Physiological
Communication
Biosci. Biotech. Res. Comm. 9(3): 341-348 (2016)
Cognitive type susceptibility in ICR mice model of
chronic cerebral hypoperfusion.
Sirilak Somredngan
1
and Wachiryah Thong-asa
1
*
1
Physiology Division, Department of Zoology, Faculty of Science, Kasetsart University, Bangkok, Thailand.
ABSTRACT
Animal model of chronic cerebral hypoperfusion (CCH) was found to be bene cial as pathophysiological provided
relevance to vascular dementia (VD) and subcortical ischemic vascular dementia (SIVD). There were many evidence
kinds of CCH animal model and all were useful. The present study investigated the pathophysiology of mild CCH in
ICR mice during 2 to 8 weeks of permanent right common carotid artery occlusion Sensorimotor, cognitive abilities
and anxiety-like behavior were assessed in Morris water maze (MWM) and elevated plus maze (EPM), respectively.
Frontal cortex, striatum and hippocampus infarctions were evaluated using 2% 2,3,5-triphenyltetrazolium chloride
(TTC) at 2, 4 and 8 weeks of permanent right common carotid arteryocclusion. Sensorimotor, spatial learning and
memory in the acquisition paradigm of the MWM and anxiety-like behavior assessed in the EPM were not affected
during the experimental period of 2, 4 and 8 weeks of arterial occlusion.Signi cant de cit of learning exibility and
memory of reverse platform location in the reversal paradigm were found, though reversible of learning exibility
de cit presented at 8 weeks. Signi cant infarction early appeared in striatum since 4 weeks while the frontal cortex
and hippocampus stated at 8 weeks. The present study suggested that mild CCH induced by permanent right com-
mon carotid artery occlusion in ICR mice induced reversible learning exibility de cit but not memory of the reverse
platform location in the MWM.
KEY WORDS: CHRONIC CEREBRAL HYPOPERFUSION, ELEVATED PLUS MAZE,LEARNING FLEXIBILITY, SPATIAL LEARNING AND MEMORY.
341
ARTICLE INFORMATION:
*Corresponding Author: fsciwyth@ku.ac.th
Received 20
th
Aug, 2016
Accepted after revision 7
th
Sep, 2016
BBRC Print ISSN: 0974-6455
Online ISSN: 2321-4007
Thomson Reuters ISI ESC and Crossref Indexed Journal
NAAS Journal Score 2015: 3.48 Cosmos IF : 4.006
© A Society of Science and Nature Publication, 2016. All rights
reserved.
Online Contents Available at: http//www.bbrc.in/
INTRODUCTION
Animal model of chronic cerebral hypoperfusion (CCH)
can be induced by permanent ligation of major cerebral
arterial supplies. Severity of pathological appearances
depended on the number of vessel occlusion and persis-
tent of cerebral reduction. CCH study in animal model
is associated with cerebral blood ow (CBF) reduction,
metabolic insuf cient, oxidative stress, neuroin am-
mation, neurotransmitter system dysfunction, mental
confusion, cognitive decline, white matter and neu-
ronal degeneration. These pathological relevancies of
342 COGNITIVE TYPE SUSCEPTIBILITY IN ICR MICE OF CCH MODEL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Sirilak Somredngan and Wachiryah Thong-asa
CCH model were variable due to the difference of vessel
occlusion type and animal strain. Among rodent strain,
the difference in susceptibility has been reported, (Reid
et al. 2010). The rapid onset and signi cant pathophysi-
ological appearance clearly revealed in permanent bilat-
eral common carotid artery occlusion (BCCAO) model.
BCCAO model has provided bene cial data of causative
role played by cerebral hypoperfusion in neurodegen-
erative diseases and can be further useful in neuropro-
tective and therapeutic researches, (Farkas et al. 2007
and Du et al. 2016).
Knowing of pathomechanism is relevant to vascu-
lar dementia (VD) and subcortical ischemic vascular
dementia (SIVD) using a milder model of CCH such as
unilateral carotid artery occlusion (UCO) model. There
was frequency used of right common carotid artery
occlusion (rCCAO), and it revealed useful data of CCH
outcome both in rats and mice. Pathological relevance
for instance signi cant reduction of ipsilateral CBF,
activation of pro-in ammatory cytokine (IL-1, TNF-
), inhibition of anti-in ammatory cytokine (IL-4, 10),
downregulation of A1 adenosine receptors, white matter
damage, hippocampal neuronal degeneration,and corre-
lation with cognitive impairments such as spatial learn-
ing and memory (Yoshizaki et al. 2008, Thong-asa et al.
2013, Thong-asa and Tilokskulchai 2014, Cheng et al.
2015, Thong-Asa 2015).
Reversible of cognitive de cit was reported in short-
term but not long-term study of rCCAO without neu-
ronal degeneration correlation (Thong-asa et al. 2013,
Thong-asa and Tilokskulchai 2014, Thong-Asa 2015).
Cognitive ability outcomes from rodent assessed in cog-
nitive tasks might improve by repetitive test, previous
experience and compensatory mechanisms of cerebral
ischemia in CCH model were also suggested. Data com-
paring from rats and mice rCCAO model, focus only on
cognitive which varied due to the difference of maze
paradigm, repetitive test or previous experience and
effect of global ischemia on behavioral measures of
emotion, locomotion as well as habituation(Choy et al.
2006, Coyle and Panzenbeck 1990, Dellu et al. 1997,
Kim et al. 2008). Rats exhibited more susceptibility than
mice as more spatial ability de cits were found since 6
days of rCCAO(Thong-asa et al. 2013) but it was only 4
weeks in mice, (Cheng et al. 2015).
There are reports about global cerebral ischemia
induced hyperactivity, anxiety and locomotion which
might help reversible of cognitive ability de cit as
well(Milot and Plamondon 2009, Plamondon and Khan
2005). It is interesting that strain of rat and mouse used
as CCH model provide differences of pathophysiologi-
cal outcome. It is important to clarify the pathomecha-
nism, susceptibility and neuropathology correlated with
behavioral de cit in each CCH rodent model. Regarding
the importance of basic knowledge, the present study
aimed to investigate the pathophysiologyof CCH induced
by permanent right common carotid artery occlusion
focus on ICR mice strain.
MATERIAL AND METHODS
Animals:
Thirty-six male ICR mice, 40 – 50 grams, were
obtained from the National Laboratory Animal Cen-
tre, Mahidol University, Salaya, Nakornprathom.Mice
were housed under 12h/12h light-dark cycle with well-
controlled temperature (23 ± 2
o
C), humidity (55 ± 5%)
with appropriated ventilation. Mice were allowed free
access to standard food pellets and RO water. The pre-
sent study was conducted in accordance with interna-
tionally accepted principles for laboratory animal use
and care of the European Community (EEC directive of
1986; 86/609/EEC) and the experimental protocol was
approved by theAnimal Ethics Committee, Kasetsart
University Research and Development Institute (KURDI),
Kasetsart University, Bangkok, Thailand (ID#OACKU
04559).
Experimental protocol:
In brief, mice were randomly
assigned to two main groups of Sham and unilateral
(right) common carotid artery occlusion (UCO). After
fasting, mice were anesthetized by sodium pentobarbi-
tal (45 mg/kg) intraperitoneal injection. After checking
their re exes, a skin incision was made on the midline
ventral neck, right common carotid artery was exposed,
and it was cleared from nerves and surrounding connec-
tive tissues then permanently occluded with silk suture.
After wound sutured, antibiotic was given intramuscular
injection and mice were placed under heat lamps and
blankets in a recovery chamber. Sham and UCO main
groups were further randomly divided into 3 experi-
mental groups based on the period of arterial occlusion
at 2, 4 and 8weeks.Evaluation of sensorimotor, cogni-
tive abilities usingthe Morris water maze (MWM), anx-
iety-like behavior in the elevated plus maze(EPM), and
infarction volume of brain tissues were conducted at
these period as well.
Sensorimotor and cognitive abilities evaluation in the
Morris water maze:
The Morris water maze was a 150
cm diameter plastic pool and 50 cm tall. It was lled
with 30 cm depth of water (25
o
C). Prior to the cognitive
tests, the sensorimotor evaluation was done in order to
assess visual and motor abilities. Sensorimotor test was
conducted using the visible platform paradigm (cue test).
Brie y, a visible platform was placed and clearly seen-
above the water surface about 2 cm. Mice were given
four trials to swim, search, climb and sit on the visible
platform. The maximum time for each trail was 120 sec-

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COGNITIVE TYPE SUSCEPTIBILITY IN ICR MICE OF CCH MODEL 343
Sirilak Somredngan and Wachiryah Thong-asa
onds. The swimming speed of each group was compared
for sensorimotor evaluation. On the following day, spa-
tial learning was tested and continued for ve consecu-
tive days as the acquisition trial. Brie y, the pool was
divided into four quadrants: northeast (NE), northwest
(NW), southeast (SE), and southwest (SW) on the com-
puter monitorthat was connected to a ceiling camera.
The hidden platform was placed under the water surface
about 2 cm in the center of the NW quadrant (the target
quadrant of acquisition trial).
A variety of visual cues were placed outside and
around the pool. Mice were continuously given four tri-
als a day with 120 minutes maximum timeof each trial.
For ran out time case, mice were guided to the hidden
platform by the experimenter. When the acquisition
trial was completed, the probe trial began in order to
determine spatial memory capacity. The hidden platform
was removed from the target quadrant, and mice were
allowed to swim for 60 seconds, then the time spent in
each quadrant was recorded. The time spent in the target
quadrant was then further converted to percentage of
time spent in the target quadrant and represented as spa-
tial memory capacity. After nish acquisition trial and
probe, learning exibility was continuously assessed in
the reversal trial for three consecutive days. The only
difference from the acquisition trial was the switching of
hidden platform to the opposite quadrant (SE). The probe
trial was delivered on the last test day as well in order to
assess memory capacity of reverse platform location. All
data were record by using Smart
©
3.0.04 (Planlab/Har-
vard Apparatus).
Anxiety-like behavior assessment in the elevated plus
maze:
After nishing the cognitive tests in the MWM,
the anxiety-like behavior was further evaluated. The
EPM was a cross shape maze comprised of two open
(25x5x0.5cm) and two closed (25x5x16cm) arms with
a central platform (5x5x0.5cm). EPM was placed in dry
circular tank (normally used as the MWM) and about 40
cm above the oor. The EPM was started at 6.00 pm. by
transferring all mice into the experimental room 30 min
prior to the test. Illumination was maintained at 100 lux
in the experimental room. The testing of anxiety-like
behavior was started when mice were placed on the cen-
tral platform facing to the closed arm. Mice were allowed
to move freely in the EPM for 5 min with continuously
video recording. Arm entries de ned as the center of
mass of the mouse enters the arm.The number of open
arm entries and duration were analyzed and served as
an index of anxiolytic behavior, (Komada et al. 2008).
Infarction area analysis: After nishing all behav-
ioral tests in each experimental period, all mice were
sacri ced by lethal dose of intraperitoneal injection of
sodium pentobarbital (>60 mg/kg). Brains were quickly
removed after decapitation, and brie y washed in cold
0.9 % normal saline solution (NSS) and cut with surgi-
cal blade to yield 2 mm of thickness. Brain pieces were
stained with 2 % 2, 3, 5-triphenyltetrazolium chloride
(TTC) at 37
o
C for 10 min. After staining with TTC, brain
pieces were kept in 10 % neutral buffer formalin (NBF)
for 24 h, then images were captured and analyzed.
Infarction area was calculated by using UTHSCSA Image
Tool 3.0by differentiated pale tissue area among reddish
areas. About TTC technique, colorless TTC was reduced
to a deep-red precipitate by dehydrogenases in the pres-
ence of NADH of viable tissue. The lethally damaged
cells do not retain these reactants, nonviable areas were
not stained and appear pale, while viable cells were stain
red(Fishbein et al. 1981).
Statistical analysis:
All data were interpreted as mean
± SEM., spatial learning ability and learning exibil-
ity (represented by escape latencies) were analyzed by
repeated-measure analysis of variance (ANOVA) fol-
lowed by Fisher’s PLSD post hoc test. Memory capac-
ity (% time spent in the target quadrant), anxiety-like
behavior index (represented by open arm entries and
duration), infarction area (represented by % infarction)
were analyzed by ANOVA followed by Fisher’s PLSD
post hoc test. Statistical signi cance was accepted at
p-value < 0.05.
RESULTS
Sensorimotor evaluation at 2, 4 and 8 weeks: All mice
exhibited normal ability of swimming, seeing and
climbing on the platform during cue test in the MWM.
Results indicated no signi cant difference of the swim-
ming speed (cm/min) at 2 (Sham 2W = 17.18±1.97, UCO
2W = 17.81±1.48, p > 0.05), 4 (Sham 4W = 19.25±0.99,
UCO 4W = 19.87±1.37, p > 0.05) and 8 (Sham 8W =
18.89±1.09, UCO 8W = 19.23±0.81, p > 0.05) weeks after
permanent right common carotid artery occlusion.
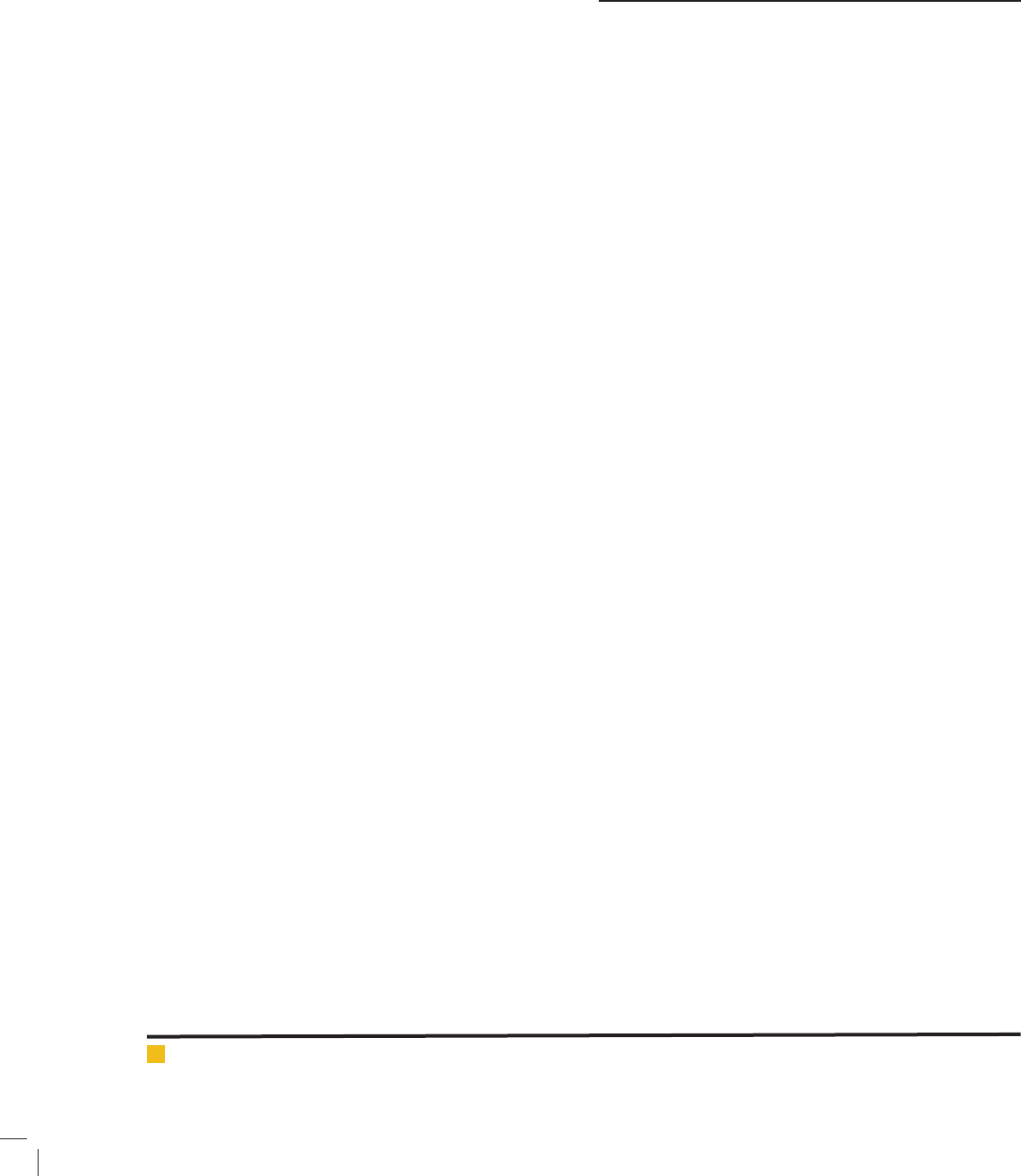
Spatial cognitions and learning exibility at 2, 4 and
8 weeks:
Swimming pathof acquisition and reversal tri-
als, the target selected quadrants, spatial learning abil-
ity, spatial memory capacity, learning exibility and
memory capacity of reverse platform location at 2, 4
and 8 weeks of right common carotid artery occlusion
showed in Fig.1. Spatial learning ability indicated by
the escape latency was not difference at all period of the
experiment (p > 0.05).Similar to spatial memory capac-
ity indicated by the percentage of time spent in the tar-
get quadrant of the acquisition probe (p > 0.05). These
results imply that the permanent right common carotid
artery occlusion for a period of 2, 4 and 8 weeks did not
induce de cit on spatial learning ability and memory
capacity of ICR mice CCH model.
344 COGNITIVE TYPE SUSCEPTIBILITY IN ICR MICE OF CCH MODEL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Sirilak Somredngan and Wachiryah Thong-asa
common carotid artery occlusion at 2, 4 and 8 weeks did
not induce difference in anxiety-like behavior (p > 0.05).
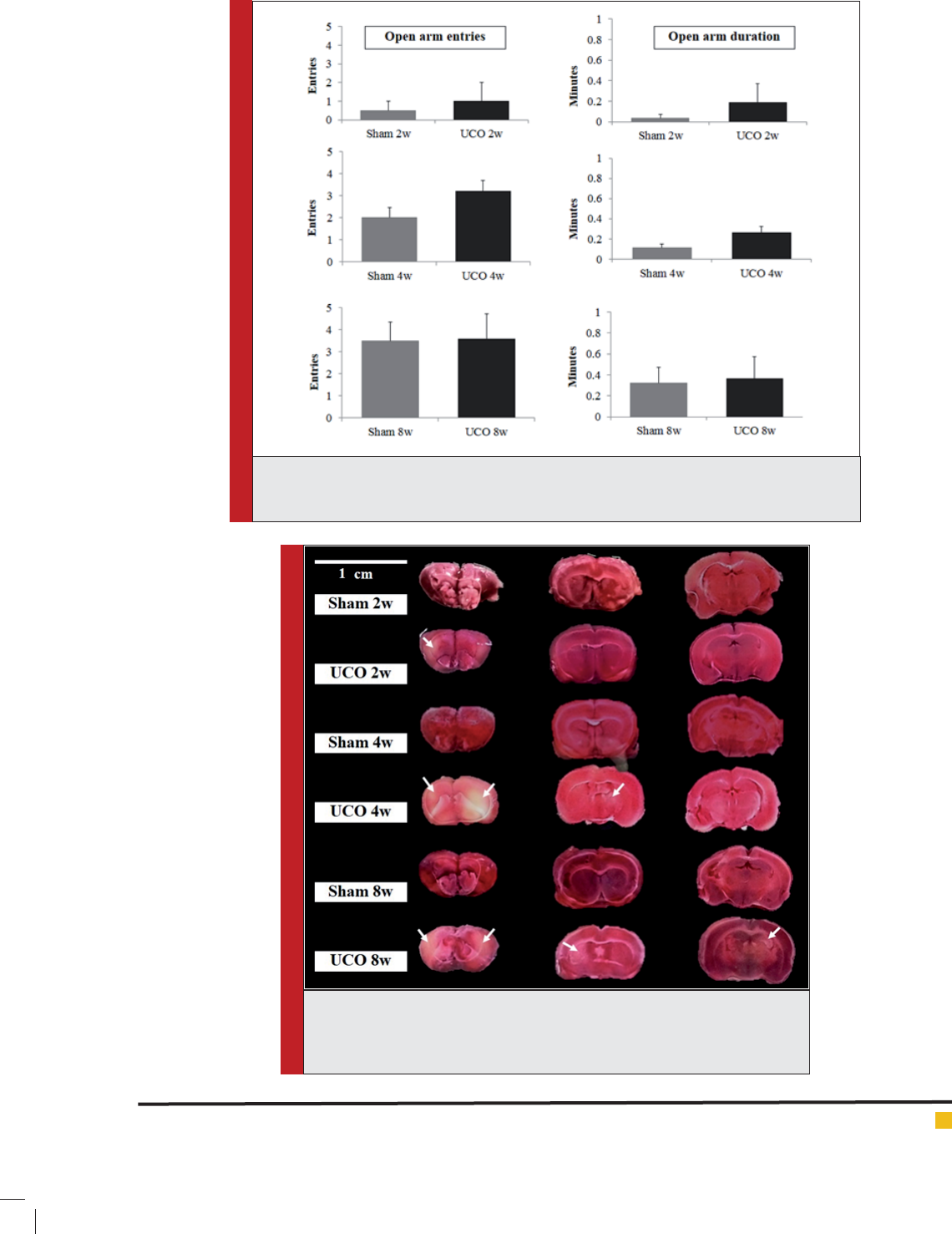
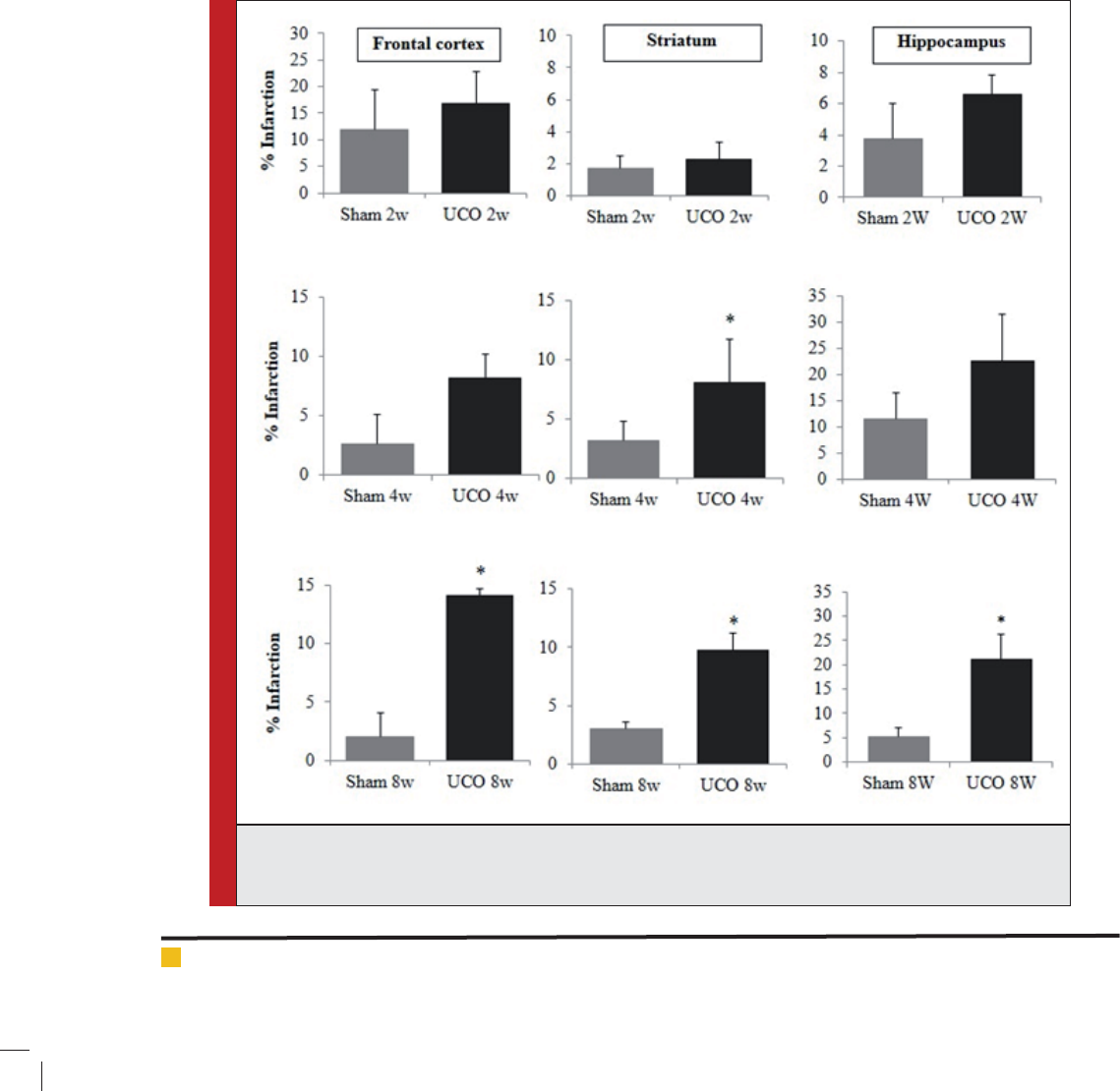
Infarction area at 2, 4 and 8 weeks: Percentage of infarc-
tion area in Fig. 3 indicated the signi cant difference
in the frontal cortex and hippocampal areas found at 8
weeks (p < 0.05). Striatum infarction found signi cantly
at 4 and 8 weeks of arterial occlusion (p < 0.05). Infarc-
tion areas from TTC staining in our present study sug-
gested about early appearance of infarction on striatum
in ICR mice CCH model.
DISCUSSION
The present study used ICR mice as CCH model induced
by permanent right common carotid artery occlusion-
and persistent from 2 to 8 weeks. This CCH model did
not induce spatial learning and memory de cits, but
revealed about cognitive type susceptible to CCH that-
was the learning exibility. The present study also
found reversible of exibility de cit but not the mem-
ory capacity of new platform location. Mice strain that
used as CCH model induced by permanent right commo n
carotid artery occlusion such as ICR and B57BL/6 gave
such differences of outcome. Using of ICR mice as the
present study revealed about CCH did not induce spa-
tial learning and memory de cits during 2 to 8 weeks,
but B57BL/6 mice the de cit of spatial memory capac-
ity appeared since 2 weeks and spatial learning de cit
found at 4 weeks, (Cheng et al. 2015).
It was not surprise because of B57BL/6 was the most
susceptible to global cerebral ischemia rather than ICR
or other mouse strains, based on neurological signs, his-
tological ndings, cortical microcirculatory and perfu-
sion patterns(Yang et al. 1997).There were reports about
the differences of pathophysiology and behavioral out-
come among mouse strains(Adams et al. 2002, Brosnan-
Watters et al. 2000). Not only strain difference, sex also
concern especially in the cognitive behavioral tests(Ge et
al. 2013). There was evidence indicated that locomotor
activities such as the basal open- eld activity of the ICR
was greater than that of the C57BL/6, the hippocampal-
dependent learning and memory such as novel object
was lower in the ICR and the strength of memory reten-
tion in the ICR mice was relatively weak (Kim et al.
2008). This evidence correlated with our present study
that the exibility of learning and memory retention
especially for the new platform location were impaired.
As the differences of brain circuit and mechanism of
cognitions, affected of cognitive functions depended on
location of circuit area andneuronal cell damage.
We suggest that cognitive type speci c factor that
early affected in ICR mice CCH model was the exibil-
ity.Adaptive behavior associated with prefrontal cortex-
FIGURE 1. Representation of swimming paths,
target quadrants, spatial learning and memory in
the acquisition paradigm, learning exibility and
memory in the reversal paradigm assessed in Morris
water maze (MWM); spatial learning ability and
learning exibility represented by escape latencies
(sec); memory capacity represented by the percent-
age of time spent in the target quadrant (%); at 2,
4 and 8 weeks of permanent right common carotid
artery occlusion, * p < 0.05.
The escape latency in the reversal trial(learning ex-
ibility) signi cantly increased in UCO group at 2 (p <
0.05) and 4 (p < 0.05)but not 8 weeks (p > 0.05) of per-
manent right common carotid artery occlusion which
indicated reversible of learning exibility de cit at 8
weeks. Memory capacity of the reverse platform loca-
tion in the reversal probe signi cantly decreased in UCO
group at all periods (p < 0.05) with no such the reversible
as found in learning exibility. Our data revealed about
cognitive type susceptible to CCH that was induced by
permanent right common carotid artery occlusion and it
was the cognitive exibility.
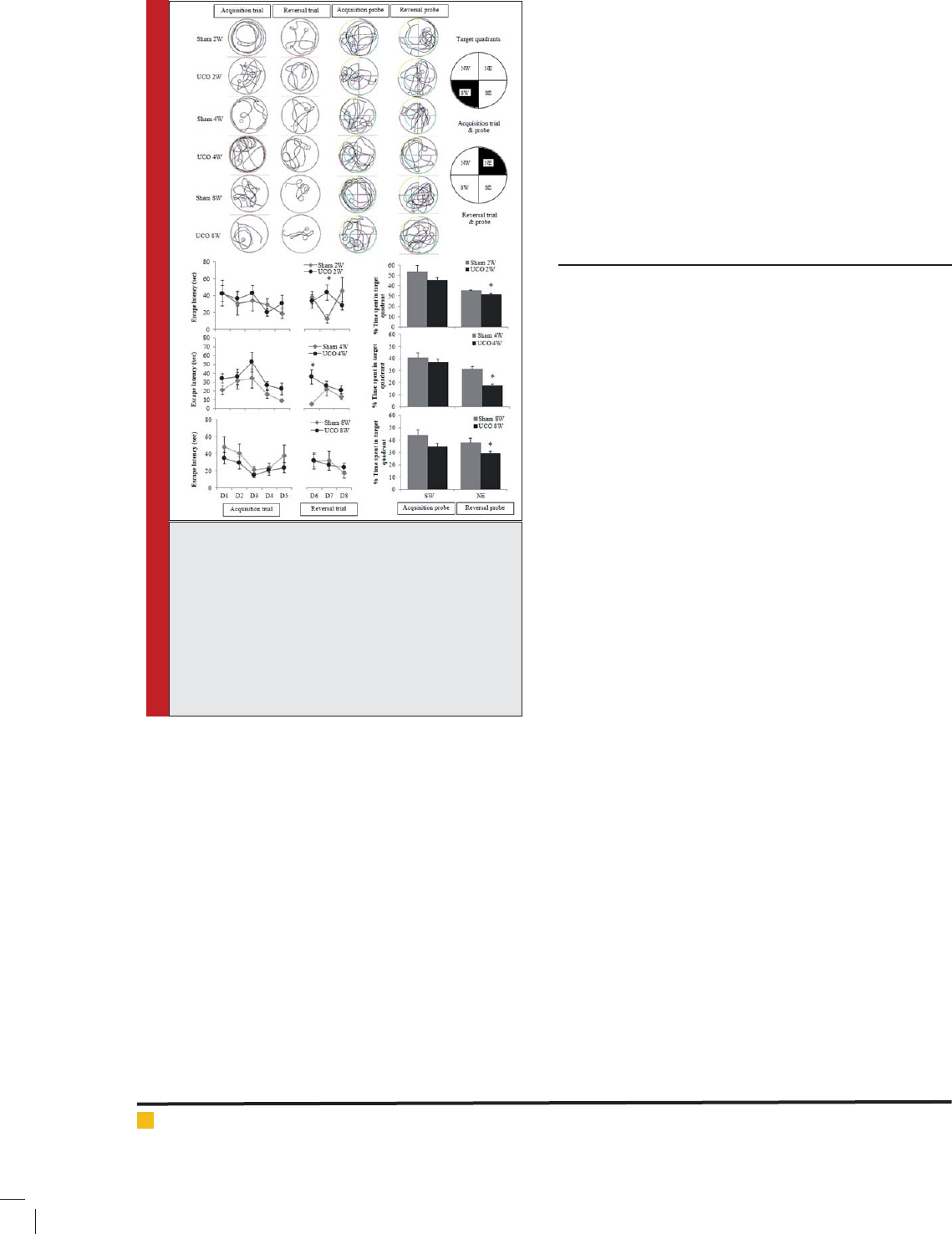
Anxiety-like behavior at 2, 4 and 8 weeks: Anxiety-like
behavior indicated by the number of open arm entries
and duration at 2, 4 and 8 weeks showed in Fig.2. The
result indicated that CCH induced by permanent right
BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COGNITIVE TYPE SUSCEPTIBILITY IN ICR MICE OF CCH MODEL 345
Sirilak Somredngan and Wachiryah Thong-asa
FIGURE 2. Representation of anxiety-like behavior assessed in the elevated plus maze (EPM).
Data interpreted as open arm entries and duration at 2, 4 and 8 weeks of right common
carotid artery occlusion.
FIGURE 3. Representation of brain tissue stained with 2 % 2, 3, 5-triph-
enyltetrazolium chloride (TTC) of frontal cortex, striatum and hippocampus.
White arrow indicated example of pale TTC area as identi cation of tissue
infarction. Scale bar = 1 cm.
346 COGNITIVE TYPE SUSCEPTIBILITY IN ICR MICE OF CCH MODEL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Sirilak Somredngan and Wachiryah Thong-asa
basal ganglia circuitry, facilitating a shift in strategies
and response pattern(Ragozzino et al. 2009).The present
study found signi cant infarction of striatum since 4
weeks that might correlated with this exibility de -
cit. However, reversible of learning exibility de cit did
not correlate with signi cant frontal cortex and stria-
tum infarction at 8 weeks. TTCtechnique use the reac-
tion of dehydrogenase to reduce TTC and turn to red
color in healthy tissue (Fishbein et al. 1981). Tissue lack
of blood perfusion was found to lead to lack of O
2
and
further NADH in cellular respiration. TTC technique had
high degree identi cation of infarct area and volume
and suitable for producing accurate measurements of
cerebral experimental infarcts as the use of cresyl violet
staining (Tureyen et al. 2004).
The present study identi ed pale area of TTC as not a
pure white, but pink-white, so we got a trend. It might
describe as penumbra area of ischemic tissue, perfu-
sion still present and cells are viable with hypofunc-
tion because of metabolic insuf cient. This area was
destined as delayed cell dead or survive (Heiss and Graf
1994). As we had found in the frontal cortex and stri-
atum, the reversible of learning exibility de cit at 8
weeks that not correlated with infarction volume might
involve compensatory mechanisms such as vascular
remodeling and improvement of collateral blood sup-
FIGURE 4. Representation of infarction area of frontal cortex, striatum and hippocampus using TTC staining
technique. The infarction area indicated by the percentage of infarction at 2, 4 and 8 weeks of permanent
right common carotid artery occlusion (UCO), * p < 0.05.

BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS COGNITIVE TYPE SUSCEPTIBILITY IN ICR MICE OF CCH MODEL 347
Sirilak Somredngan and Wachiryah Thong-asa
ply that help survive of cells in this penumbra-like area
in CCH model(Choy et al. 2006, Coyle and Panzenbeck
1990).The ipsilateral cerebral blood reduction in rCCAO
mice returned to normal level with 4 weeks was reported
(Yoshizaki et al. 2008), however, hemispheric perfusion
asymmetry was found in some challenge situation such
as hypercapnia. It was report about progressively less
pronounced with time but a slight asymmetry still per-
sists one month after unilateral carotid occlusion(Ley et
al. 1985). This hemodynamic insuf cient might last long
as the artery was occluded. Early affected of CCH was
on cognitive behavior while pathological appearance
of damaged neuron not signi cantly presented as has
been reported by Thong-asa et al. (2013), Thong-asa and
Tilokskulchai (2014) and Thong-Asa (2015).
Unlike present study, spatial learning and memory in
the acquisition trial and probe did not affected but learn-
ing exibility. The difference between acquisition and
reversal paradigm in the MWM produced a difference
outcome of stress and lead to difference effect on syn-
aptic density of the hippocampus. The divergent effects
of experiences on CA3 hippocampal synaptic activity,
i.e. stress as a suppressor and learning as a promoter
of synaptic plasticity (Sandi et al. 2003). Susceptibil-
ity of cognition type to CCH found in the present study
might involve stress inducing during reversal paradigm
as well. Learning exibility associated with prefrontal
cortex-basal ganglia circuitry while spatial acquisition
was hippocampal-dependent cognitive type. It was not
surprising that hippocampus-dependent learning ability
in acquisition trial did not correlate with hippocampus
infarction found in the present study.
The percentage of infarction area in the hippocam-
pus higher than the frontal cortex and striatum but it
was not signi cantly different until 8 weeks. There was
a report suggested that hippocampal-dependent spatial
learning only requires a minislab (down to 26% of total)
of dorsal hippocampal tissue (Moser et al. 1995).
The present study found infarction area not more
than 25% and not all of this area appear dead. It is
interesting that how long after appearance of signi cant
infarction in the hippocampus that the spatial learning
and memory in the acquisition paradigm will appear?
There are reports suggesting about increase of locomo-
tor activity and mental confusion such as anxiety-like
behavior in the ischemic experimental animals(Milot
and Plamondon 2009, Plamondon and Khan 2005) and
these factors might involve facilitation of cognitive abil-
ity in the cognitive tasks. Early after ischemic onset as
24 hours and 1 week, it appeared signi cantly anxiolytic
promoting of ischemia.This anxiolytic promoting disap-
peared in long term as time-dependent effects of global
cerebral ischemia. These reports provided data only
ischemic-reperfusion model unlike in the present study.
We assessed anxiety-like behavior at 2, 4 and 8 weeks of
CCH model and we found only trend that UCO mice had
higher open arm entries and duration than Sham with
no signi cant difference. We rst report about anxiety-
like behavior in CCH mice model, and it did not involve
or facilitate the cognitive abilities in this study.
In conclusion, the present study suggested that mild
CCH induced by permanent right common carotid artery
occlusion in ICR mice induce reversible learning ex-
ibility de cit but not memory of the reverse platform
location paradigm of the MWM. Our study imply about
cognitive type susceptible to mild CCH in ICR mice and
it was the learning exibility.
ACKNOWLEDGEMENTS
We would like to thank Department of Zoology, Fac-
ulty of Science, Kasetsart University for research facility
and assistance. This work was supported by a grant from
Graduate School, Kasetsart University.
Con ict of interest:
None.
REFERENCES
Adams, B., Fitch, T., Chaney, S. and Gerlai, R. (2002). Altered
performance characteristics in cognitive tasks: comparison
of the albino ICR and CD1 mouse strains.Behavioural Brain
Research, 133(2): 351-361.
Brosnan-Watters, G., Ogimi, T., Ford, D., Tatekawa, L., Gil-
liam, D., Bilsky, E. J. and Nash, D. (2000). Differential effects of
MK-801 on cerebrocortical neuronal injury in C57BL/6J, NSA,
and ICR Mice. Progress in Neuro-Psychopharmacology and
Biological Psychiatry, 24(6): 925-938.
Cheng, P., Ren, Y., Bai, S., Wu, Y., Xu, Y., Pan, J., Chen, J., Zhu,
X., Qi, Z., Shao, W., Tang, W., Liu, M., Xie, P. and Huang, W.
(2015). Chronic Cerebral Ischemia Induces Downregulation of
A1 Adenosine Receptors During White Matter Damage in Adult
Mice.Cell MolNeurobiol, 35(8): 1149-56.
Choy, M., Ganesan, V., Thomas, D. L., Thornton, J. S., Proctor,
E., King, M. D., van der Weerd, L., Gadian, D. G. and Lyth-
goe, M. F. (2006). The chronic vascular and haemodynamic
response after permanent bilateral common carotid occlusion
in newborn and adult rats. J Cereb Blood Flow Metab, 26(8):
1066-75.
Coyle, P. and Panzenbeck, M. J. (1990). Collateral development
after carotid artery occlusion in Fischer 344 rats. Stroke, 21(2):
316-21.
Dellu, F., Mayo, W., Vallee, M., Le Moal, M. and Simon, H.
(1997). Facilitation of cognitive performance in aged rats by
past experience depends on the type of information processing
involved: a combined cross-sectional and longitudinal study.
Neurobiol Learn Mem, 67(2): 121-8.
Du, S.-Q., Wang, X.-R., Xiao, L.-Y., Tu, J.-F., Zhu, W., He, T. and
Liu, C.-Z. (2016). Molecular Mechanisms of Vascular Dementia:
348 COGNITIVE TYPE SUSCEPTIBILITY IN ICR MICE OF CCH MODEL BIOSCIENCE BIOTECHNOLOGY RESEARCH COMMUNICATIONS
Sirilak Somredngan and Wachiryah Thong-asa
What Can Be Learned from Animal Models of Chronic Cerebral
Hypoperfusion? Molecular Neurobiology, 1-13.
Farkas, E., Luiten, P. G. M. and Bari, F. (2007). Permanent,
bilateral common carotid artery occlusion in the rat: A model
for chronic cerebral hypoperfusion-related neurodegenerative
diseases. Brain Research Reviews, 54(1): 162-180.
Fishbein, M. C., Meerbaum, S., Rit, J., Lando, U., Kanmatsuse,
K., Mercier, J. C., Corday, E. and Ganz, W. (1981). Early phase
acute myocardial infarct size quanti cation: validation of the
triphenyltetrazolium chloride tissue enzyme staining tech-
nique. Am Heart J, 101(5): 593-600.
Ge, J. F., Qi, C. C., Qiao, J. P., Wang, C. W. and Zhou, N. J.
(2013). Sex differences in ICR mice in the Morris water maze
task.Physiol Res, 62(1): 107-17.
Heiss, W. D. and Graf, R. (1994). The ischemic penumbra.Cur-
rOpinNeurol, 7(1): 11-9.
Kim, J.-S., Yang, M., Son, Y., Kim, S.-H., Kim, J.-C., Kim, S.,
Lee, Y., Shin, T. and Moon, C. (2008). Strain-dependent dif-
ferences of locomotor activity and hippocampus-dependent
learning and memory in mice.Toxicol. Res, 24(3): 183-188.
Komada, M., Takao, K. and Miyakawa, T. (2008). Elevated Plus
Maze for Mice. Journal of Visualized Experiments:JoVE, (22):
1088.
Ley, G. D., Nshimyumuremyi, J.-B. andLeusen, I. (1985). Hemi-
spheric blood ow in the rat after unilateral common carotid
occlusion: evolution with time. Stroke, 16: 69-73.
Milot, M. R. and Plamondon, H. (2009). Time-dependent effects
of global cerebral ischemia on anxiety, locomotion, and habit-
uation in rats.Behav Brain Res, 200(1): 173-80.
Moser, M. B., Moser, E. I., Forrest, E., Andersen, P. and Morris,
R. G. (1995). Spatial learning with a minislab in the dorsal hip-
pocampus.ProcNatlAcadSci U S A, 92(21): 9697-701.
Plamondon, H. and Khan, S. (2005). Characterization of anxi-
ety and habituation pro le following global ischemia in rats.
Physiology & Behavior, 84(4): 543-552.
Ragozzino, M. E., Mohler, E. G., Prior, M., Palencia, C. A. and
Rozman, S. (2009). Acetylcholine activity in selective striatal
regions supports behavioral exibility. Neurobiology of Learn-
ing and Memory, 91(1): 13-22.
Reid, W. M., Rolfe, A., Register, D., Levasseur, J. E., Churn, S.
B. and Sun, D. (2010). Strain-Related Differences after Experi-
mental Traumatic Brain Injury in Rats. J Neurotrauma, 27(7):
1243-1253.
Sandi, C., Davies, H. A., Cordero, M. I., Rodriguez, J. J., Popov,
V. I. and Stewart, M. G. (2003). Rapid reversal of stress induced
loss of synapses in CA3 of rat hippocampus following water
maze training.Eur J Neurosci, 17(11): 2447-56.
Thong-asa, K., Chompoopong, S., Tantisira, M. H. and Tilok-
skulchai, K. (2013). Reversible short-term and delayed long-
term cognitive impairment induced by chronic mild cerebral
hypoperfusion in rats.J Neural Transm, 120(8): 1225-35.
Thong-Asa, W. (2015). Early onset effects of mild chronic cere-
bral hypoperfusion on the dorsal hippocampus and white mat-
ter areas: The use of male sprague-dawley rats as a UCO model.
Journal of Neurological Sciences, 32(1): 030-039.
Thong-asa, W. and Tilokskulchai, K. (2014). Neuronal damage
of the dorsal hippocampus induced by long-term right com-
mon carotid artery occlusion in rats. Iran J Basic Med Sci,
17(3): 220 - 226.
Tureyen, K., Vemuganti, R., Sailor, K. A. and Dempsey, R. J.
(2004). Infarct volume quanti cation in mouse focal cerebral
ischemia: a comparison of triphenyltetrazolium chloride and
cresyl violet staining techniques. Journal of Neuroscience
Methods, 139(2): 203-207.
Yang, G., Kitagawa, K., Matsushita, K., Mabuchi, T., Yagita, Y.,
Yanagihara, T. and Matsumoto, M. (1997). C57BL/6 strain is
most susceptible to cerebral ischemia following bilateral com-
mon carotid occlusion among seven mouse strains: selective
neuronal death in the murine transient forebrain ischemia.
Brain Res, 752(1-2): 209-18.
Yoshizaki, K., Adachi, K., Kataoka, S., Watanabe, A., Tabira, T.,
Takahashi, K. and Wakita, H. (2008). Chronic cerebral hypoper-
fusion induced by right unilateral common carotid artery occlu-
sion causes delayed white matter lesions and cognitive impair-
ment in adult mice. Experimental Neurology, 210(2): 585-591.