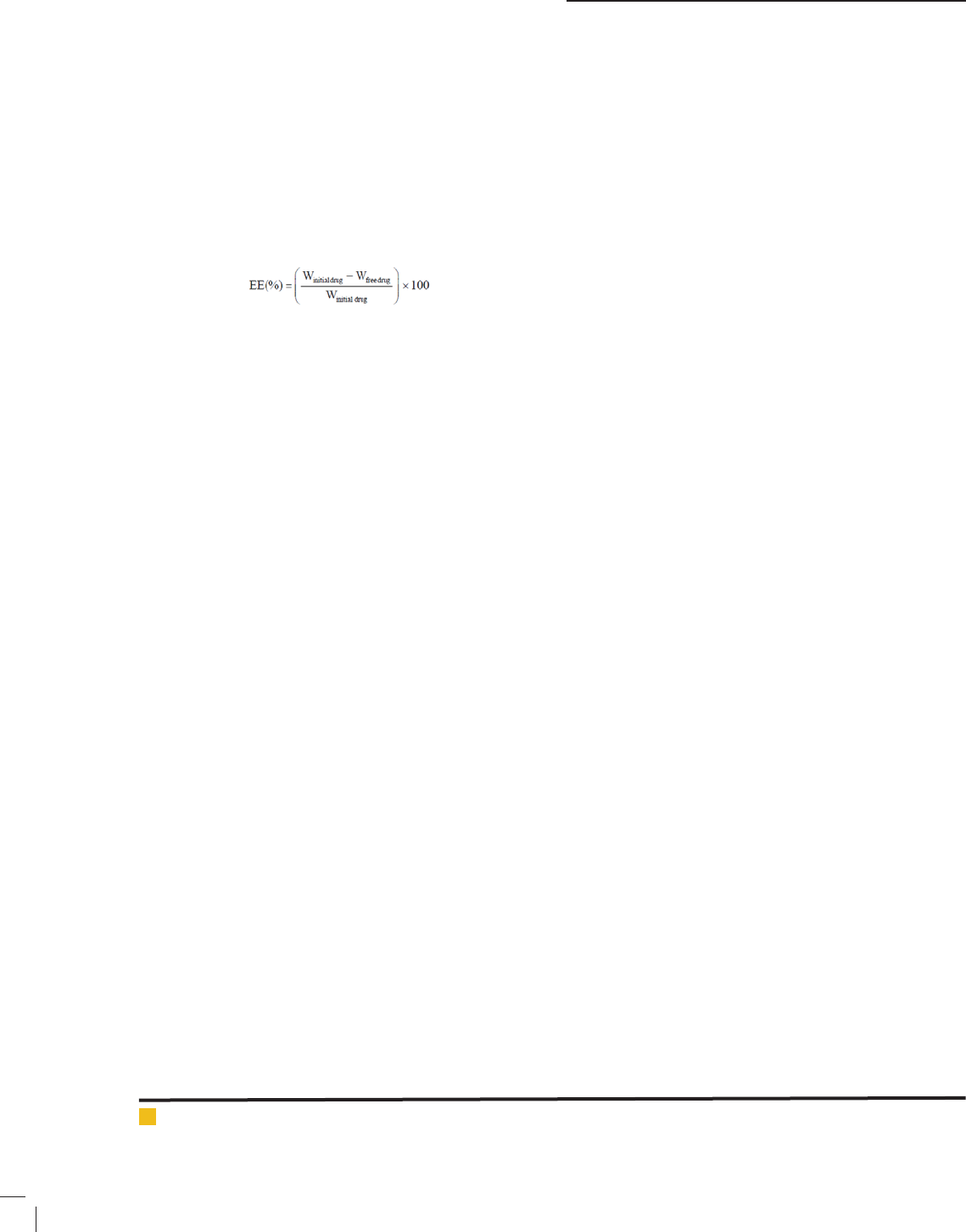
DRUG ENTRAPMENT EFFICIENCY (DE)
To check the encapsulated drug in the prepared liposomal
formulations, the maximum absorption of uconazole
in the UV, 255 nm was obtained. Then, the calibration
curve was drawn, and a speci c amount of formulation
is placed above the amicon lter and centrifuged for
5 minutes at 4 ° C and 4000 × g. A certain amount of
free drug passes on the underside of Falcon removed by
pipette and unloaded uconazole absorption was meas-
ured by spectrophotometer at a wavelength of 255 nm,
and the percent of uconazole loading was obtained by
the following formula:
RESULTS AND DISCUSSION
Eighty-seven positive cultures of yeast isolated from
cutaneous, mucosal and systemic fungal lesions of
patients referring Razi hospital in Tehran, Sina Hospital
in Tabriz city and another private lab in Tabriz were
collected. Due to the fact that more than one type of
yeast wereisolated from some patient samples, the total
number of 91 yeast colonies were isolated and tested in
the next steps. The relevant results and methods of iden-
tifying can be seen in Table 1.
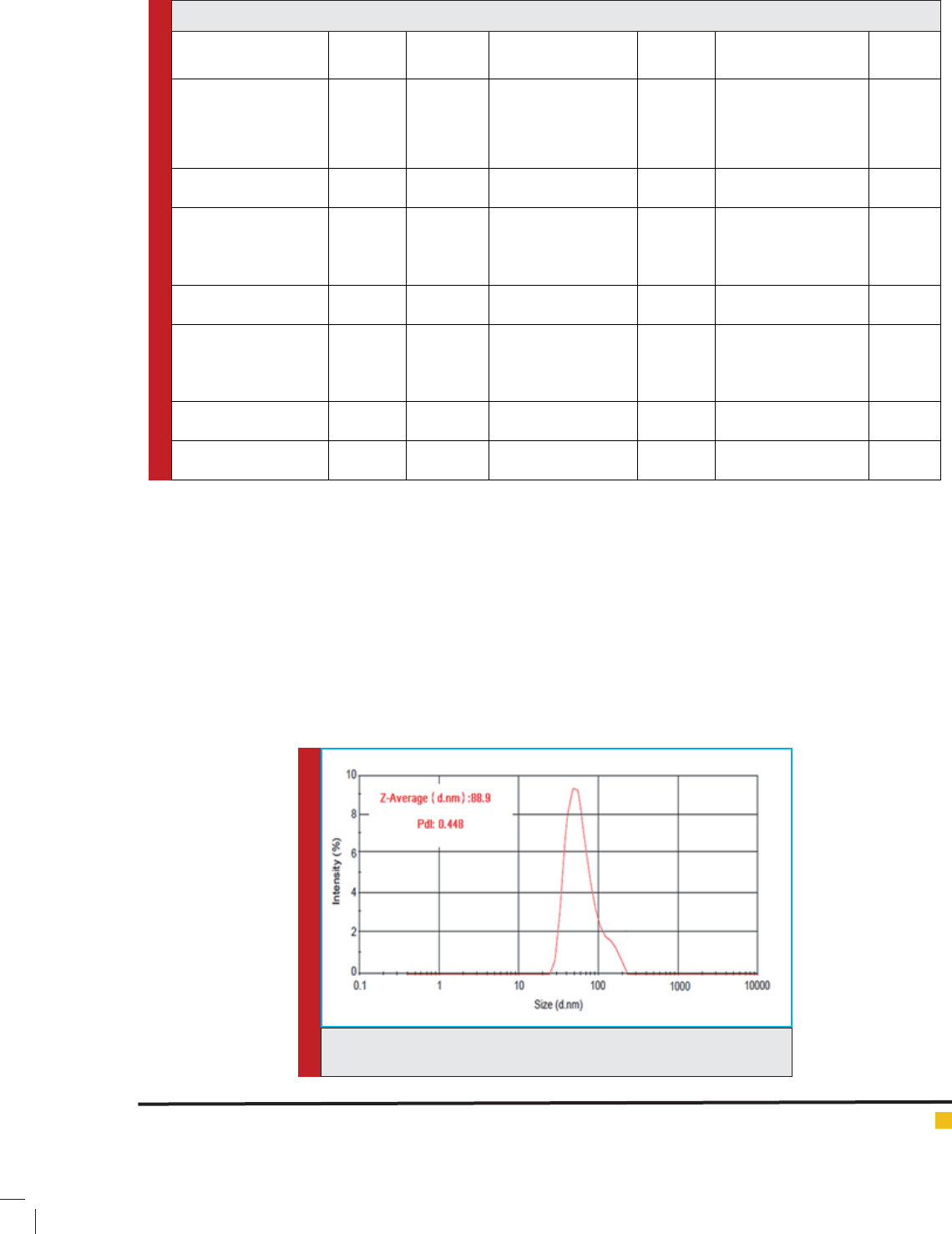
The particle size of liposomal nanoparticles: In the
present study, our goal was to obtain an ideal formula-
tion in terms of particle size and the uconazole encap-
sulation amount for the evaluation of the antifungal
effects and its comparison with the normal form of
the drug, which is reached. As can be seen, the particle
size of liposome containing uconazole is 88.9± 12.1
nm(Figure 3). Zeta potential for the uconazole lipo-
some formulation is -12.20± 1.88mv (Figure 4).
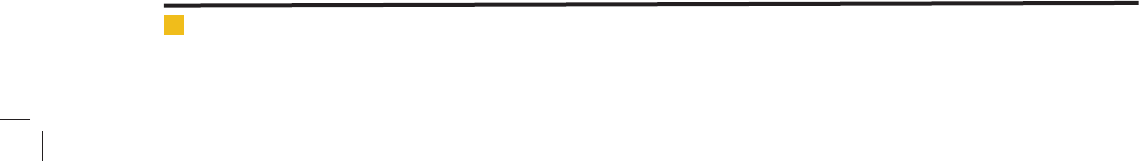
The SEM results for the nanoliposomes contain-
ing uconazole, to study the structure and form of the
nanoliposomes the scanning electron microscope was
used. The results are presented in Figure 5,6. Figure 5 is
captured with a lower magni cation and to show a uni-
form distribution of particle size and Figure 6 is captured
with a higher magni cation to better show the structure
and form of the nanoliposomes, which are spheral.
The amount of uconazole loaded in the liposomal
formulation is obtained as 75.1% in accordance with the
aforementioned method.
ANTIFUNGAL SUSCEPTIBILITY TESTING
Table 2 represents the susceptibility of the examined
Candida species to the uconazole and nanoliposomal
uconazole medicines.In the present study, the produced
liposomal formulation for more loading of the ucona-
zole drug as well as its antifungal effects on the u-
conazole susceptible and resistant Candida species iso-
lated from patients in the current form of uconazole
were created.Poly dispersity index or PDI of the prepared
nanoparticles:
The results of the measurements using malvernzeta-
sizer indicated that the formulation had a narrow par-
ticle distribution domain and the samples prepared to
apply this method had a proper PDI and particle dis-
tribution. About the examination of the results of poly
dispersity index or PDI of the prepared nanoparticles,it
can be said that the lower the PDI, the lower the distri-
bution of particle sizes is and the system will be more
homogeneous. Generally, if PDI is in the range of 0 to
0.5, the suspension is mono disperse, and if it is higher
than 0.5, it is poly disperse.
ANTIFUNGAL SUSCEPTIBILITY TESTING
In this study, the standard method of Broth Microdilu-
tion described in CLSI M27-A3-S4 is presented and the
standard quality control of C. parapsilosis ATCC 22019
was used to evaluate the MIC of uconazole and u-
conazole nanolipososmal formulation (J.H. Rex et al.,
2008).
To perform this test, 96-well microplateswere used. In
order to dilute the uconazole stock solution, we used
the growth medium RPMI1640 (with glutamine, with-
out bicarbonate and buffered to pH 7.0). To prepare this
medium, we solved 10.43 grams of RPMI powder and
34.53 grams of MOPS buffer (N-MorpholinoPropane-
sulfoni Acid) in one liter of distilled water with gentle
shaking on the ame. We used normal saline for dilu-
tion of the Nanoliposomes containing uconazole. In
the case of the two drugs, after adding to each drug the
yeast cell suspension which had been prepared accord-
ing to CLSI M27-A3 protocol and contained 2.5×10
3
to
0.5 × 10
3
cfu/ml of yeast, we obtained the highest and
lowest nal concentrations as 64μg / ml and 0.063μlg
/ml respectively. The well No. 12 was used as posi-
tive control. Each of the isolates was examined as dual
series.
After 48 hours of incubation, to determine the Mini-
mum Inhibitory Concentration (MIC) of uconazole and
nanoliposomal uconazole that the fungus growing
inside were not observed in the microplates and 80% of
its growth was blocked, examined and the results were
read.To ensure of the non-antifungal activity of nanoli-
posomes particles, blank nanoparticles liposome were
used in this test and no antifungal effect was observed.
We have used SPSS version 18 and Student’s T-test in
order to analyze and compare the antifungal effects of
the liposomal nanoparticles containing uconazole and
the free uconazole.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE 637
Asadi Mehrdad et al.
Table 1:
Identifying method Percent Quantity Species Total (%) Candida Species
(quantity)
Clinical
species
Chrome Candida Agar,
the production of
chlamydocanidia
61.4 56 Candida albicans 29 (31.8) Albicans (16),
parapsilosis (5),
tropicalis (3), glabrata
(1), krusei (2),
guilliermondii (2)
Fingernail
PCR-RFLP 16.5 15 Candida parapsilosis 11 (12) Albicans (6), parapsilosis
(3), tropicalis(2)
Toenail
Chrome Candida Agar,
PCR-RFLP
8.8 8 Candida tropicalis 15 (16.5) Albicans (9), parapsilosis
(3), tropicalis (2), krusei
(1)
Hand
PCR-RFLP 6.6 6 Candida glabrata 6 (6.6) Albicans (4),
parapsilosis(2)
Foot
Chrome Candida
Agar,PCR-RFLP
4.4 4 Candida krusei 19 (20.9) Albicans (15), glabrata
(3), krusei (1)
Vagina
PCR-RFLP 2.2 2 Candida guilliermondii 6 (6.6) Albicans (4),
parapsilosis(2)
Groin
100 91 Total 5 (5.5) Albicans (2), tropicalis
(1), glabrata (2)
BAL
FIGURE 3. Curve of Particle size distribution of liposomes containing
uconazole.
Zeta potential of the uconazole liposome formula-
tion:
Zeta potential is an important factor in determin-
ing the stability of the colloidal system and is the best
indicator to determine the status of super cial electrical
dispersions. Usually, cholesterol is used to improve the
stability of liposomes. Adding cholesterol increases the
zeta potential of the colloidal system; as the zeta poten-
tial increases in the particles, the particles will be more
stable, and on the other hand, adding cholesterol will
increase the rigidity of bilayer phospholipid (Mcnaught
and Wilkinson, 1997, Panyam and V. Labhasetwar,
2003).
In liposomal formulations, in the lipid composition of
which there is cholesterol in addition to the lecithin, zeta
potential was negative. About the stability of stearic and
electrostatic combined, a minimum of ±20 ZP is desir-
able (Tang et al., 2014). In a research by El-Nesr et al.
in 2010 on multilayer liposomes containing uconazole,
formulations with negative, neutral and positive zeta
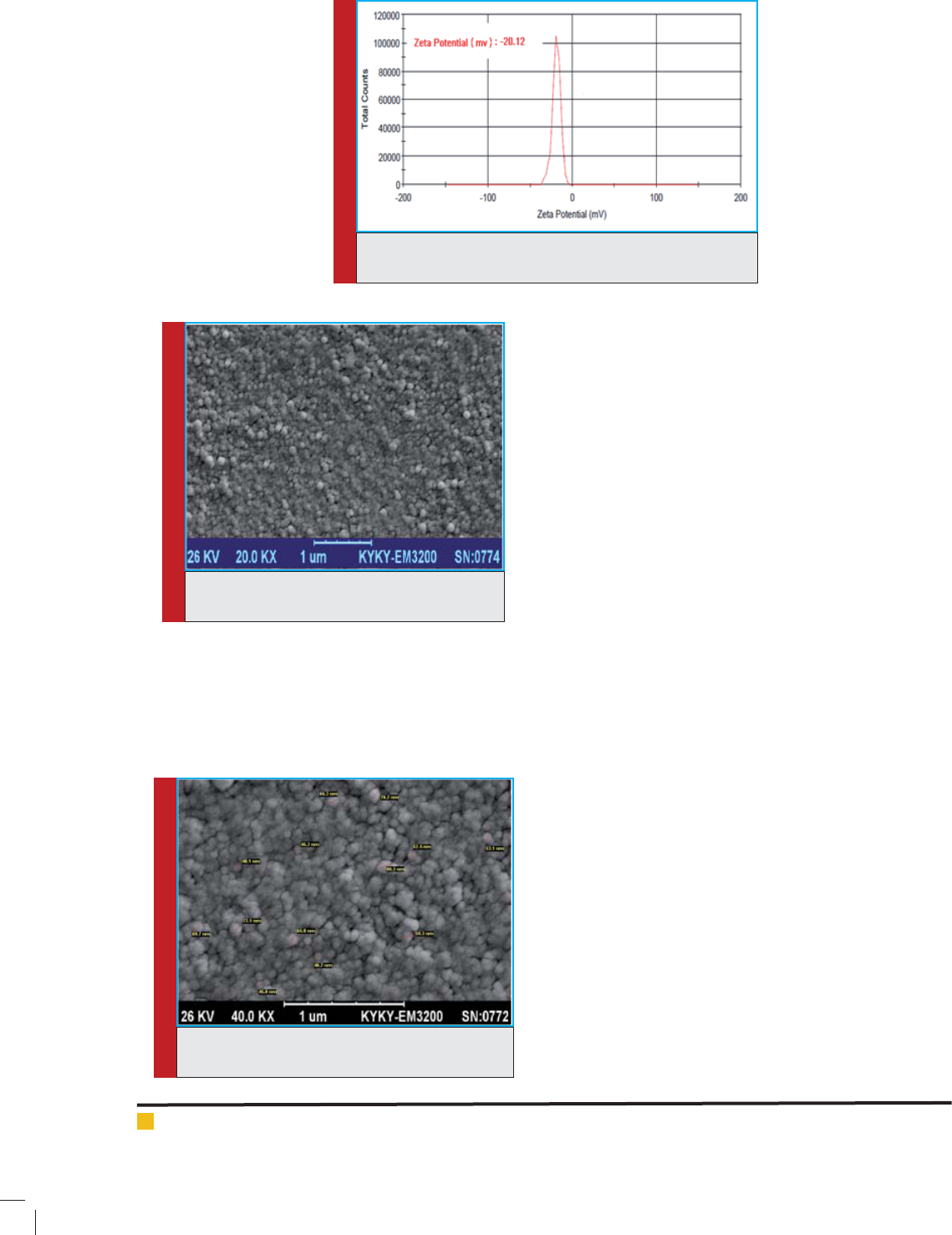
Asadi Mehrdad et al.
638 IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
the stearylamine particles in liposome and the slightly
negative charge of the uconazole in comparison with
the liposome particles with negative and neutral charge,
(Ola et al., 2010).
The amount of drug loaded in the liposomal formula-
tion of uconazole:
The amount of drug loaded in the liposomal formula-
tion was 75.11%. In fact, a signi cant amount of u-
conazole was encapsulated in the nanoliposomes, which
is due to the hydrophilic nature of uconazole that are
not willing to be present in the external phase and as a
result, a signi cant amount of uconazole is encapsu-
lated during the formation of nanoparticles.
The SEM results of nanoliposomes containing u-
conazole:
In the scanning electron microscope images, the
narrow range of particle distribution and the spherical
shape of the formed particles was clearly observable.
According to the results of SEM, the prepared particles
were in the same particle size range that was obtained
by nanozetasizer.
The comparison of the antifungal effects of ucona-
zole and liposomal nanoparticles containing ucona-
zole:
Table 2 shows the results of in vitro susceptibility of 6
susceptible and resistant Candida isolates to the ucona-
zole comparing to the uconazole containing nanolipo-
some. As can be seen in the table 2, the use of the lipo-
somal form of uconazole signi cantly decreased the
MIC of C.albicans species (P <0.0001), C.parapsilosis (P
<0.05), C.tropicalis (P = 0.0005), C.glabrata (P = 0.002)
and C.guilliermondii (P <0.05), but no difference was
observed in the amount of MIC of the two forms of drug
in the case of C.krusei.
The MIC
50
amount for C.glabrata was obtained as
8μg/ml, which had the highest amount of MIC relative
to the nanoliposomes containing uconazole among the
isolated Candida species of patients. The resistant strain
FIGURE 4. Curve of Zeta Potential of liposomes containing
uconazole.
FIGURE 5. Image of nanoparticles with lower
magni cation.
FIGURE 6. Image of nanoparticles with greater
magni cation.
potential were evaluated. This examination indicated
that the nanoliposomes having positive, neutral and
negative electric charge had the highest drug loading
amount, respectively, and this can be possibly related
to the electrostatic force between the positive charge of

Asadi Mehrdad et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE 639
to the uconazole of C.glabrata which has MIC equal to
64 μg/ml, had 4 times decrease in the MIC and placed in
the susceptible species after exposing to the uconazole
liposomal form. The amount of MIC
50
for the ucona-
zole susceptible strains of C.albicans, C.parapsilosis,
C.tropicalis, and C.guilliermondii against to the ucona-
zole liposomal form, were 1μg/ml, 1μg/ml, 1.5μg/ml and
2μg/ml, respectively. The amount of MIC
50
for the resist-
ant strains of C.albicans, C.parapsilosis, C.krusei were
3μg/ml, 4μg/ml and 64 μg/ml, respectively.
There is a high possibility that the reason for the
low amount of MIC in the uconazole resistant Can-
dida strains relative to the nanoliposomal uconazole
is related to the identi ed resistance mechanisms of
pathogenic fungi to drugs; the mechanisms that make
fungi survive in the vicinity of a series of toxic sub-
strates as well as antifungal drugs( Mishra et al., 2007,
Morschhäuser, 2010). Various molecular mechanisms
have been identi ed in the development of strains resist-
ant to antifungal drugs (Franz et al., 1998), that these
mechanisms include reducing the transmission and dif-
fusion of the drug into the cell, changes in the enzymes
of the pathway of ergosterol Biosynthesis, changes in
the target enzyme (point mutations, expression increase
Table 2: In vitro antifungal susceptibility of all yeast isolates (n = 91)
Species/Antifungal
agent
MIC Range
MIC (μ/ml)
MIC50 MIC90 GM
C. albicans (30)
Fluconazole 0.5-4 1 4 1.33
LE-Fluconazole 0.063-4 0.125 2 0.21
C. albicans(26)*
Fluconazole 8-64 32 64 28.64
LE-Fluconazole 0.063-8 4 8 4.34
C. parapsilosis(13)
Fluconazole 0.25-4 1 2 0.89
LE-Fluconazole 0.063-0.5 0.125 0.45 0.13
C.parapsilosis(2)*
Fluconazole 8-32 20 -- 16
LE-Fluconazole 2-4 3 -- 2.82
C.tropicalis(8)
Fluconazole 0.5-2 1.5 -- 1.29
LE-Fluconazole 0.125-0.5 0.25 -- 0.27
C. glabrata (5)
Fluconazole 4-16 8 -- 6.96
LE-Fluconazole 1-8 2 -- 2
C.glabrata(1)*
Fluconazole 64 64 -- --
LE-Fluconazole 8 8 -- --
C.krusei (4)*
Fluconazole 64 64 -- 64
LE-Fluconazole 64 64 -- 64
C.guilliermondii(2)
Fluconazole 2 2 -- 2
LE-Fluconazole 0.125 0.125 -- 0.125
C.parapsilosis 22019
Fluconazole 0.5 0.5 -- --
LE-Fluconazole 0.125 0.125 -- --
*Percent resistance using interpretive breakpoint criteria of the CLSI(M27-A3s4); Fluconazole
resistance C. albicans, C.parapsilosis ≥ 8 μg/ml and C.glabrata≥ 64 μg/ml MIC(minimum
inhibitory concentration), GM (Geometricmean)

Asadi Mehrdad et al.
640 IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
and gene change) and the increase of drug release to
the outside by membrane diffusion pumps, activation of
the enzymes that break down drugs outside the cell wall
(Favre et al., 1999, Hitchcock et al., 1990, Li et al., 2004,
Löf er et al., 1997, Sanglard and Odds, 2002, White
et al., 1998).
From the mentioned cases, the most important reason
for the resistance of Candida to the azole drugs is related
to the expression of the high amount of transporter
membrane proteins that lead to the pumping of azoles to
the outside of the cell. The result is the reduction of drug
concentration in cell and in this case, the drug concen-
tration inside the cell is under the necessary level needed
for creating the inhibitory effect on Erg11p (Caira et al.,
2004). Two main types of drug release pumps from the
fungus cells causes their resistance to drugs: ABS and
MFS (Lamping et al., 2010). Cdr1 and Cdr2 are among
the important transporters of the drug to the outside of
the cell in C.albicans, C.glabrata and C.parapsilosis and
the increase in their expression leads to the release of
drug from the cell, which nally leads to the resistance
of mentioned species to the uconazole (White et al.,
2002, Bennett et al., 2004 and Souza et al., 2015).
In the examination conducted by us, the lipid nano-
particles are fused quickly with the cell membrane of
yeast due to the hydrophobic outer surface, and as they
are small in terms of size, they are capable of pene-
trating to the yeast cell and causes the drug protection
against enzymes and ef ux pumps. Also, the lipid drug
delivery systems due to the use biodegradable lipids are
low toxic, have the ability to get lyophilized (Garud et
al., 2012) and are capable of controlled release of the
drugs, (Bose et al., 2013).
In a recent study conducted by Moazeni et al. in 2016,
using lipid nanoparticles against uconazole suscepti-
ble and resistant species in vitro, the results indicated
the satisfying effect of lipid nanoparticles contain-
ing uconazole on the resistant strains of C.albicans,
C.parapsilosis and C.glabrata (M. Moazeni et al., 2016).
Similarly, Gupta et al. had observed that the use of
drug delivery system can lead to the increase of u-
conazole penetration from the skin surface (Gupta et al.,
2013). As the uconazole prevents the synthesis of ergos-
terol by affecting the -demethylase14 enzyme, in the
investigations of Venkateswarlu et al. it was determined
that the -demethylase14 existing in the C.krusei differs
the -demethylase14 of C.albicans (Venkateswarlu et al.,
1997, Venkateswarlu et al., 1996).
Studies by Orozco et al. indicated that the intra-
cellular accumulation of uconazole of C.krusei and
C.albicans are similar (Orozco et al., 1998). This in turn
could explain the reason why C.krusei was resistant to
the uconazole exposure as well as the form of ucona-
zole nanoparticles.
CONCLUSION
In the current study, the function of a new drug delivery
system was evaluated on the uconazole resistant and
susceptible Candida strains isolated from the patients.
Although different mechanisms of resistance to ucona-
zole are expressed, it seems that the resistance is due to
the pumps releasing drug from the cell and it is one of the
main reasons for these resistances. Therefore, it is likely
that we can use the new system for drug delivery to pre-
vent drug release from the cell. In addition, this is the rst
research using uconazole lipid nanoparticles against
C.krusei.
REFERENCES
A ggarwal, N. and S. Goindi (2013) Preparation and in vivo
evaluation of solid lipid nanoparticles of griseofulvin for der-
mal use. Journal of biomedical nanotechnology Vol. 9 No 4:
Page 564-576.
B ennett, J.E., K. Izumikawa and K.A. Marr (2004) Mechanism
of increased uconazole resistance in Candida glabrata during
prophylaxis. Antimicrobial Agents and Chemotherapy Vol. 48
No 5: Page 1773-7.
B ose, S., Y. Du, P. Takhistov and B. Michniak-Kohn (2013) For-
mulation optimization and topical delivery of quercetin from
solid lipid based nanosystems. International Journal of Phar-
maceutics Vol. 441 No 1-2: Page 56-66.
C aira, M.R., K.A. Alkhamis and R.M. Obaidat (2004) Prepara-
tion and crystal characterization of a polymorph, a monohy-
drate, and an ethyl acetate solvate of the antifungal ucona-
zole. Journal of Pharmaceutical Sciences Vol. 93 No 3: Page
601-11.
F alkiewicz-Dulik, M. and A. Macura (2008) Nanosilver as sub-
stance biostabilising footwear materials in the foot mycosis
prophylaxis. Mikologia Lekarska Vol. 15 No: Page 145-150.
F avre, B., M. Didmon and N.S. Ryder (1999) Multiple amino
acid substitutions in lanosterol 14-demethylase contribute to
azole resistance in Candida albicans. Microbiology Vol. 145 No
10: Page 2715-2725.
F ranz, R., S.L. Kelly, D.C. Lamb, D.E. Kelly, M. Ruhnke and
J. Morschhauser (1998) Multiple molecular mechanisms con-
tribute to a stepwise development of uconazole resistance in
clinical Candida albicans strains. Antimicrobial Agents and
Chemotherapy Vol. 42 No 12: Page 3065-72.
G arud, A., D. Singh and N. Garud (2012) Solid Lipid Nanopar-
ticles (SLN): Method, Characterization and Applications. Inter-
national Current Pharmaceutical Journal Vol. 1 No 11: Page
384-393.
G upta, M., S. Tiwari and S.P. Vyas (2013) In uence of various
lipid core on characteristics of SLNs designed for topical deliv-
ery of uconazole against cutaneous candidiasis. Pharmaceu-
tical development and technology Vol. 18 No 3: Page 550-559.
H itchcock, C.A., K. Dickinson, S. Brown, E. Evans and D.
Adams (1990) Interaction of azole antifungal antibiotics with

Asadi Mehrdad et al.
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE 641
cytochrome P-450-dependent 14-sterol demethylase puri ed
from Candida albicans. Biochemical Journal Vol. 266 No 2:
Page 475-480.
J enning, V., M. Schäfer-Korting and S. Gohla (2000) Vitamin
A-loaded solid lipid nanoparticles for topical use: drug release
properties. Journal of controlled release Vol. 66 No 2: Page
115-126.
L amping, E., P.V. Baret, A.R. Holmes, B.C. Monk, A. Goffeau
and R.D. Cannon (2010) Fungal PDR transporters: Phylogeny,
topology, motifs and function. Fungal Genetics and Biology
Vol. 47 No 2: Page 127-42.
L i, X., N. Brown, A.S. Chau, J.L. Lopez-Ribot, M.T. Ruesga, G.
Quindos, C.A. Mendrick, R.S. Hare, D. Loebenberg, B. Dido-
menico and P.M. Mcnicholas (2004) Changes in susceptibil-
ity to posaconazole in clinical isolates of Candida albicans.
The Journal of antimicrobial chemotherapy Vol. 53 No 1: Page
74-80.
L öf er, J., S.L. Kelly, H. Hebart, U. Schumacher, C. Lass-Flörl
and H. Einsele (1997) Molecular analysis of cyp51 from ucon-
azole-resistant Candida albicans strains. FEMS microbiology
letters Vol. 151 No 2: Page 263-268.
M cnaught, A.D. and A. Wilkinson (1997)Compendium of
chemical terminology (Vol. 1669). Blackwell Science,Oxford.
M ehnert, W. and K. Mader (2001) Solid lipid nanoparticles:
production, characterization and applications. Advanced Drug
Delivery Reviews Vol. 47 No 2-3: Page 165-96.
M endes, A.I., A.C. Silva, J.A. Catita, F. Cerqueira, C. Gabriel
and C.M. Lopes (2013) Miconazole-loaded nanostructured lipid
carriers (NLC) for local delivery to the oral mucosa: improving
antifungal activity. Colloids Surf B Biointerfaces Vol. 111 No:
Page 755-63.
M ishra, N.N., T. Prasad, N. Sharma, A. Payasi, R. Prasad, D.K.
Gupta and R. Singh (2007) Pathogenicity and drug resistance
in Candida albicans and other yeast species. A review. Acta
Microbiologica et Immunologica Hungarica Vol. 54 No 3: Page
201-35.
M oazeni, M., H.R. Kelidari, M. Saeedi, K. Morteza-Semnani,
M. Nabili, A.A. Gohar, J. Akbari, E. Lotfali and A. Nokhod-
chi (2016) Time to overcome uconazole resistant Candida
isolates: Solid lipid nanoparticles as a novel antifungal drug
delivery system. Colloids and S urfaces B: Biointerfaces Vol.
142 No: Page 400-407.
M ohammadi, F., P. Dehghan, S. Nekoeian and S.J. Hashemi
(2016) Determination of antifungal susceptibility patterns
among the environmental isolates of Aspergillus fumigatus in
Iran. Advanced biomedical research Vol. 5 No: Page.
M ohanty, B., D.K. Majumdar, S.K. Mishra, A.K. Panda and S.
Patnaik (2015) Development and characterization of itracona-
zole-loaded solid lipid nanoparticles for ocular delivery. Phar-
maceutical Development and Technology Vol. 20 No 4: Page
458-64.
M orschhäuser, J. (2010) Regulation of multidrug resistance in
pathogenic fungi. Fungal Genetics and Biology Vol. 47 No 2:
Page 94-106.
N eppelenbroek, K., N. Campanha, D.M.P. Spolidório, L.C. Spo-
lidorio, R. Seó and A.C. Pavarina (2006) Molecular ngerprint-
ing methods for the discrimination between C. albicans and C.
dubliniensis. Oral diseases Vol. 12 No 3: Page 242-253.
O la, H., S.A. Yahiya and O.N. El-Gazayerly (2010) Effect of
formulation design and freeze-drying on properties of ucon-
azole multilamellar liposomes. Saudi pharmaceutical journal
Vol. 18 No 4: Page 217-224.
O midi, B., S.J. Hashemi, M. Bayat and K. Larijani (2014) Bio-
synthesis of Silver Nanoparticles by Lactobacillus fermentum.
Bulletin of Environment, Pharmacology and Life Sciences Vol.
3 No: Page 186-192.
O rozco, A.S., L.M. Higginbotham, C.A. Hitchcock, T. Parkin-
son, D. Falconer, A.S. Ibrahim, M.A. Ghannoum and S.G. Filler
(1998) Mechanism of Fluconazole Resistance in Candida kru-
sei. Antimicrobial agents and chemotherapy Vol. 42 No 10:
Page 2645-2649.
P anyam, J. and V. Labhasetwar (2003) Biodegradable nanopar-
ticles for drug and gene delivery to cells and tissue. Advanced
Drug Delivery Reviews Vol. 55 No 3: Page 329-47.
P faller, M.A. (1995) Epidemiology of candidiasis. J Hosp Infect
Vol. 30 Suppl No: Page 329-38.
P rice, M.F., M.T. Larocco and L.O. Gentry (1994) Fluconazole
susceptibilities of Candida species and distribution of species
recovered from blood cultures over a 5-year period. Antimi-
crobial Agents and Chemotherapy Vol. 38 No 6: Page 1422-4.
R evankar, S.G., W.R. Kirkpatrick, R.K. Mcatee, O.P. Dib, A.W.
Fothergill, S.W. Redding, M.G. Rinaldi and T.F. Patterson
(1996) Detection and signi cance of uconazole resistance in
oropharyngeal candidiasis in human immunode ciency virus-
infected patients. Journal of Infectious Diseases Vol. 174 No
4: Page 821-7.
R ex, J.H., B. Alexander, D. Andes, B. Arthington-Skaggs, S.
Brown, V. Chaturveli, A. Espinel-Ingroff, M. Ghannoum, C.
Knapp and M. Motyl (2008) Reference method for broth dilu-
tion antifungal susceptibility testing of lamentous fungi:
approved standard. Vol. No: Page.
S anglard, D. and F.C. Odds (2002) Resistance of Candida spe-
cies to antifungal agents: molecular mechanisms and clinical
consequences. Lancet Infectious Diseases Vol. 2 No 2: Page
73-85.
S chultz, S., D.R. Smith, J.J. Mock and D.A. Schultz (2000) Sin-
gle-target molecule detection with nonbleaching multicolor
optical immunolabels. Proceedings of the National Academy
of Sciences of the United States of America Vol. 97 No 3: Page
996-1001.
S ilva, S., M. Negri, M. Henriques, R. Oliveira, D.W. Williams
and J. Azeredo (2012) Candida glabrata, Candida parapsilosis
and Candida tropicalis: biology, epidemiology, pathogenicity
and antifungal resistance. FEMS Microbiology Reviews Vol. 36
No 2: Page 288-305.
S outo, E.B. and R.H. Muller (2005) SLN and NLC for topical
delivery of ketoconazole. Journal of Microencapsulation Vol.
22 No 5: Page 501-10.
Asadi Mehrdad et al.
642 IN VITRO EVALUATION OF ANTIFUNGAL EFFECTS OF NANOLIPOSOMAL FLUCONAZOLE BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
S ouza, A.C., B.B. Fuchs, H.M. Pinhati, R.A. Siqueira, F. Hagen,
J.F. Meis, E. Mylonakis and A.L. Colombo (2015) Candida par-
apsilosis Resistance to Fluconazole: Molecular Mechanisms and
In Vivo Impact in Infected Galleria mellonella Larvae. Antimi-
crobial Agents and Chemotherapy Vol. 59 No 10: Page 6581-7.
T ang, X., H. Zhu, L. Sun, W. Hou, S. Cai, R. Zhang and F. Liu
(2014) Enhanced antifungal effects of amphotericin B-TPGS-
b-(PCL-ran-PGA) nanoparticles in vitro and in vivo. Interna-
tional journal of nanomedicine Vol. 9 No: Page 5403.
T hassu, D., M. Deleers and Y.V. Pathak (2007)Nanoparticulate
drug delivery systems. CRC Press.
V enkateswarlu, K., D.W. Denning and S.L. Kelly (1997) Inhibi-
tion and interaction of cytochrome P450 of Candida krusei
with azole antifungal drugs. Journal of Medical and Veterinary
Mycology Vol. 35 No 1: Page 19-25.
V enkateswarlu, K., D.W. Denning, N.J. Manning and S.L.
Kelly (1996) Reduced accumulation of drug in Candida krusei
accounts for itraconazole resistance. Antimicrobial Agents and
Chemotherapy Vol. 40 No 11: Page 2443-6.
V erma, V., R. Kharwar and A. Gange (2009) Biosynthesis of
noble metal nanoparticles and their application. CAB Reviews:
Perspectives in Agriculture, Veterinary Science, Nutrition and
Natural Resources Vol. 4 No 026: Page 1-17.
W hite, T.C., S. Holleman, F. Dy, L.F. Mirels and D.A. Stevens
(2002) Resistance mechanisms in clinical isolates of Candida
albicans. Antimicrobial Agents and Chemotherapy Vol. 46 No
6: Page 1704-13.
W hite, T.C., K.A. Marr and R.A. Bowden (1998) Clinical, cel-
lular, and molecular factors that contribute to antifungal drug
resistance. Clinical microbiology reviews Vol. 11 No 2: Page
382-402.
W ingard, J.R. (1995) Importance of Candida species other than
C. albicans as pathogens in oncology patients. Clinical Infec-
tious Diseases Vol. 20 No 1: Page 115-125.
Y adav, N., S. Khatak and U.V.S. Sara (2013) Solid lipid nano-
particles-a review. International Journal of Applied Pharma-
ceutics Vol. 5 No 2: Page 8-18.
Z ur Muhlen, A., C. Schwarz and W. Mehnert (1998) Solid
lipid nanoparticles (SLN) for controlled drug delivery-
-drug release and release mechanism. European Journal of
Pharmaceutics and Biopharmaceutics Vol. 45 No 2: Page
149-55.